Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Columnar propeller-like 1,3,5-triphenylbenzenes: the missing link of shape-persistent hekates†
Tobias
Wöhrle
a,
Stuart James
Beardsworth
a,
Christopher
Schilling
a,
Angelika
Baro
a,
Frank
Giesselmann
b and
Sabine
Laschat
*a
aInstitut für Organische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D-70569 Stuttgart, Germany. E-mail: sabine.laschat@oc.uni-stuttgart.de
bInstitut für Physikalische Chemie, Universität Stuttgart, Pfaffenwaldring 55, D-70569 Stuttgart, Germany
First published on 4th March 2016
Abstract
Triphenylbenzenes with different substitution patterns at the outer phenyl rings have been successfully synthesised. Sixfold n-alkoxy substitution was insufficient for mesomorphism, but already increasing the number of side chains by three methoxy groups led to liquid crystalline behaviour and mesophase formation. Symmetrical triphenylbenzenes with nine n-alkoxy side chains (≥C9) formed broad enantiotropic mesophases. The symmetry of the liquid crystalline phases was unambiguously determined by X-ray diffraction measurements as Colh and Colho for symmetry-reduced methoxy–alkoxy derivatives and symmetrical nona-alkoxy-triphenylbenzenes, respectively. Based on X-ray diffraction data a stacking model was proposed in which the single molecules aggregate to helical columns forming a mesophase.
Introduction
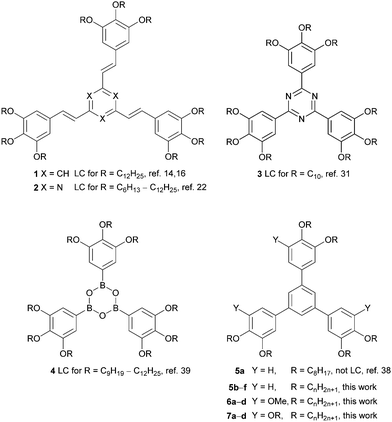
Discotic liquid crystals, which self-assemble into columnar mesophases, have been intensively studied over the last few decades, because their anisotropic properties, high 1D charge carrier mobility and self-healing of defects make them attractive for molecular electronics.1,2 Star-shaped mesogens with three or more arms pointing to the vortices of 2D polygons or 3D polyhedra are among the various discotic liquid crystals. The star topology provides access to oligomers and dendrimers with a well-defined size and allows further functionalization of the arms.3 A particularly interesting subfamily are the shape-persistent hekates, i.e. star-shaped mesogens with three arms such as phenyl, oligophenylene, oligo(het)arylene, or oligo(phenylenevinylene) groups, because the space between the arms cannot be compensated by folding. Thus, simple columnar stacking of the central aromatic cores does not provide sufficient nanosegregation required for the formation of stable columnar mesophases. In such systems space filling and nanosegregation are often achieved by rotating neighboring molecules by a certain angle along the intracolumnar axis. This self-assembly can be further enforced by hydrogen bonding. In some cases helical columnar stacking is possible, even in the absence of chiral substituents.4–11 Therefore, these compounds allow a detailed view into the interplay between the molecular structure and modes of the self-assembly. While star-shaped tris-styrylbenzene 112–20 and the corresponding tris-styryltriazine 221–23 as well as 1,3,5-triphenyltriazine 3 have been extensively studied,24–34 the parent 1,3,5-triphenylbenzenes 5 and 7 are much less explored24,35–37 (Scheme 1).In particular, Scherowsky38 and Kotha24 reported that bisalkoxy-substituted 1,3,5-triphenylbenzenes do not show any mesomorphic properties. Only for the corresponding donor–acceptor complexes with trinitrofluorenone (TNF) and C8 chains in the triphenylbenzene columnar mesophases were observed. In light of these results24,38 triphenylbenzenes 5 with only six short alkoxy substituents (<C9) were deemed unsuitable as mesogens and thus only hexa- and nona-substituted derivatives bearing longer side chains (C9–C18) were considered further. We were curious whether the missing link in star shaped discotics, i.e. the simple bis- or trisalkoxy-substituted triphenylbenzenes with columnar mesomorphism in the neat form, could be obtained. In addition, due to the fact that the corresponding planar tris(trialkoxyphenyl)triazine 331 and tris(trialkoxyphenyl)boroxine 439 display stable columnar mesophases over a broad temperature range, it was of interest how the propeller-shape of triphenylbenzenes 5–7 affects the self-assembly behaviour in comparison with 3 and 4. The results are reported below.
Synthesis of triphenylbenzenes
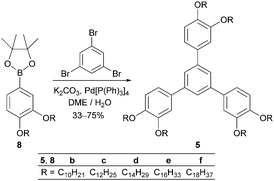
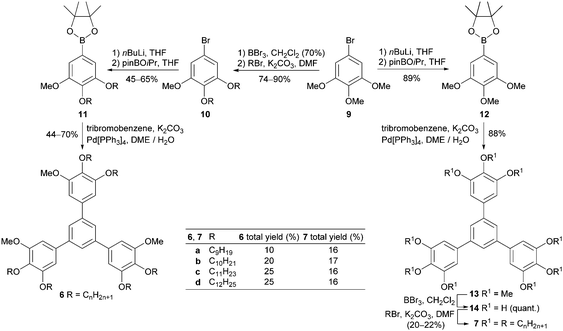
Hexaalkoxy-1,3,5-triphenylbenzenes 5 were prepared by Suzuki cross-coupling of borolanes 840 with 1,3,5-tribromobenzene in moderate to good yields (33–75%) (Scheme 2). Derivative 5f with C18 alkoxy side chains, however, was obtained in only 7% due to solubility problems during purification.Triphenylbenzenes 6 with decreased symmetry in the substitution pattern of the aryl ring were synthesized from commercially available 1,2,3-trimethoxy-5-bromobenzene 9 (Scheme 3). Selective demethylation of 9,41 Williamson etherification and borylation gave methoxy-bisalkoxy-substituted borolanes 11, which were submitted to Suzuki cross-coupling providing the desired triphenylbenzenes 6 in 10–25% total yield.
The symmetrically nona-alkoxy-substituted triphenylbenzenes 7 were accessible in 16–17% total yield from 9via borolane 1240 followed by Suzuki cross-coupling, demethylation and finally Williamson etherification (Scheme 3). This route is advantageous due to the less amount of expensive reagent BBr3 for the demethylation of 13 to 1438 and fewer purification steps.
DSC and POM investigations
The liquid crystalline properties of compounds 5–7 were first studied by differential scanning calorimetry (DSC). As the known sixfold octyloxy-substituted triphenylbenzene 5a,38 the higher homologues 5b–f did not show any mesomorphic behaviour. In contrast, both the non-symmetrical derivatives 6 and symmetrical compounds 7 with nine peripheral alkoxy side chains form stable enantiotropic mesophases. The results of the DSC investigations are summarised in Table 1 (for original DSC traces see ESI†).| Compd | Phase | T m (°C) (ΔH/kJ mol−1) | Phase | T c (°C) (ΔH/kJ mol−1) | Phase | Cycles |
|---|---|---|---|---|---|---|
| a Melting point (Tm), clearing point (Tc), glass transition temperature (Tg), crystalline phase (Cr), and glassy state (G). Colh: columnar hexagonal phase, Colho: ordered columnar hexagonal phase, and I: isotropic liquid. | ||||||
| 6a | G | 53 (Tg) | Colho | 126 (30.0) | I | 2nd heating |
| G | 90 (Tg) | Colho | 123 (30.4) | I | 2nd cooling | |
| 6b | G | 51 (Tg) | Colho | 121 (35.4) | I | 2nd heating |
| G | 86 (Tg) | Colho | 119 (34.3) | I | 2nd cooling | |
| 6c | Cr | 35 (33.2) | Colh | 113 (27.7) | I | 2nd heating |
| Cr | 5 (20.1) | Colh | 113 (30.5) | I | 2nd cooling | |
| 6d | Cr | 52 (55.1) | Colh | 110 (25.3) | I | 2nd heating |
| Cr | 23 (41.7) | Colh | 110 (24.5) | I | 2nd cooling | |
| 7a | Cr | 29 (41.3) | Colho | 143 (63.3) | I | 2nd heating |
| Cr | 28 (42.5) | Colho | 139 (68.8) | I | 2nd cooling | |
| 7b | Cr | 41 (40.9) | Colho | 140 (67.0) | I | 2nd heating |
| Cr | 38 (40.8) | Colho | 135 (69.7) | I | 2nd cooling | |
| 7c | Cr | 49 (74.1) | Colho | 136 (66.9) | I | 2nd heating |
| Cr | 47 (52.3) | Colho | 131 (67.1) | I | 2nd cooling | |
| 7d | Cr | 59 (98.3) | Colho | 130 (63.1) | I | 2nd heating |
| Cr | 52 (82.0) | Colho | 127 (58.9) | I | 2nd cooling | |
In contrast to the sixfold substituted derivatives 5 tris(dialkoxy-methoxyphenyl)benzenes 6 displayed different phase behaviour depending on their chain lengths. For 6a,b with shorter alkyl chains (C9 and C10) no melting points were visible, neither in the heating nor in the cooling scans. Both derivatives showed distinctive glass transitions at 53 °C and 51 °C, respectively, in the second heating cycle. Upon further heating clearing from the mesophase to the isotropic liquid took place at 126 °C for 6a and 121 °C for 6b. In the cooling cycle formation of the mesophase took place at 123 °C and 119 °C, respectively. The glass transitions were shifted to higher temperatures (90 °C for 6a and 86 °C for 6b) reducing the mesophase width by half to 33 K for both compounds.
Triphenylbenzenes 6c and 6d with longer alkoxy side chains (C11 and C12) displayed reproducible melting, at 35 °C and 52 °C, and clearing peaks at 113 °C and 110 °C, respectively, upon heating. Whilst the temperatures of the clearing points are maintained in the cooling cycle with respect to those measured upon heating supercooling was observed for the mesophase-to-crystalline transitions. Crystallisation took place at 5 °C and 23 °C, respectively, giving mesophase widths of 108 K for 6c and 87 K for 6d.
All tris(trialkoxyphenyl)benzenes 7a–d formed stable mesophases, displaying increasing melting points from 29 °C (7a) to 59 °C (7d) with increasing chain lengths. Conversely, the clearing points decreased with increasing chain length from 143 °C (7a) to 130 °C (7d) whereby the mesophase widths decrease linearly through the series with increasing alkoxy chain length. This behaviour is in good agreement with the structurally related triphenylboroxine 4.39
Under a polarizing optical microscope (POM) all compounds gave textures typical of those for columnar mesophases (Fig. 1). 6a and 7a displayed broken fan textures, 6c displayed a pseudo-focal conic texture, and 6b, 6d and 7b–d displayed fan-shaped textures.42 All textures also exhibited small homeotropic areas which are common for uniaxial columnar mesophases.
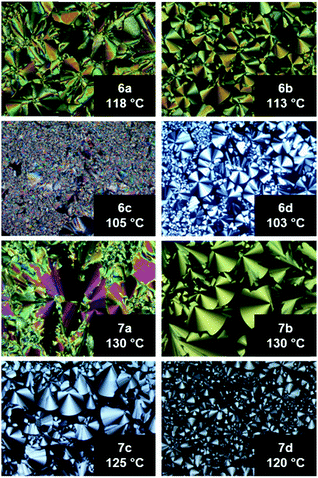 | ||
| Fig. 1 POM images of 6a–d and 7a–d upon cooling from the isotropic liquid (cooling rate 1 K min−1 and magnification ×100). | ||
X-ray diffraction (XRD) investigations
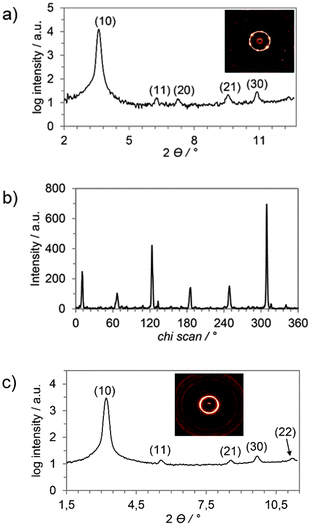
Further insight into the mesophase geometries of 6a–d and 7a–d came from X-ray diffraction measurements. Two derivatives of each type of mesogen with different substitution patterns, namely 6a, 6d, 7b and 7d, were representatively investigated (SAXS and WAXS, for further details see Table S2, ESI†). The SAXS profile and the corresponding diffraction pattern of 6a showed two reflexes which were assigned as (10) and (20) as they are in a relative ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2 (Fig. 3a).43 This set of signals might be assigned to a discotic lamellar phase geometry44 due to the missing (11) peak which would be necessary for an unambiguous assignment of Colh phase geometry. However, the partially oriented SAXS pattern (Fig. 2a, inset) clearly indicates a hexagonal lattice. In the SAXS diffractogram of 6d four reflexes in a ratio of 1
1/2 (Fig. 3a).43 This set of signals might be assigned to a discotic lamellar phase geometry44 due to the missing (11) peak which would be necessary for an unambiguous assignment of Colh phase geometry. However, the partially oriented SAXS pattern (Fig. 2a, inset) clearly indicates a hexagonal lattice. In the SAXS diffractogram of 6d four reflexes in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√3
1/√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√7
1/√7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√9 were visible (Fig. 2b), which have been unambiguously assigned as (10), (11), (21) and (30). Despite the missing (20) peak, which might occur due to superimposing of areas with different orientation in the sample, the observed reflexes were in good agreement with a Colh lattice.
1/√9 were visible (Fig. 2b), which have been unambiguously assigned as (10), (11), (21) and (30). Despite the missing (20) peak, which might occur due to superimposing of areas with different orientation in the sample, the observed reflexes were in good agreement with a Colh lattice.
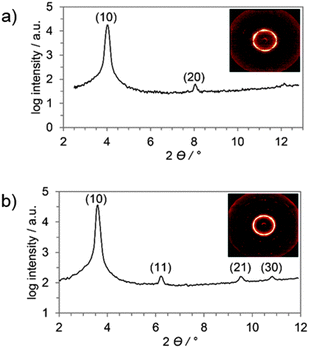 | ||
| Fig. 2 SAXS profile and diffraction pattern (inset) during cooling of (a) 6a at 105 °C and (b) 6d 85 °C. | ||
The small-angle X-ray scattering profile and the diffraction pattern of 7b are depicted in Fig. 3a. The SAXS pattern (Fig. 3a inset) is highly ordered and exhibits an oriented hexagon of the innermost reflection together with four additional sets of oriented reflections. Integration of the inner hexagon over 2Θ is shown in Fig. 3b. The relative angle of 60(±2)° between the peaks indicates a hexagonal columnar mesophase. In the small-angle region five reflections were visible in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√3
1/√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2
1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√7
1/√7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√9 which were indicated as (10), (11), (20), (21) and (30), confirming a hexagonal lattice (Fig. 3a).43
1/√9 which were indicated as (10), (11), (20), (21) and (30), confirming a hexagonal lattice (Fig. 3a).43
The SAXS investigations of 7d (Fig. 3c) confirmed the results of the previous XRD experiments. The SAXS profile displayed five reflections in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√3
1/√3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2
1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√7
1/√7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/√12 which fit to a hexagonal lattice and were therefore indicated as (10), (11), (21), (30), and (22) (Fig. 3c).
1/√12 which fit to a hexagonal lattice and were therefore indicated as (10), (11), (21), (30), and (22) (Fig. 3c).
In the wide-angle region of 6d and 7b a broad halo at d = 4.5 Å and d = 4.3 Å was visible related to the liquid-like alkoxy chains (Fig. 4a and b). In addition, the wide-angle X-ray scattering profile of 7b showed a multitude of discernable reflections labelled (10), (11), (20), (21), (30), (22) and (40) besides the halo at d = 4.6 Å and a peak at d = 4.3 Å, which originate from π–π interactions between the aromatic cores and correspond to their mean distance within the columns. The latter reflection suggested the formation of an ordered columnar hexagonal mesophase.
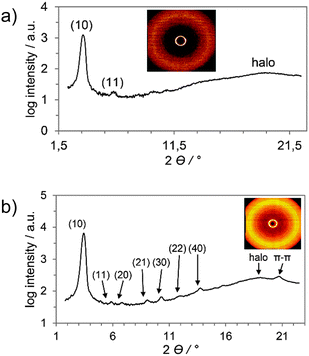 | ||
| Fig. 4 (a) WAXS profile and diffraction pattern (inset) of 6d during cooling at 85 °C and (b) WAXS profile and diffraction pattern (inset) of 7b during cooling at 88 °C. | ||
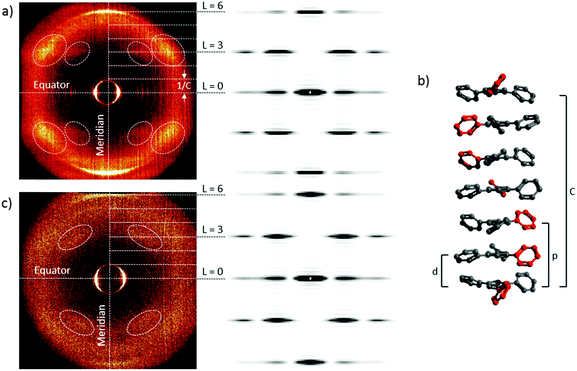
For compounds 6a and 7d the WAXS samples were prepared by fibre extrusion. Due to the better alignment of the sample in the WAXS of 7d as compared to 6a the former was analysed first. The WAXS pattern of 7d showed two sets of diffraction peaks, one in the equatorial and one in the meridional region (Fig. 5a on the left side). The reflexes in the equatorial region, perpendicular to the fibre axis, correspond to the lattice geometry of the mesophase which was determined as columnar hexagonal (Fig. 3c). The meridional region displayed four pairs of scattering peaks on the left and right hand side of the meridian and two distinct reflexes on the meridian axis, typical for helical superstructures. The obtained WAXS pattern is in good agreement with the simulated one shown in Fig. 5a on the right.45 For this simulation it was assumed that, because of their C3 symmetry, the molecules stack in a manner where they are rotated by φ = 60°. Thus the inner benzene rings are piled up on top of each other while the outer phenyl groups describe a triple helix around the inner column. Owing to the C3-symmetry of the triphenylbenzenes only the reflexes on the layer lines L = 3 and L = 6 are visible. The reflex on the meridional axis on the layer line L = 6 corresponds to the stacking inside of the column giving a stacking distance of d = 4.3 Å. The layer line L = 3 corresponds to the pitch p of the helix, which is three molecules or p = 8.6 Å. Altogether the WAXS pattern of 7d indicates that a triple 61 helix with an azimuthal rotation angle of φ = 60°, a pitch p = 8.6 Å and a repeat C of three pitches, giving C = 25.8 Å, is formed (Fig. 5b). Although this resembles an antiparallel “zig-zag” like packing, the high orientation in the WAXS pattern indicated a periodical setup where the peripheral phenyl rings are twisted in the same direction respective to each other. In an antiparallel stacking a random orientation would be expected.
The WAXS diffractogram of 6a (Fig. 5c on the left side) displayed a similar structure to that of 7d, although the scattering peaks on the equatorial axis were not visible. In the meridional region four peaks on the right and left hand side of the axis were visible as well as two distinct reflexes on the meridional axis. With the assumption of the same rotational angle as for 7d (φ = 60°) a diffraction pattern was simulated which is in good agreement with the measurement.45 The stacking distance inside the column at L = 6 is d = 4.0 Å which leads to slightly different helical coefficients from those for 7d.
The analysis of the WAXS pattern indicates the formation of a triple 61 helix with a rotational angle of φ = 60°, a pitch of p = 8.0 Å and a repeat of C = 24.0 Å. In both cases, indication of the handedness could not be obtained. The helix formation seems to be a statistical process due to the absence of chiral information in the triphenylbenzenes, in contrast to the recent work of Casado et al.46 who studied C3 tricarboxamides bearing chiral side chains by ECD, VPC and ECL techniques.
Proposed packing model
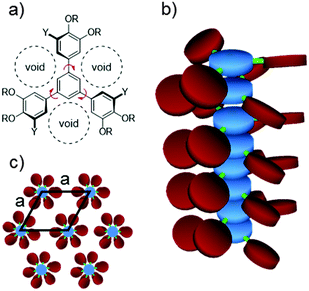
The peripheral phenyl rings of 1,3,5-triphenylbenzene derivatives are well known to be twisted by up to 48° in the solid state.47–49 According to Scherowsky this non-planar structure suppresses mesomorphism in hexaoctyloxy- and various ester-functionalized triphenylbenzenes 5.38 As discussed above, side chain elongation in the prepared novel sixfold substituted triphenylbenzenes 5b–f did not promote liquid crystalline behaviour. However, introducing a third alkoxy group in each peripheral phenyl ring led to the formation of enantiotropic mesophases in derivatives 6a–d and 7a–d. For this purpose, even the minimalistic OCH3 group is sufficient, as was demonstrated for 6a–d. To explain this finding we considered two aspects, the influence of the non-planar triphenylbenzene core system and the varying substitution patterns.The X-ray data of 6a and 7d indicate a helical structure of the formed columns. In a helical setup parts of the voids resulted from the rigid star shape (Fig. 6a) can be compensated by the twisted outer phenyl rings above and beneath each disk in the column, respectively (Fig. 6b). The remaining empty spaces then have to be filled by the peripheral alkoxy chains. This can be achieved either by backfolding of the side chains or by their interdigitation.11,50 Both effects should lead to a reduced diameter of the discs (determined by the lattice spacings a of the Colh phase geometry) as can be seen in Table 2. For example, the estimated diameters for 6d and 7d are the same yet the measured matrix parameter of 6d is considerably smaller than that of 7d, indicating that the compensation of the minimalistic methoxy groups requires more backfolding or interdigitation of the remaining alkoxy chains. Taking into account that the rigid core cannot take part in these effects, the resulting reduction amounts 47% for 6d and 37% for 7d, respectively.
| Compd | a calcd/Å | a obsd/Å | Δa/Å | Δdalk (%) |
|---|---|---|---|---|
| 6a | 36.4 | 25.3 | 11.1 | 44 |
| 6d | 43.4 | 28.4 | 15 | 47 |
| 7b | 37.8 | 28.3 | 9.5 | 38 |
| 7d | 43.4 | 31.8 | 11.6 | 37 |
The absence of mesomorphism in the series of hexa-substituted triphenylbenzenes 5b–f is not uncommon among shape persistent hekates. For structurally related hexa- and nona-alkoxy substituted triphenylboroxines39 as well as for hexa-30 and nona-alkoxy31 substituted triphenyltriazine similar behaviour has been found. This indicates that the formation of a liquid crystalline phase in these small star shaped mesogens strongly depends on the number of alkoxy substituents and not so much on their length. Apparently nine side chains are necessary for successful nanosegregation. This might originate from two effects: the additional group at each peripheral aryl unit increases the shielding of the aromatic core, thus improving segregation of the cores and stabilising columnar mesophases. Alternatively, the additional methoxy group deplanarises the remaining alkoxy chains, forcing them to fill the void between the rings.51 Considering this, both possibilities lead to the fact that any substituent in the 5-position of the outer phenyl groups should promote mesomorphism. To elucidate this interesting topic further investigations are currently in progress.
Conclusions
In conclusion, we have synthesised triphenylbenzenes with 3,4-bisalkoxy- and 3,4,5-trisalkoxyphenyl moieties, which were not liquid crystalline (5), if only two alkoxy substituents were present at each peripheral phenyl ring. However, derivatives 7 with three alkoxy substituents on each phenyl ring showed enantiotropic hexagonal columnar mesophases with phase widths of 75–114 K depending on the chain lengths. Surprisingly, even methoxy served as a third minimalistic alkoxy unit at the peripheral phenyl rings, resulting in enantiotropic hexagonal columnar mesophases with phase widths of 58–80 K for compounds 6. Extensive XRD studies indicate that a packing model with helical self-assembly seems very likely. The mesomorphic properties indicate that compounds 6 and 7 are indeed the missing links in the series of shape-persistent hekates and might be useful for applications such as light-emitting diodes or photovoltaic cells.Acknowledgements
Generous financial support by the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie (PhD fellowship for S. J. Beardsworth), the Ministerium für Wissenschaft und Kunst des Landes Baden-Württemberg and the Bundesministerium für Bildung und Forschung (shared instrumentation grant 01 RI05177) is gratefully acknowledged.References
- Review: S. Laschat, A. Baro, N. Steinke, F. Giesselmann, C. Hägele, G. Scalia, R. Judele, E. Kapatsina, S. Sauer, A. Schreivogel and M. Tosoni, Angew. Chem., Int. Ed., 2007, 46, 4832 CrossRef CAS PubMed.
- Review: S. Sergeyev, W. Pisula and Y. H. Geerts, Chem. Soc. Rev., 2007, 36, 1902 RSC.
- Review: M. Lehmann, in Handbook of Liquid Crystals, ed. J. W. Goodby, P. J. Collings, T. Kato, C. Tschierske, H. F. Gleeson and P. Raynes, Wiley-VCH, Weinheim, 2nd edn, 2014, vol. 5, p. 243 Search PubMed.
- O. Simalou, P. Xue and R. Lu, Tetrahedron Lett., 2010, 51, 3685 CrossRef CAS.
- C. Bao, M. Jin, R. Lu, Z. Song, X. Yang, D. Song, T. Xu, G. Liu and Y. Zhao, Tetrahedron, 2007, 63, 7443 CrossRef CAS.
- M. Tominaga, H. Masu, K. Katagiri and I. Azumaya, Tetrahedron Lett., 2007, 48, 4369 CrossRef CAS.
- C. Bao, R. Lu, M. Jin, P. Xue, C. Tan, T. Xu, G. Liu and Y. Zhao, Chem. – Eur. J., 2006, 12, 3287 CrossRef CAS PubMed.
- J. Bae, J.-K. Kim, N.-K. Oh and M. Lee, Macromolecules, 2005, 38, 4226 CrossRef CAS.
- J.-K. Kim, M.-K. Hong, J.-H. Aku and M. Lee, Angew. Chem., Int. Ed., 2005, 44, 328 CrossRef CAS PubMed.
- C.-J. Jong, J.-H. Ryu, J.-D. Lee, D. Sohn and M. Lee, Chem. Mater., 2004, 16, 4226 CrossRef.
- S. K. Pathak, R. K. Gupta, S. Nath, D. S. S. Rao, S. K. Prasad and A. S. Achalkumar, J. Mater. Chem. C, 2015, 3, 2940 RSC.
- M. Lehmann and M. Huegel, Angew. Chem., Int. Ed., 2015, 54, 4110 CrossRef CAS PubMed.
- M. Lehmann, C. Koehn, H. Meier, S. Renker and A. Oehlhof, J. Mater. Chem., 2006, 16, 441 RSC.
- M. Lehmann, I. Fischbach, H. W. Spiess and H. Meier, J. Am. Chem. Soc., 2004, 126, 772 CrossRef CAS PubMed.
- S. Xu, Q. Zeng, J. Lu, C. Wang, L. Wan and C.-L. Bai, Surf. Sci., 2003, 538, L451 CrossRef CAS.
- H. Meier, M. Lehmann, C. Schnorpfeil, M. Fetten and H. Meier, J. Inf. Rec., 2000, 25, 259 Search PubMed.
- H. Meier, M. Lehmann and U. Kolb, Chem. – Eur. J., 2000, 6, 2462 CrossRef CAS.
- M. Lehmann, B. Schartel, M. Hennecke and H. Meier, Tetrahedron, 1999, 55, 13377 CrossRef CAS.
- H. Meier and M. Lehmann, Angew. Chem., Int. Ed., 1998, 37, 643 CrossRef CAS.
- G. Zerban and H. Meier, Z. Naturforsch., 1993, 48b, 171 Search PubMed.
- H. Meier, E. Karpuk and H. C. Holst, Eur. J. Org. Chem., 2006, 2609 CrossRef CAS.
- H. C. Holst, T. Pakula and H. Meier, Tetrahedron, 2004, 60, 6765 CrossRef CAS.
- H. Meier, H. C. Holst and A. Oehlhof, Eur. J. Org. Chem., 2003, 4173 CrossRef CAS.
- S. Kotha, D. Kashinath and S. Kumar, Tetrahedron Lett., 2008, 49, 5419 CrossRef CAS.
- H. K. Dambal and C. V. Yelamaggad, Tetrahedron Lett., 2012, 53, 186 CrossRef CAS.
- Y. Kudo, M. Sakuragi, S. Hashida, R. Kuwakara, T. Ishi, H. Masunaga and K. Sakurai, Polym. J., 2010, 42, 812 CrossRef CAS.
- T. Ishi, R. Kuwahara, A. Takata, Y. Jeong, K. Sakurai and S. Mataka, Chem. – Eur. J., 2006, 12, 763 CrossRef PubMed.
- A. C.-A. Chen, J. U. Wallace, S. K.-H. Wie, L. Zeng, S. H. Chen and T. N. Blanton, Chem. Mater., 2006, 18, 204 CrossRef CAS.
- A. C.-A. Chen, J. U. Wallace, L. Zeng, S. K.-H. Wie and S. H. Chen, Proc. SPIE, 2005, 5936 Search PubMed.
- D. Goldmann, A. Nordsieck, D. Janietz, T. Frese, C. Schmidt and J. H. Wendorff, Mol. Cryst. Liq. Cryst., 2004, 411, 1379 CAS.
- H. Lee, D. Kim, H.-K. Lee, W. Qiu, N.-K. Oh, W.-C. Zin and K. Kim, Tetrahedron Lett., 2004, 45, 1019 CrossRef CAS.
- C.-H. Lee and T. Yamamoto, Mol. Cryst. Liq. Cryst. Sci. Technol., Sect. A, 2002, 378, 13 CrossRef CAS.
- C.-H. Lee and T. Yamamoto, Bull. Chem. Soc. Jpn., 2002, 75, 615 CrossRef CAS.
- C.-H. Lee and T. Yamamoto, Tetrahedron Lett., 2001, 42, 3993 CrossRef CAS.
- O. Simalou, P. Xue and R. Lu, Tetrahedron Lett., 2010, 51, 3685 CrossRef CAS.
- B. P. Dash, R. Satapathy, J. A. Maguire and N. S. Hosmane, Org. Lett., 2008, 10, 2247 CrossRef CAS PubMed.
- A. N. Cammidge and A. S. H. King, Tetrahedron Lett., 2006, 47, 5569 CrossRef CAS.
- E. Frackowiak and G. Scherowsky, Z. Naturforsch., 1997, 52b, 1539 Search PubMed.
- T. Wöhrle, A. Baro and S. Laschat, Materials, 2014, 7, 4045 CrossRef.
- M. Kaller, S. Tussetschläger, P. Fischer, C. Deck, A. Baro, F. Giesselmann and S. Laschat, Chem. – Eur. J., 2009, 15, 9530–9542 CrossRef CAS PubMed.
- B. Schmidt and M. Riemer, J. Org. Chem., 2014, 79, 4104–4118 CrossRef CAS PubMed.
- I. Dierking, Textures of Liquid Crystals, Wiley-VCH, Weinheim, 2003 Search PubMed.
- S. K. Prasad, D. S. S. Rao, S. Chandrasekhar and S. Kumar, Mol. Cryst. Liq. Cryst., 2003, 396, 121–139 CrossRef CAS.
- M. Lehmann, M. Jahr and J. Gutmann, J. Mater. Chem., 2008, 18, 2995 RSC.
- C. Knupp and J. M. Squire, J. Appl. Crystallogr., 2004, 37, 832 CrossRef CAS.
- B. Nieto-Ortega, F. García, G. Longhi, E. Castiglioni, J. Calbo, S. Abbate, J. T. López Navarrete, F. J. Ramírez, E. Orti, L. Sánchez and J. Casado, Chem. Commun., 2015, 51, 9781 RSC.
- M. S. Farag, Acta Crystallogr., 1954, 7, 117–121 CrossRef CAS.
- L. M. C. Beltran, C. Cui, D. H. Leung, J. Xu and F. J. Hollander, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2002, 58, o782–o783 CAS.
- V. Vergadou, G. Pistolis, A. Michaelides, G. Varvounis, M. Siskos, N. Boukos and S. Skoulika, Cryst. Growth Des., 2006, 6, 2486–2492 CAS.
- M. Lehmann, Chem. – Eur. J., 2009, 15, 3638 CrossRef CAS PubMed.
- We are very grateful to one of the referees, who proposed this hypothesis in order to explain the difference between hydrogen and an extra methoxy (or alkoxy) group.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sm02489g |
| This journal is © The Royal Society of Chemistry 2016 |