Shape control of mesoporous silica nanomaterials templated with dual cationic surfactants and their antibacterial activities†
Nanjing
Hao
,
Xuan
Chen
,
Kalana W.
Jayawardana
,
Bin
Wu
,
Madanodaya
Sundhoro
and
Mingdi
Yan
*
Department of Chemistry, University of Massachusetts, 1 University Ave., Lowell, MA 01854, USA. E-mail: mingdi_yan@uml.edu; Fax: +1-978-934-3013; Tel: +1-978-934-3647
First published on 14th September 2015
Abstract
Mesoporous silica nanomaterials of different shapes (film, platelet, sphere, rod) were synthesized simply by tuning the mole ratio of dual cationic surfactant templates, cetyltrimethylammonium bromide (CTAB) and tetrabutylammonium iodine (TBAI). The film showed the most potent antibacterial activity against mycobacteria.
Mesoporous silica nanomaterials (MSNs) have shown a wide range of potential applications in drug delivery,1 protein transportation,2 bioimaging,3 cancer therapy,4 catalysis,5 renewable energy,6 and biosensing.7 MSNs are generally prepared from alkoxysilanes or silicates using a base or acid catalyst, and an organic surfactant or a block copolymer as the structure-directing template.8 Recent work has shown that the particle shape plays a critical role in successfully realizing many of the aforementioned applications.9
In this context, much effort has been devoted to the synthesis of MSNs of different shapes. For example, MSNs of various aspect ratios from sphere to rod can be prepared by changing the concentration of the cationic surfactant and/or catalyst,10 the catalyst type,11 and the stirring rate,12 or introducing an anionic surfactant as the co-template,13 an organic solvent as the co-solvent,14 or an organoalkoxysilane as the co-precursor.15 However, shapes other than sphere or rod have not been reported following these general synthetic strategies. Mesoporous silica platelets and films are two mesoporous silica structures that have shown promise in separation, catalysis, and biomedical applications.16 There are limited methods for the synthesis of well-defined mesoporous silica platelets.17 One strategy is to use a cationic/anionic surfactant as the confining bilayer and then let the Pluronic 123/silicate nanocomposite intercalate between the bilayers.17a Another strategy involved cocondensation of silicate and aminopropyltriethoxysilane in a surfactant solution under strongly acidic and microwave irradiation conditions.17b Mesoporous silica films have been prepared by self-assembly at solid/liquid/vapor interfaces by dip-/spin-coating on the solid substrate.18 However, these strategies are relatively complex and tedious, and most importantly difficult to alter the particle shape. Herein, we report a general strategy to synthesize MSNs of various shapes, including film-, platelet-, sphere-, and rod-like MSNs, by simply tuning the mole ratio of dual cationic surfactant templates, cetyltrimethylammonium bromide (CTAB) and tetrabutylammonium iodine (TBAI). These MSNs were further tested against mycobacteria (M. smegmatis strain mc2 651) to study the role of the particle shape on the antibacterial activity.
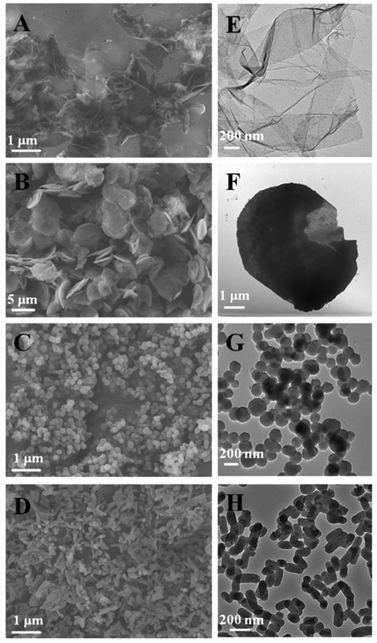
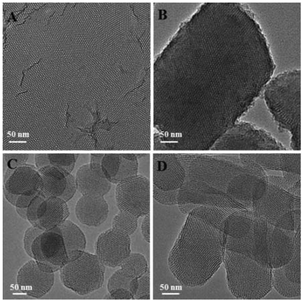
MSNs of different shapes were synthesized following a simple protocol of ammonia-catalyzed condensation of tetraethyl orthosilicate (TEOS) using CTAB and TBAI as co-templates (see the ESI† for details). As revealed by the scanning electron micrographs (SEM) and transmission electron micrographs (TEM) in Fig. 1, by varying the mole ratio of CTAB to TBAI (R = [CTAB]/[TBAI]), MSNs of different shapes were obtained. At R = 0.8, mesoporous silica films (FMSN) having an average thickness of 20 nm were formed (Fig. 1A). When R was changed to 1.5, the product turned into a platelet-like nanostructure (PMSN) with an average particle size of ∼5 μm and a thickness of 100–300 nm (Fig. 1B). At R = 2.5, spheres (SMSN) with an average particle size of ∼150 nm were obtained (Fig. 1C). Further increasing of R to 4 led to rods (RMSN) having an average particle size of ∼100 nm in width and ∼250 nm in length (Fig. 1D). After removing the templates by solvent extraction in acidic ethanol, the pore channels could be clearly seen in these materials (Fig. 2). Nitrogen adsorption–desorption measurement of all four samples showed the typical type IV isotherm (Fig. S1 and Table S1†), which corresponded to ordered cylindrical mesostructures.19 These materials possessed a relatively high Brunauer–Emmett–Teller (BET) specific surface area and pore volume, ranging from 606 to 1121 m2 g−1 and 0.48 to 1.03 cm3 g−1, respectively (Table S1†). All the four MSNs displayed a narrow pore size distribution, which centered around 2.8–3.4 nm as determined by the Barrett–Joyner–Halenda (BJH) method (Table S1†).
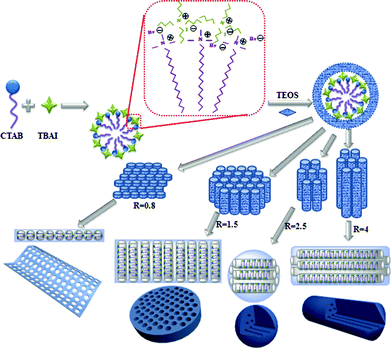
A mechanism was proposed to account for the formation of these MSNs (Scheme 1). At the initial stage of the reaction, TBAI, being a smaller surfactant having a shorter chain length, could insert into the CTAB micelles to form a self-assembled template structure.20 This hypothesis is further supported by the results that the pore size increased with increasing mole ratio of TBAI to CTAB (Table S1†). Ammonia-catalyzed hydrolysis of TEOS yielded negatively charged oligomeric silicate species that interact with the CTAB/TBAI micelle surface through electrostatic interactions to form a cylindrical CTAB/TBAI–silicate complex.21 When R is around 0.8, the relatively higher concentration of TBAI is expected to decrease the critical micelle concentration (CMC),22 which leads to a rapid aggregation of cylindrical micelles. The higher amount of TBAI also inhibited the growth of the micelles along the longitudinal axis, likely due to the reduced interactions of the CTAB alkyl tails caused by the diffusion of TBAI into the CTAB micelles.18a These led to thinner micelle aggregates, and when used as the template, gave a film-like nanostructure with perpendicular mesoporous channels. A similar film structure was also obtained at R = 1.0 (Fig. S2†). When R was increased to 1.5, the increased CTAB concentration led to continuous growth of micelles along the longitudinal direction of the cylindrical micelles. This type of template gave platelet-like nanostructures with mesoporous channels running along the thickness direction of PMSNs. A similar platelet structure was also obtained at R = 1.3 (Fig. S3†). At R = 2.5 and 4, sphere- and rod-shaped nanostructures were obtained. These are similar to the MSNs synthesized by using CTAB alone, indicating that the role of TBAI was significantly weakened and the CTAB dominated the template structure. TBAI plays a significant role in the assembly of micelles. Without TBAI, spherical particles were formed (Fig. S4†). However, TBAI alone as the template did not give any well-defined particles (Fig. S5†).
Because these nanomaterials are synthesized under the same conditions, they provide an excellent platform to study the effect of shape on their biological performance. The as-synthesized MSNs contain quaternary ammonium halides, which allow us to use their antimicrobial activity as a tool to investigate the shape effect. Cationic surfactants are found in many household products, such as soaps, shampoos, detergents, and cosmetics. Their antibacterial activities could be attributed to (i) adsorption and penetration into the cell wall; (ii) reaction with the cytoplasmic membrane followed by membrane disruption; (iii) leakage of intrabacterial materials; (iv) degradation of nucleic acids and proteins.23 Halide ions can rapidly penetrate into microorganisms and cause bacteria death by attacking nucleotides, proteins, and fatty acids.23 The as-synthesized differently shaped MSNs with surfactants embedded inside displayed positive zeta potentials from +9.43 to +16.56 mV (Fig. S6†), which allow them to capture the negatively charged bacterial cells by electrostatic interactions (Fig. S7†).24 After the surfactants were removed by extraction in acidic ethanol, the zeta potentials of the MSNs became negative (−34.89 to −19.39 mV, Fig. S8†). The adsorption peaks in the infrared spectra at 2925 cm−1 and 2847 cm−1 suggest that CTAB and TBAI surfactants were embedded inside MSNs (Fig. 3A and S9†). In the decomposition temperature range of CTAB and TBAI at 200–550 °C, the weight loss of the as-synthesized MSNs was determined as 16%, 23%, 31%, and 40% for FMSN, PMSN, SMSN, and RMSN, respectively (Fig. 3B). This shows that the amount of surfactants entrapped was the lowest for the films, followed by platelets and spheres. Rods contained more than twice the surfactants than the films.
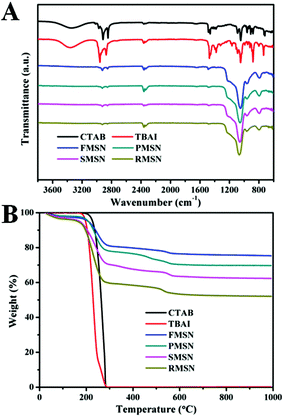 | ||
| Fig. 3 (A) FTIR spectra of CTAB, TBAI, and as-synthesized FMSN, PMSN, SMSN, and RMSN. (B) Thermogravimetric analysis (TGA) curves of CTAB, TBAI, and as-synthesized FMSN, PMSN, SMSN, and RMSN. | ||
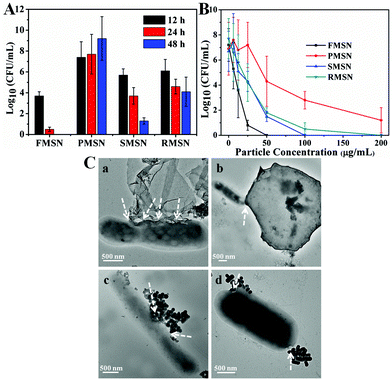
The antibacterial activity of the as-synthesized MSNs was tested against mycobacteria (M. smegmatis strain mc2 651) at different particle concentrations and incubation times. The minimal inhibitory concentration (MIC) of CTAB and TBAI was determined to be 18 and 90 μM, respectively. The antibacterial activity of FMSN, PMSN, SMSN, and RMSN was measured by dilution plate counting at different incubation times (Fig. 4A) and particle concentrations (Fig. 4B). The half inhibitory concentrations (IC50) of mycobacteria treated with the as-synthesized FMSN, PMSN, SMSN, and RMSN are 15, 93, 36, and 39 μg mL−1, respectively. The results showed that films exhibited the highest and platelets exhibited the lowest antibacterial activity. FMSN completely inhibited the formation of colonies at a low concentration of 25 μg mL−1 after 48 h treatment. The release profiles of CTAB and TBAI from differently shaped materials were further determined by suspending equal quantities of each material in PBS (pH 7.4), and the amount of surfactants released was determined by comparing with standard calibration curves from bromide and iodide ion standard solutions (see the ESI†). The results showed that the amount of released surfactants increased with time, and was highly dependent on the particle shape. The films released the fastest, followed by rods and spheres, and finally platelets (Fig. S10†). These results further supported that the antibacterial activity difference could be attributed to the shape-regulated release behavior of mesoporous silica materials.25 The films have the shortest and vertical pore channels which enabled the fastest release of antibacterial agents.
The interactions between the as-synthesized mesoporous materials and mycobacteria were further investigated under TEM. As is shown in Fig. 4C, the as-synthesized MSNs bound to the bacterial surface, likely due to the electrostatic attractions of surfactant-embedded MSNs with mycobacteria.24,26 The direct interaction facilitated the release of CTAB and TBAI, and increased the local concentration of quaternary ammonium cations and halide ions. The film exhibited high binding to mycobacteria. In addition, the interactions between FMSN and mycobacteria occurred mostly at the film edges (Fig. 4C and S11†). Similar observation has been reported for other films and 2D materials, where the edge-mediated interactions with the cell membrane cause effective cell death.27
In summary, we developed a new and general strategy to synthesize MSNs of different shapes including film, platelet, sphere and rod simply by tuning the mole ratio of dual cationic surfactant templates CTAB and TBAI. The antibacterial activity of these as-synthesized MSNs against mycobacteria (M. smegmatis strain mc2 651) is shape-dependent. FMSNs exhibited higher and faster antibacterial activity than other particle shapes, albeit the films contained the least amount of surfactants. Films also showed strong edge interactions with mycobacteria. Because these materials are synthesized using the same protocol, the results provide strong evidence for shape-dependent antimicrobial activities of nanomaterials.
The authors acknowledge the financial support from the National Institutes of Health (R01GM080295, R21AI109896), and a startup grant from the University of Massachusetts Lowell. We thank Professor William R. Jacobs of Albert Einstein College of Medicine for the kind gift of M. smegmatis strain mc2 651.
Live subject statement
All bacteria handling and experimental protocols were performed in accordance with the University of Massachusetts Lowell guidelines. The procedures were conducted using approved Institutional Biosafety Committee (IBC) procedures (registration no. 15-06-YAN).Notes and references
- M. Colilla, B. González and M. Vallet-Regí, Biomater. Sci., 2013, 1, 114 RSC.
- S. Hudson, J. Cooney and E. Magner, Angew. Chem., Int. Ed., 2008, 47, 8582 CrossRef CAS PubMed.
- J. A. Barreto, W. O'Malley, M. Kubeil, B. Graham, H. Stephan and L. Spiccia, Adv. Mater., 2011, 23, H18 CrossRef CAS PubMed.
- T. Yoshitomi and Y. Nagasaki, Biomater. Sci., 2015, 3, 810 RSC.
- A. Stein, B. J. Melde and R. C. Schroden, Adv. Mater., 2000, 12, 1403 CrossRef CAS.
- J. Liu, S. Z. Qiao, J. S. Chen, X. W. Lou, X. R. Xing and G. Q. Lu, Chem. Commun., 2011, 47, 12578 RSC.
- N. J. Hao, K. Neranon, O. Ramström and M. Yan, Biosens. Bioelectron. DOI:10.1016/j.bios.2015.07.031.
- F. Hoffmann, M. Cornelius, J. Morell and M. Fröba, Angew. Chem., Int. Ed., 2006, 45, 3216 CrossRef CAS PubMed.
- (a) N. J. Hao, L. F. Li and F. Q. Tang, J. Mater. Chem. A, 2014, 2, 11565 RSC; (b) K. Yang and Y. Q. Ma, Nat. Nanotechnol., 2010, 5, 579 CrossRef CAS PubMed; (c) N. J. Hao, L. F. Li and F. Q. Tang, J. Biomed. Nanotechnol., 2014, 10, 2508 CrossRef CAS PubMed.
- (a) N. J. Hao, H. H. Yang, L. F. Li, L. L. Li and F. Q. Tang, New J. Chem., 2014, 38, 4258 RSC; (b) N. J. Hao, L. L. Li, Q. Zhang, X. L. Huang, X. W. Meng, Y. Q. Zhang, D. Chen, F. Q. Tang and L. F. Li, Microporous Mesoporous Mater., 2012, 162, 14 CrossRef CAS; (c) G. Lelong, S. Bhattacharyya, S. Kline, T. Cacciaguerra, M. A. Gonzalez and M. L. Saboungi, J. Phys. Chem. C, 2008, 112, 10674 CrossRef CAS.
- Q. Cai, Z. S. Luo, W. Q. Pang, Y. W. Fan, X. H. Chen and F. Z. Cui, Chem. Mater., 2001, 10, 258 CrossRef.
- H. Jin, Z. Liu, T. Ohsuna, O. Terasaki, Y. Inoue, K. Sakamoto, T. Nakanishi, K. Ariga and S. Che, Adv. Mater., 2006, 18, 593 CrossRef CAS.
- H. M. Chen and J. H. He, Chem. Commun., 2008, 4422 RSC.
- D. Zhao, J. Sun, Q. Li and G. D. Stucky, Chem. Mater., 2000, 12, 275 CrossRef CAS.
- S. Huh, J. W. Wiench, J.-C. Yoo, M. Pruski and V. S.-Y. Lin, Chem. Mater., 2003, 15, 4247 CrossRef CAS.
- (a) K. J. Edler and B. Yang, Chem. Soc. Rev., 2013, 42, 3765 RSC; (b) N. Ehlert, P. P. Mueller, M. Stieve, T. Lenarz and P. Behrens, Chem. Soc. Rev., 2013, 42, 3847 RSC.
- (a) B. C. Chen, H. P. Lin, M. C. Chao, C. Y. Mou and C. Y. Tang, Adv. Mater., 2004, 16, 1657 CrossRef CAS; (b) Sujandi, S. E. Park, D. S. Han, S. C. Han, M. J. Jin and T. Ohsuna, Chem. Commun., 2006, 4131 RSC.
- (a) Z. G. Teng, G. F. Zheng, Y. Q. Dou, W. Li, C. Y. Mou, X. H. Zhang, A. M. Asiri and D. Y. Zhao, Angew. Chem., Int. Ed., 2012, 51, 2173 CrossRef CAS PubMed; (b) A. Walcarius, E. Sibottier, M. Etienne and J. Ghanbaja, Nat. Mater., 2007, 6, 602 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- P. L. He, B. Xu, H. L. Liu, S. He, F. Saleem and X. Wang, Sci. Rep., 2013, 3, 1833 Search PubMed.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710 CrossRef CAS.
- H. O. Pastore, M. Munsignatti, D. R. S. Bittencourt and M. M. Rippel, Microporous Mesoporous Mater., 1999, 32, 211 CrossRef CAS.
- G. McDonnell and A. D. Russell, Clin. Microbiol. Rev., 1999, 12, 147 CAS.
- N. J. Hao, K. W. Jayawardana, X. Chen and M. Yan, ACS Appl. Mater. Interfaces, 2015, 7, 1040 CAS.
- B. G. Trewyn, C. M. Whitman and V. S.-Y. Lin, Nano Lett., 2004, 4, 2139 CrossRef CAS.
- T. J. Kinnari, J. Esteban, E. Gomez-Barrena, N. Zamora, R. Fernandez-Roblas, A. Nieto, J. C. Doadrio, A. López-Noriega, E. Ruiz-Hernández, D. Arcos and M. Vallet-Regí, J. Biomed. Mater. Res., Part A, 2009, 89, 215 Search PubMed.
- (a) Y. F. Li, H. Y. Yuan, A. von dem Bussche, M. Creighton, R. H. Hurt, A. B. Kane and H. J. Gao, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 12295 CrossRef CAS PubMed; (b) Y. S. Tu, M. Lv, P. Xiu, T. Huynh, M. Zhang, M. Castelli, Z. R. Liu, Q. Huang, C. H. Fan, H. P. Fang and R. H. Zhou, Nat. Nanotechnol., 2013, 8, 594 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, material characterization and additional data. See DOI: 10.1039/c5bm00197h |
| This journal is © The Royal Society of Chemistry 2016 |