Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Determination of catecholamines and related compounds in mouse urine using column-switching HPLC
Takahiro
Kanamori
,
Takashi
Funatsu
and
Makoto
Tsunoda
*
Graduate School of Pharmaceutical Sciences, University of Tokyo, Tokyo, Japan. E-mail: makotot@mol.f.u-tokyo.ac.jp; Tel: +81 3 5841 4761
First published on 31st March 2016
Abstract
We have developed an analytical method for the determination of catecholamines and related compounds in mouse urine by column-switching HPLC. Selective extraction of the catechol compounds was performed using a precolumn modified with phenylboronic acid, which has a pH dependent affinity for the catechol structures. The pretreatment buffer, which facilitated binding of the catechols to the precolumn, was optimized to ensure high analyte recoveries and good peak shapes. We found that using the same acetonitrile content in the pretreatment buffer and hydrophilic interaction liquid chromatography mobile phase was necessary to improve peak shapes. Eight catechol compounds were selectively extracted and separated using 100 mmol L−1 ammonium formate/acetonitrile (20/80 v/v, pH 8.0) for the extraction step, and 20 mmol L−1 ammonium formate (pH 2.5)/acetonitrile (20/80 v/v) for elution and separation. Native fluorescence of the separated catechol compounds was monitored, and the limits of detection, corresponding to a signal to noise ratio of 3, were 9–58 nmol L−1. Five catechol compounds (dopamine, epinephrine, norepinephrine, 3,4-dihydroxyphenylglycol, and 3,4-dihydroxymandelic acid) were successfully quantified in mouse urine. Intra- and inter-day precisions were less than 10%, and performance was superior to that afforded by manual sample pretreatment.
Introduction
Catecholamines are monoamines with a catechol structure and play important roles as neurotransmitters and hormones.1 Determination of these compounds is required for the elucidation of physiological regulation pathways that are controlled by the sympathetic nervous system. Furthermore, the measurement of catecholamines and their metabolites is used for disease diagnosis, such as pheochromocytomas and neuroblastomas; these are disease states that result in elevated levels of catecholamines.2,3 The amounts of catecholamines in biological fluids such as urine and plasma are very low, so there is demand for highly sensitive and selective analytical methods for the determination of catecholamines.High-performance liquid chromatography (HPLC) is widely used for the simultaneous determination of catecholamines and their metabolites.4,5 Reversed-phase liquid chromatography (RPLC) has been used in most studies to separate catechol compounds.6–9 However, the simultaneous measurement of multiple catechol compounds, including catecholamines and their precursors and metabolites, is challenging because catechols are hydrophilic and are weakly retained under RPLC conditions.
Hydrophilic interaction liquid chromatography (HILIC) is a chromatographic technique that uses a hydrophilic stationary phase and an organic-rich mobile phase.10 Under HILIC conditions, hydrophilic compounds are strongly retained by hydrogen bonding, ion exchange, and hydrophilic partitioning.11–14 On this basis, we therefore supposed that HILIC may be suitable for the separation of hydrophilic catechol compounds and accordingly developed a HILIC method with fluorescence detection for determination of catechol compounds in mouse urine.15 The new method allowed the separation of eight catechol compounds within 15 min. Furthermore, high sensitivity was realized without the use of fluorescence derivatization, probably because of an enhancement in the fluorescence intensity in the acetonitrile-rich mobile phase used for HILIC separation.
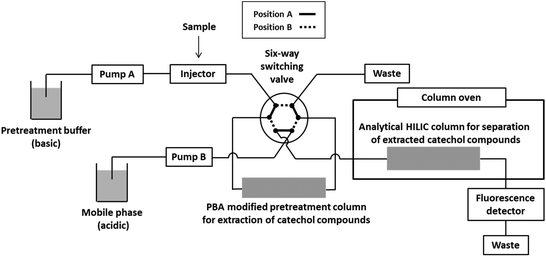
It is important to account for the potential effects of interfering substances present when analyzing biological fluids, such as urine and plasma. Interfering substances may lower the selectivity and sensitivity for the target compounds. Sample pretreatment is a strategy used to remove interfering substances and/or enrich target compounds.16 Protein precipitation, liquid–liquid extraction, and solid phase extraction (SPE) are widely used for sample pretreatments. We have previously used selective SPE with a phenylboronic acid (PBA)-modified spin column as a pretreatment for samples containing catechol compounds.15,17,18 PBA forms a negatively charged complex with the cis-hydroxy groups of catechol compounds in basic conditions. The bound catechol compounds can be subsequently released by lowering the pH with an acidic media.19 However, the pretreatment of samples by SPE with the spin column technique is time-consuming and tedious. Furthermore, variations in the timing of each step in SPE procedures can affect recoveries because catechol compounds are unstable in basic conditions. To overcome this problem, it is necessary to use a column-switching system whereby the precolumn for selective extraction of catechols is directly coupled to the analytical column. In this study, we developed a method for determining catechol compounds (Fig. 1) by column-switching using a PBA modified column, followed by separation on a HILIC column. An acidic mobile phase was used for HILIC and this served as an effective eluent for the desorption of the analytes from the precolumn. Systematic studies concerning the composition of the pretreatment buffer and the adsorption time were first undertaken so that thereafter, satisfactory results were obtained for determining catechol compounds in mouse urine samples.
 | ||
| Fig. 1 Chemical structure of catechol compounds; 1, DA; 2, NE; 3, E; 4, DOPA; 5, DOPAC; 6, DHPG; 7, DHMA; 8, N-MeDA. | ||
Experimental
Chemicals and reagents
Dopamine (DA), epinephrine (E), norepinephrine (NE), 3,4-dihydroxyphenylalanine (DOPA), 3,4-dihydroxyphenylacetic acid (DOPAC), 3,4-dihydroxyphenylglycol (DHPG), 3,4-dihydroxymandelic acid (DHMA), deoxyepinephrine (N-MeDA), 3-methoxytyramine (3-MT), and acetonitrile (ACN, HPLC grade) were obtained from Sigma-Aldrich (St. Louis, MO, USA). Other reagents used were analytical grade. Water was purified using a Milli-Q system (Merck Millipore, Darmstadt, Germany).HPLC apparatus and chromatographic conditions
Fig. 2 shows a schematic diagram of the column-switching system. The system used consisted of two pumps (PU-2080 Plus, JASCO, Tokyo Japan), a high-pressure six-way switching valve (SI-2, Shiseido, Tokyo, Japan), a column oven (860-CO, JASCO), and a fluorescence detector (RF-20A, Shimadzu, Kyoto, Japan). A PBA-modified precolumn (30 mm × 3.0 mm i.d., 7 μm, GL Sciences, Tokyo, Japan) for extraction of catechols and a HILIC analytical column (Inertsil Amide, 150 mm × 3.0 mm i.d., 5 μm, GL Sciences) were connected as illustrated in Fig. 2. The pretreatment buffer for retaining catechols on the precolumn was 100 mmol L−1 ammonium formate/acetonitrile (20/80 v/v, pH 8.0). The pH of the pretreatment buffer was adjusted by measuring the pH of the mixture of acetonitrile and aqueous ammonium formate. The mobile phase for elution from the precolumn and analytical separation on the HILIC column was 20 mmol L−1 ammonium formate (pH 2.5)/acetonitrile (20/80 v/v). The flow rates of the pretreatment buffer and the mobile phase were 0.4 and 0.5 mL min−1, respectively. The temperature of the column oven was set to 35 °C, and the fluorescence detection of catechol compounds was performed using excitation and emission wavelengths of 280 and 320 nm, respectively. Chromatograms were analyzed using Chromato-Pro software (Run Time Corporation, Kanagawa, Japan).Sample preparation
Urine samples were obtained from male C57BL/6J mice (6–7 months old). Hydrochloric acid solution (6 mol L−1) was added to urine samples to preserve the catechol compounds. Urine samples were diluted 2-fold with 1 mol L−1 sodium phosphate buffer (pH 8.0) before analysis. The diluted urine solutions were injected into the column-switching system.Selective extraction and separation of catechol compounds
The valve was initially set to position A (Fig. 2), and the pretreatment buffer and the mobile phase were carried from Pump A and Pump B, to the precolumn and the analytical column, respectively. The sample solution was injected and carried to the precolumn, where the catechol compounds in the sample were selectively bound. Potentially interfering compounds, which had no affinity for the precolumn, were flushed to waste from the precolumn by the pretreatment buffer. The valve was changed to position B 120 s after sample injection, resulting in reverse flow of the mobile phase through the precolumn to induce desorption and transport of the catechol compounds to the analytical column.Validation
The limit of detection (LOD) and limit of quantification (LOQ) were calculated for a signal-to-noise ratio (S/N) of 3 and 10, respectively. Quantification was performed using a relative peak height basis with N-MeDA as the internal standard. Five standard concentrations of the catechol compounds (DHPG: 0.05–5 μmol L−1, DHMA: 0.25–25 μmol L−1, DA, E, NE: 0.025–2.5 μmol L−1, and DOPA: 0.1–10 μmol L−1) were analyzed to prepare calibration curves. Analyte recoveries were studied by spiking additional catechol compounds into mouse urine at two different concentrations (0.55 and 1.1 μmol L−1 for DHPG, DHMA, and NE; 1.1 and 2.2 μmol L−1 for DA; 0.275 and 0.55 μmol L−1 for E). The recovery values were calculated as the ratio of the increase in the amount of the catechol compounds, quantified using the calibration curve, to the amount of added catechol compounds. Urine samples were analyzed five times on the same day and on consecutive days to assess the intra- and inter-day precisions, respectively.Results and discussion
The extraction and separation conditions were optimized for our experimental system (Fig. 2). First, the composition of the mobile phase for separation of the catechols on the HILIC column was studied. Next, the composition of the pretreatment buffer and adsorption time were investigated to ensure high analyte recoveries and achieve efficient removal of interfering compounds from the mouse urine samples. The pH-controlled binding of catechol compounds to PBA was exploited to retain analytes on the precolumn. A basic pretreatment buffer ensured retention of catechols on the precolumn while an acidic mobile phase induced release and elution of the analytes.HILIC column selection and optimization of the mobile phase
We have previously used a phosphorylcholine-modified HILIC column (ZIC-cHILIC) for the separation of eight catechol compounds—DA, E, NE, DOPA, DHPG, DOPAC, DHMA, and 3,4-dihydroxybenzylamine (DHBA, internal standard).15 For this study, we chose an amide-modified column (Inertsil Amide) because it offered good separation of the eight catechol compounds when using N-MeDA as the internal standard with a shorter analysis time compared with that of the ZIC-cHILIC column. The composition of the mobile phase (acetonitrile content, pH, and salt concentration) was investigated, and the optimal mobile phase consisted of 20 mmol L−1 ammonium formate (pH 2.5)/acetonitrile (20/80 v/v).Optimization of pretreatment buffer
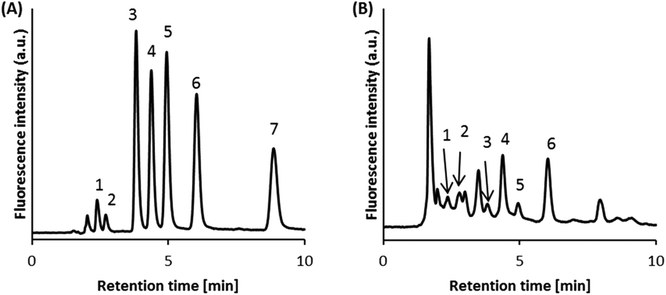
We initially used a 5 mmol L−1 phosphate buffer (pH 8.0) without any organic solvent as the pretreatment buffer. Fig. 3A shows the chromatograms for the catechols separated on the HILIC column after on-line pretreatment. DOPAC, DHPG, and DHMA were not detected, and the peak widths for N-MeDA, DA, E, NE, and DOPA were broader than those obtained without the use of a precolumn. The recoveries of N-MeDA, DA, E, NE, and DOPA were 12%, 18%, 16%, 22%, and 15%, respectively. The poor recoveries and peak broadening may be related to the large differences in the amounts of organic solvent in the pretreatment buffer (0%) and the mobile phase (80%). The poor peak shapes observed might be caused by the water-rich pretreatment buffer, because water-rich samples have been reported to distort the peak shapes of HILIC.20–23 We investigated a 5 mmol L−1 phosphate pretreatment buffer/acetonitrile (20/80 v/v, pH 8.0) in an attempt to improve peak shape. Using this pretreatment buffer, all catechol compounds were detected and separation efficiency was excellent (Fig. 3B). The recoveries were also markedly improved: DOPAC, 8%; DHPG, 43%; DHMA, 42%; N-MeDA, 59%; DA, 49%; E, 64%; NE, 51%; and DOPA, 84%. The improved results indicate that the composition of the pretreatment buffer is important for achieving efficient separation.Optimization of pretreatment conditions
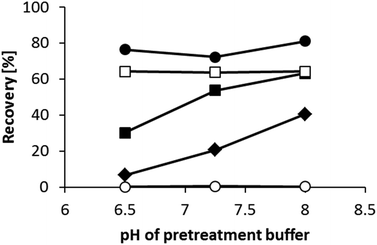
We next investigated the effects of pretreatment buffer pH on the recoveries of the analytes. Fig. 4 shows the recovery data for DOPAC (acidic), DHPG (neutral), DA (basic), DOPA (zwitterionic), and 3-MT (O-methylated dopamine). We analyzed 3-MT to confirm the selectivity of the precolumn for catechol structures. When the pH of the pretreatment buffer was 8.0, catechol compounds with a positive charge (DA and DOPA) had an improved retention on the precolumn compared with compounds without positive charge (DOPAC and DHPG). This was partly caused by the electrostatic interaction between the catechols and the negative charge of PBA. We found that 3-MT was barely retained, indicating that the PBA-modified column was selective for catechol structures. The retention of DHPG and DOPAC decreased with lower pH pretreatment buffers, indicating the interaction between the catechols and PBA is stronger at higher pH. We selected pH 8.0 as the optimal pH for the pretreatment buffer.Optimization of the adsorption time (from sample injection to valve switching) was evaluated by analyzing a mouse urine sample. We found that 120 s was sufficient to remove the interfering compounds in the sample. Fig. 5 shows typical chromatograms for a standard solution and a mouse urine sample analyzed using the optimal conditions. The concentrations of the catechols quantified in mouse urine by the developed method were as follows: 0.04 ± 0.01 μmol L−1 for DHPG, 0.38 ± 0.27 μmol L−1 for DHMA, 1.31 ± 0.27 μmol L−1 for DA, 0.03 ± 0.004 μmol L−1 for E, and 0.56 ± 0.03 μmol L−1 for NE (n = 3). These values are similar to those found in previous studies.24,25 DOPAC and DOPA could not be quantified in mouse urine because of interferences by impurities and low concentrations, respectively.
Validation of the developed method
The linear ranges, the LODs, and the LOQs for the developed method are shown in Table 1. Although sensitivity was lower than that for chemiluminescence detection,26,27 it was sufficient for quantification of the analytes in the mouse urine samples. High sensitivity was obtained compared with RPLC separation, without the need for fluorescence derivatization, probably because of enhanced fluorescence intensity in the acetonitrile-rich mobile phase.15Table 2 summarizes precision and recovery data obtained in the present study. The recovery of DHPG was lower than the recovery of the other catechol compounds. This may be attributed to a sample matrix effect, and/or to the fact that DHPG had a lower affinity for PBA than the other catechols.| LOD [nmol L−1] | LOQ [nmol L−1] | Linearity [μmol L−1] | |||
|---|---|---|---|---|---|
| S/N = 3 | LOD [fmol] | S/N = 10 | LOQ [fmol] | r 2 > 0.99 | |
| DHPG | 9 | 109 | 30 | 365 | 0.05–5 |
| DHMA | 58 | 700 | 194 | 2330 | 0.25–25 |
| DA | 3 | 38 | 11 | 127 | 0.025–2.5 |
| E | 5 | 59 | 16 | 196 | 0.025–2.5 |
| NE | 4 | 49 | 14 | 163 | 0.025–2.5 |
| DOPA | 13 | 152 | 42 | 508 | 0.1–10 |
| Intra-day precision | Inter-day precision | |||||||
|---|---|---|---|---|---|---|---|---|
| Catechol compounds | Concentration (μmol L−1) | Concentration (μmol L−1) | ||||||
| (n = 5) (added/μmol L −1) | Mean | SD | C.V. (%) | Recovery (%) | Mean | SD | C.V. (%) | Recovery (%) |
| DHPG | ||||||||
| 0 | 0.04 | 0.004 | 8.4 | — | 0.03 | 0.002 | 7.6 | — |
| 0.55 | 0.17 | 0.01 | 6.3 | 23 | 0.19 | 0.01 | 5.6 | 29 |
| 1.1 | 0.24 | 0.02 | 6.5 | 18 | 0.29 | 0.01 | 3.8 | 23 |
| DHMA | ||||||||
| 0 | 0.22 | 0.02 | 8.0 | — | 0.11 | 0.01 | 7.8 | — |
| 0.55 | 0.74 | 0.04 | 6.0 | 95 | 0.57 | 0.03 | 4.6 | 85 |
| 1.1 | 1.09 | 0.07 | 6.3 | 79 | 1.03 | 0.03 | 2.5 | 84 |
| DA | ||||||||
| 0 | 1.69 | 0.02 | 1.4 | — | 1.09 | 0.02 | 1.7 | — |
| 1.1 | 2.62 | 0.05 | 1.9 | 84 | 2.04 | 0.04 | 1.8 | 86 |
| 2.2 | 3.30 | 0.08 | 2.4 | 73 | 2.92 | 0.07 | 2.5 | 83 |
| E | ||||||||
| 0 | 0.03 | 0.002 | 8.1 | — | 0.02 | 0.001 | 3.9 | — |
| 0.275 | 0.26 | 0.01 | 4.1 | 83 | 0.27 | 0.01 | 4.5 | 92 |
| 0.55 | 0.57 | 0.05 | 8.8 | 99 | 0.56 | 0.01 | 2.6 | 98 |
| NE | ||||||||
| 0 | 0.70 | 0.02 | 2.3 | — | 0.58 | 0.01 | 2.0 | — |
| 0.55 | 1.20 | 0.03 | 2.6 | 92 | 1.05 | 0.02 | 1.8 | 87 |
| 1.1 | 1.78 | 0.04 | 2.3 | 99 | 1.62 | 0.05 | 3.0 | 94 |
Advantages of the column-switching system for the analysis of catechols
The analytical method we have developed for the determination of catechol compounds possesses two advantages. One is that the extraction procedure is convenient and reproducible compared with SPE using the spin column technique.15 In the present method, samples can be injected directly, so the centrifugation procedure needed for spin column SPE can be omitted. Furthermore, the precision of the developed method was less than 10%, while the precision for extraction using the spin column technique was greater than 10%, which showed that the reproducibility of extraction of catechols by column-switching was superior to manual pretreatment.The other advantage is that a dilution of sample by acetonitrile, which would result in a decrease in method sensitivity, is unnecessary. In HILIC analysis, the sample composition should be acetonitrile-rich. If a water-rich sample is injected into the HILIC column, peak shapes are distorted. The use of a high proportion of acetonitrile in the pretreatment buffer prevents the water in the aqueous samples from distorting peak shapes.
Conclusions
We have developed an analytical method for determination of catechol compounds in mouse urine samples utilizing a column-switching system with a PBA-modified precolumn to extract the analytes for separation on a HILIC column. The PBA-modified precolumn showed selectivity for catechol compounds and permitted the determination of five catechol compounds in mouse urine samples. Moreover, the extraction of the catechol compounds was simple and reproducible. Sample dilution with acetonitrile, which is typically required for HILIC, was not necessary. The proposed method should be useful in clinical chemistry and for research on catecholamine-related diseases.Acknowledgements
This research was partially supported by a Grant-in-Aid for Scientific Research (C) from the Japan Society for the Promotion of Science (JSPS) (Grant Number 26460033), Scientific Research on Innovative Areas (Grant Number 15H01527), and the Center of Innovation Program from the Japan Science and Technology Agency (JST).References
- G. Eisenhofer, I. J. Kopin and D. S. Goldstein, Pharmacol. Rev., 2004, 56, 331–349 CrossRef CAS PubMed.
- R. Subramaniam, Trends Anaesth. Crit. Care, 2011, 1, 104–110 CrossRef.
- A. Hallett and H. Traunecker, Paediatr. Child Health, 2012, 22, 103–107 CrossRef.
- J. Bicker, A. Fortuna, G. Alves and A. Falcão, Anal. Chim. Acta, 2013, 768, 12–34 CrossRef CAS PubMed.
- M. Tsunoda, Anal. Bioanal. Chem., 2006, 386, 506–514 CrossRef CAS PubMed.
- K. Takezawa, M. Tsunoda, K. Murayama, T. Santa and K. Imai, Analyst, 2000, 125, 293–296 RSC.
- M. Tsunoda, K. Takezawa, T. Santa and K. Imai, Anal. Biochem., 1999, 269, 386–392 CrossRef CAS PubMed.
- M. Tsunoda, M. Nagayama, T. Funatsu, S. Hosoda and K. Imai, Clin. Chim. Acta, 2006, 366, 168–173 CrossRef CAS PubMed.
- M. Tsunoda, K. Takezawa and K. Imai, Analyst, 2001, 126, 637–640 RSC.
- A. J. Alpert, J. Chromatogr., 1990, 499, 177–196 CrossRef CAS PubMed.
- B. Buszewski and S. Noga, Anal. Bioanal. Chem., 2012, 402, 231–247 CrossRef CAS PubMed.
- P. Hemström and K. Irgum, J. Sep. Sci., 2006, 29, 1784–1821 CrossRef.
- M. Isokawa, T. Kanamori, T. Funatsu and M. Tsunoda, Bioanalysis, 2014, 6, 2421–2439 CrossRef CAS PubMed.
- P. Jandera, Anal. Chim. Acta, 2011, 692, 1–25 CrossRef CAS PubMed.
- T. Kanamori, M. Isokawa, T. Funatsu and M. Tsunoda, J. Chromatogr., B, 2015, 985, 142–148 CrossRef CAS PubMed.
- R. M. Smith, J. Chromatogr., A, 2003, 1000, 3–27 CrossRef CAS PubMed.
- M. Tsunoda, C. Aoyama, S. Ota, T. Tamura and T. Funatsu, Anal. Methods, 2011, 3, 582–585 RSC.
- M. Tsunoda, M. Hirayama, K. Ohno and T. Tsuda, Anal. Methods, 2013, 5, 5161–5164 RSC.
- I. D. Wilson and P. Martin, in Solid-Phase Extraction: Principles, Techniques, and Applications, ed. N. J. K. Simpson, Marcel Dekker, New York, 2000, ch. 11, pp. 331–349 Search PubMed.
- J. Ruta, S. Rudaz, D. V. McCalley, J.-L. Veuthey and D. Guillarme, J. Chromatogr., A, 2010, 1217, 8230–8240 CrossRef CAS PubMed.
- M. Isokawa, T. Funatsu and M. Tsunoda, Analyst, 2013, 138, 3802–3808 RSC.
- M. Isokawa, T. Funatsu and M. Tsunoda, Chromatographia, 2014, 77, 1553–1556 CAS.
- T. Kanamori, T. Funatsu and M. Tsunoda, Chromatography, 2015, 36, 123–126 CrossRef.
- M. Moreira-Rodrigues, J. Quelhas-Santos, R. Roncon-Albuquerque, P. Serrao, A. Leite-Moreira, B. Sampaio-Maia and M. Pestana, Exp. Biol. Med., 2012, 237, 949–955 CrossRef CAS PubMed.
- S. M. Kim, F. Theilig, Y. Qin, T. Cai, D. Mizel, R. Faulhaber-Walter, H. Hirai, S. Bachmann, J. P. Briggs, A. L. Notkins and J. Schnermann, Am. J. Physiol. Renal Physiol., 2009, 296, F382–F389 CrossRef CAS PubMed.
- M. Tsunoda and K. Imai, Anal. Chim. Acta, 2005, 541, 13–23 CrossRef CAS.
- M. Tsunoda and T. Funatsu, Anal. Bioanal. Chem., 2012, 402, 1393–1397 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |