Synthesis of BiOI nanosheet/coarsened TiO2 nanobelt heterostructures for enhancing visible light photocatalytic activity
Zhanjun Liab,
Meiting Wang*ab,
Jianxing Shenab,
Zhiwen Zhuab and
Yu Liuab
aInstitute of Materials Science and Engineering, Qilu University of Technology, Jinan 250353, China. E-mail: meiting_wang@163.com; Fax: +86 531 89631227; Tel: +86 531 89631225
bKey Laboratory of Pulp and Paper Science & Technology of Ministry of Education of China, Qilu University of Technology, Jinan, 250353, China. E-mail: leoliuyu@163.com
First published on 11th March 2016
Abstract
For high photocatalytic activity, BiOI nanosheets were deposited on acid-corroded TiO2 nanobelts, where a large number of BiOI nanosheet/TiO2 nanobelt heterojunctions could be built. The structure, morphology and optical properties of the BiOI nanosheet/coarsened TiO2 nanobelt heterostructure composites (BiOI/TiO2 CNHs) were characterized by XRD, XPS, SEM, HRTEM, DRS, PL, TOC and nitrogen sorption. The XRD results show that only two phases of TiO2 and BiOI were found in the composites. The HRTEM image shows clear lattice fringes, which prove the formation of heterojunctions at the interface between BiOI and coarsened TiO2. The photocatalytic activity of the BiOI/TiO2 CNHs for the degradation of methyl orange under visible-light irradiation was evaluated. The results reveal that the BiOI/TiO2 CNHs exhibited much higher photocatalytic activity compared with coarsened TiO2 nanobelts and pure BiOI nanosheets due to the introduction of BiOI onto the surface of the coarsened TiO2 nanobelts and the formation of heterojunctions. BiOI acts as a visible light photosensitizer in the BiOI/TiO2 CNHs. In addition, the large surface area and matched energy bands of the BiOI/TiO2 CNHs both contribute to enhancing the photocatalytic activity. In addition, the possible mechanism of promotion of the photocatalytic performance of the new heterostructure system is explained.
Introduction
Nanometer TiO2 with various morphologies, including nanobelts, nanowires and nanotubes, has been at the core of current nanoscience and nanotechnology and has experienced increasing interest in environmental protection and green energy fields.1–4 For instance, TiO2, as well as Ga2O3 obtained by calcination of GaOOH, are both able to photocatalyse the overall splitting of water by using solar energy.5–9 Because of its favorable electronic band structure, biochemical compatibility, strong oxidizing power, nontoxicity and long-term stability against photochemical corrosion,10 TiO2 has been considered to be the most promising material and has been applied in photocatalysts,11,12 solar cells,13,14 gas sensors,15,16 and lithium batteries.17,18 Nano-TiO2 is considered to be a very important photocatalyst for removal of organic pollutants in dye wastewater. However, one of the critical drawbacks of the TiO2 photocatalyst is its wide band gap of 3.2 eV, which restricts the excitation to UV light irradiation and greatly decreases the utilization of sunlight due to the small ultraviolet fraction (4–5%) of the solar spectrum. Therefore, it is very meaningful for practical application to develop effective means to expand the light absorption area.19–22Many studies on shifting the optical response of TiO2 to the visible light range and then promoting the photocatalytic efficiency have been reported, including those on nonmetallic doping, deposition of noble metals and sensitization.23–25 In addition, the formation of heterostructures by coupling nano-TiO2 with an effective visible light semiconductor photocatalyst is also an effective way to enhance the visible light photocatalytic activity. The heterostructure can not only expand the spectral response range to visible light but also improve the efficiency of the charge carrier separation of the photocatalyst. For the past few years, forming a heterojunction between TiO2 and a narrow-gap semiconductor has been frequently reported. For instance, Chai et al. prepared TiO2/BiOI photocatalysts consisting of flower-like BiOI decorated with TiO2 nanoparticles by a facile one-pot solvothermal route.26 Tian et al. reported a broad spectrum photocatalyst with 3D Bi2WO6/TiO2 nanobelt heterostructures by introducing Bi2WO6 nanosheets on surface-coarsened TiO2 nanobelts.20 Zhou et al. prepared Ag2O nanoparticles/TiO2 nanobelts by an acid-assisted hydrothermal method which exhibited enhanced photocatalytic activity.27 Liu et al. fabricated bicomponent SnO2/TiO2 nanofibers with controllable heterojunctions by the electrospinning process, improving the photocatalytic activity of TiO2.28 Zhang et al. reported a visible-light-activated photocatalyst based on BiOI/TiO2 heterostructured powders.29 Among these heterostructures, BiOI/TiO2 heterostructures show good activity for the photocatalytic degradation of organic pollutants under visible-light irradiation, for the band gap of BiOI of about 1.77 eV can be stimulated by visible light irradiation. Many BiOI-based composites have been investigated, such as BiOBr/BiOI,30 AgI/BiOI,31 and BiOCl/BiOI.32
In this work, we concentrate on working on the modified BiOI/TiO2 system. BiOI/TiO2 CNHs were prepared by using BiOI and surface-coarsened TiO2 nanobelts through a hydrothermal method at certain temperature. The surface-coarsened TiO2 nanobelts are expected to offer a large amount of active sites for the growth of BiOI nanosheets while facilitating a uniform distribution of BiOI nanosheets on the surface of the coarsened TiO2 nanobelts. The photocatalytic activity and recycling ability of the BiOI/TiO2 CNHs were investigated by the degradation of MO. Furthermore, in the process of degradation of MO, the BiOI/TiO2 CNHs show better photocatalysis efficiency than the pure coarsened TiO2 nanobelts and BiOI nanosheets. To the best of our knowledge, this is the first report on heterojunctions of BiOI/TiO2 CNHs with a unique BiOI flake-coarsened TiO2 belt structure and excellent visible light photocatalytic activity and stability.
Experimental
Materials
All the reagents used were of analytical grade. Titania P-25 (commercial TiO2, ca. 80% anatase and 20% rutile) was bought from China National Medicines Co., Ltd. Hydrochloric acid and sulfuric acid were obtained from Sino-pharm Chemical Reagent Co., Ltd. NaOH, Bi(NO3)3·5H2O, KI and ethylene glycol (EG) were purchased from Tianjin Reagent Co., Ltd.Preparation of TiO2 nanobelts and surface-coarsened TiO2 nanobelts
TiO2 nanobelts were prepared via the hydrothermal process using commercial TiO2 powder. First, 0.3 g of P-25 powder was dispersed in 40 mL of 10 M NaOH aqueous solution, followed by magnetic stirring for 30 min. After that, the solution was transferred into a 40 mL Teflon-lined stainless steel autoclave, heated at 180 °C for 72 h, and cooled down to room temperature under ambient conditions. The treated product was washed thoroughly with deionized water several times, followed by a filtration process. Subsequently, the wet product was dispersed in a 0.1 M HCl aqueous solution for 48 h, and then washed thoroughly with deionized water. The resultant hydrogen titanate nanobelts were carefully dried at 80 °C for 8 h in an oven and further heat-treated in the muffle furnace at 600 °C for 2 h, obtaining TiO2 nanobelts.The hydrogen titanate nanobelts were dispersed in 20 mL of a 0.02 M H2SO4 aqueous solution under magnetic stirring for 30 min, and then heated in a Teflon-lined stainless steel autoclave at 100 °C for 12 h. After washing and drying, the sample was annealed at 600 °C for 2 h, and finally the surface-coarsened TiO2 nanobelts were successfully synthesized.
Preparation of BiOI/TiO2 CNHs and BiOI/TiO2 nanobelt heterostructure composites (BiOI/TiO2 NHs)
BiOI/TiO2 CNHs with different molar ratios from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 3
3 to 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 were synthesized using a three-step hydrothermal method. As an example, the preparation process of 1
1 were synthesized using a three-step hydrothermal method. As an example, the preparation process of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BiOI/TiO2 CNHs is presented as follows: 0.485 g Bi(NO3)3·5H2O and 0.166 g KI were each separately dissolved in 40 mL of EG. After stirring magnetically for 30 min, the KI solution was added to the Bi(NO3)3·5H2O solution drop by drop. Next, the calculated amount of coarsened TiO2 nanobelts was added to the mixed solution, which was stirred for 30 min and then ultrasonically treated for 10 min. Then, the above mixed solution was transferred into a 40 mL Teflon-lined stainless steel autoclave, and heated at 160 °C for 6 h. Finally, BiOI/TiO2 CNHs were obtained after washing with deionized water and ethanol several times and drying at 80 °C. For comparison, individual BiOI was also synthesized by the same process.
1 BiOI/TiO2 CNHs is presented as follows: 0.485 g Bi(NO3)3·5H2O and 0.166 g KI were each separately dissolved in 40 mL of EG. After stirring magnetically for 30 min, the KI solution was added to the Bi(NO3)3·5H2O solution drop by drop. Next, the calculated amount of coarsened TiO2 nanobelts was added to the mixed solution, which was stirred for 30 min and then ultrasonically treated for 10 min. Then, the above mixed solution was transferred into a 40 mL Teflon-lined stainless steel autoclave, and heated at 160 °C for 6 h. Finally, BiOI/TiO2 CNHs were obtained after washing with deionized water and ethanol several times and drying at 80 °C. For comparison, individual BiOI was also synthesized by the same process.
BiOI nanosheet/TiO2 nanobelt heterostructure composites (BiOI/TiO2 NHs) were synthesized using the hydrothermal method. In a typical procedure, 0.485 g Bi(NO3)3·5H2O and 0.166 g KI were each separately dissolved in 20 mL of EG. After stirring magnetically for 30 min, the KI solution was added to the Bi(NO3)3·5H2O solution drop by drop. Next, the calculated amount of TiO2 nanobelts was added to the mixed solution, which was stirred for 30 min and then ultrasonically treated for 10 min. Then, the above mixed solution was transferred into a 40 mL Teflon-lined stainless steel autoclave, and heated at 160 °C for 6 h. Finally, BiOI/TiO2 NHs were obtained after washing with deionized water and ethanol several times and drying at 80 °C.
Characterization
X-ray powder diffraction (XRD) patterns of the catalysts were recorded on a Bruker D8 Advance powder X-ray diffractometer with Cu-Ka (λ = 0.15406 nm) radiation. X-ray photoelectron spectroscopy (XPS), which was carried out on an ESCALAB 250 photoelectron spectrometer, was used to analyze the surface chemical composition and valence state. The morphologies of the samples were characterized by scanning electron microscopy (SEM, Hitachi, Japan), transmission electron microscopy (TEM, TecnaiF20) and high-resolution TEM (HRTEM, Jeol, Japan). UV-Vis diffuse reflectance spectra (DRS) of the dry-pressed disk samples in the wavelength range of 200–800 nm were obtained using a UV-Vis spectrometer (Shimadzu U-3010) and BaSO4 as a reflectance standard. Photoluminescence (PL) spectra were recorded on a FLS920 fluorescence spectrometer with an excitation wavelength of 380 nm. A Micromeritics Tristar 3000 and TOC analyzer (TOC-V, Shimadzu, Japan) were employed to measure nitrogen adsorption–desorption isotherms and total organic carbon (TOC) concentration, respectively. The Brunauer–Emmett–Teller (BET) surface area was estimated using the adsorption data. The Mott–Schottky measurement was conducted on an electrochemical system (CHI 660E, Shanghai). A three electrode single compartment immersed in 0.5 M Na2SO4 solution was used for capacitance analysis. The as-prepared film coated on FTO was used as a working electrode while Ag/AgCl and platinum were used as the reference and counter electrodes, respectively.Photocatalytic activity test
The photocatalytic activity of the BiOI/TiO2 heterostructures was examined by detecting the photodegradation of MO at ambient temperature under simulated visible light. MO was selected as the model chemical to evaluate BiOI/TiO2 photocatalytic activity. The reactivity was tested with 50 mL of 20 mg L−1 MO solution illuminated by a 350 W Xe arc lamp (wavelength > 410 nm by pass filter), which was used as the light source of a homemade photoreactor. The BiOI/TiO2 concentration was 1.0 g L−1. Before irradiation, the MO suspension was placed in the dark for 60 min with magnetic stirring to achieve adsorption/desorption equilibrium. At given irradiation time intervals of 15 minutes, 4 mL MO suspension was taken out. The residual MO concentration was calculated from absorbance at 464 nm on a UV-Vis spectrophotometer.Results and discussion
Structure and morphology analysis
The crystallinity and crystal structures of the different samples were characterized by XRD. In Fig. 1a, there are four relatively wide diffraction peaks at around 29.6°, 31.7°, 45.5° and 55.2°, which correspond to (012), (110), (004) and (212), and all of the diffraction peaks can be indexed to the tetragonal phase of BiOI (space group P4/nmm, JCPDS 10-0445). As seen from Fig. 1b, the diffraction peaks located at 25.2°, 37.9°, 48.1°, 53.9° and 62.8° in the XRD pattern of the TiO2 nanobelts correspond to the (101), (004), (200), (105) and (201) planes of anatase TiO2 (JCPDS file no. 21-1272), respectively. In addition, several peaks at 28.8°, 33.3° and 43.4° are also observed and are indexed to TiO2-B (JCPDS no. 46-1237). The diffraction peaks of the coarsened TiO2 nanobelts in Fig. 1c remain almost consistent with those in Fig. 1b, which illustrates that the surface modification by H2SO4 solution does not change the crystalline phase of the TiO2 nanobelts. However, the TiO2-B peaks become sharp after acid corrosion, probably due to the formation of heterostructures composed of nanoparticles and nanobelts, which may promote the migration of the crystalline phase to be more complete.33 Fig. 1d and e show that all the diffraction peaks can be attributed to the BiOI and TiO2 phases, which indicates that BiOI and anatase TiO2 coexist and there remains good chemical compatibility between them. | ||
| Fig. 1 XRD patterns of (a) BiOI, (b) TiO2 nanobelts, (c) coarsened TiO2 nanobelts, (d) BiOI/TiO2 NHs and (e) BiOI/TiO2 CNHs. | ||
In order to further determine the surface chemical composition of the BiOI/TiO2 CNHs, XPS studies were conducted and the spectra are shown in Fig. 2. Fig. 2a shows the existence of Ti, O, Bi, I and C elements. The XPS peak for C 1s (284.6 eV) is ascribed to the adventitious carbon from the XPS instrument. In the high resolution XPS of Bi 4f (Fig. 2b), the two peaks of Bi 4f at 159.1 eV and 164.5 eV belong to Bi 4f7/2 and Bi 4f5/2, respectively, corresponding to the characteristics of Bi3+ in BiOI.34,35 The satellite peaks, with a distance of about 1.8 eV away from the Bi 4f7/2 and Bi 4f5/2 main peaks, are consistent with the reported values.36 Fig. 2c is the survey XPS spectrum of I 3d, and the two peaks at binding energies of 630.4 eV and 619.1 eV are attributed to I 3d3/2 and I 3d5/2, respectively, which can be ascribed to I− in pure BiOI.34,35 The XPS results demonstrate the coexistence of BiOI and TiO2 in the BiOI/TiO2 CNHs and are consistent with the XRD results.
SEM and EDX were performed to describe the morphologies and element compositions of the samples. As shown in Fig. 3a, the surfaces of the TiO2 nanobelts are very smooth and clean, and the TiO2 nanobelts are typically several micrometers in length, 50–300 nm in width and 25–40 nm in thickness. Compared with the TiO2 nanobelts in Fig. 3a, the surface-coarsened TiO2 nanobelts treated by the acid process (see Fig. 3b) show rough surfaces and high specific surface area, which can provide abundant nucleation sites for the assembly of BiOI nanosheets. Fig. 3c shows that the synthesized BiOI has a flower-like spherical morphology with a diameter of 2–4 μm and is composed of flakes. The typical SEM images of the BiOI/TiO2 NHs and BiOI/TiO2 CNHs are shown in Fig. 3d and e. It can be noted that low density BiOI nanosheets with non-uniform size are anchored on the surface of the untreated TiO2 nanobelts (Fig. 3d). In contrast, homogeneous BiOI nanosheets are thickly arranged on the surface-coarsened TiO2 nanobelts, just like branches covered with snow, which indicates a large number of heterojunctions formed on the surface of the coarsened TiO2 nanobelts. The morphologies of the two BiOI/TiO2 heterostructures are different, which originates from the diversity of surface roughness. The EDX spectrum of the BiOI/TiO2 CNHs given in Fig. 3f reveals that the BiOI/TiO2 CNHs only contain Bi, O, I, and Ti, without impurity elements. To further confirm the formation, EDX mapping of the BiOI/TiO2 CNHs was conducted. As shown in Fig. 4a–e, the four constituent elements O, Ti, I, and Bi can all be detected, and they are homogeneously distributed in the composite. These results imply that uniformly structured BiOI/TiO2 CNHs are obtained.
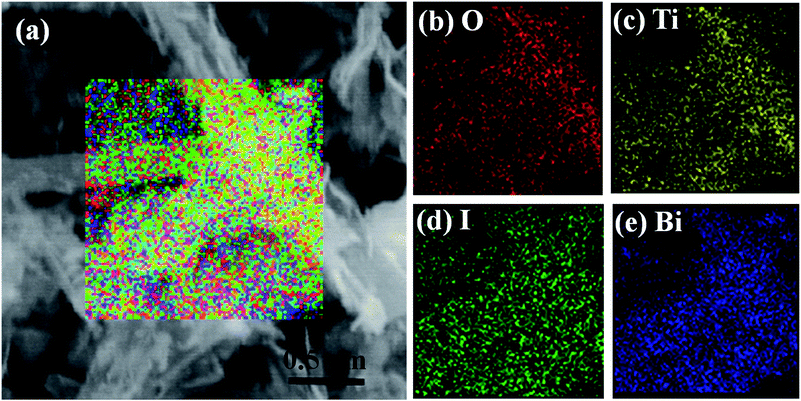 | ||
| Fig. 4 SEM image (a) and EDX mappings of BiOI/TiO2 CNHs, showing the distributions of O (b), Ti (c), I (d) and Bi (e). | ||
The typical transmission electron microscopy (TEM) and HRTEM images of TiO2 nanobelts and BiOI/TiO2 CNHs are presented in Fig. 5. The TiO2 nanobelts in Fig. 5a with a width of about 100 nm are consistent with Fig. 3a. It can be clearly observed from Fig. 5b that the BiOI nanosheets adhere on the surface of the coarsened TiO2 nanobelts and the BiOI/TiO2 CNHs still keep a one-dimensional morphology. The high-resolution TEM images of the BiOI/TiO2 CNHs are shown in Fig. 5c and d at different magnifications. The lattice spacings of TiO2 and BiOI are 0.35 nm and 0.30 nm, respectively, corresponding to the interplanar spacing of the (101) plane of TiO2 and (012) plane of BiOI, which indicates the formation of heterojunctions in the BiOI/TiO2 CNHs.
 | ||
| Fig. 5 TEM images of (a) TiO2 nanobelts and (b) BiOI/TiO2 CNHs; (c and d) HRTEM images of BiOI/TiO2 CNHs. | ||
The N2 adsorption–desorption isotherms were measured to determine the pore structure and size distribution of the as-prepared coarsened TiO2 nanobelts, BiOI/TiO2 NHs, and BiOI/TiO2 CNHs, as shown in Fig. 6. In Fig. 6b, d and f, all the isotherms are consistent with type IV, exhibit H1 hysteresis loops observed in the range of 0.5–1.0 P/P0 and illustrate the mesoporous characteristics of the samples.37 From Fig. 6a and c, it can be seen that the main pore diameters of the coarsened TiO2 nanobelts and BiOI/TiO2 NHs are in the ranges of 25–45 nm and 1–30 nm, respectively. Meanwhile, the pore diameters of the BiOI/TiO2 CNHs are mostly in the range of 2–13 nm, as shown in Fig. 6e. The difference in pore sizes may come from the crystal growth process.
By using the BET method, the surface area values of the coarsened TiO2 nanobelts, BiOI/TiO2 NHs and BiOI/TiO2 CNHs are calculated to be 26.9 m2 g−1, 29.6 m2 g−1 and 38.6 m2 g−1, respectively. Moreover, the pore volumes of the coarsened TiO2 nanobelts, BiOI/TiO2 NHs and BiOI/TiO2 CNHs are calculated to be 0.06 cm3 g−1, 0.13 cm3 g−1 and 0.18 cm3 g−1, respectively. These results indicate that the BiOI/TiO2 CNHs have a larger surface area and pore volume after coating of BiOI on the coarsened TiO2 nanobelts. The large surface area and pore volume can provide more surface active sites and facilitate the transfer of reactants, leading to an enhanced photocatalytic activity.38
Optical properties analysis
The UV-Vis diffuse reflectance spectra (DRS) of the coarsened TiO2, BiOI, BiOI/TiO2 NHs and BiOI/TiO2 CNHs are shown in Fig. 7a and b. In Fig. 7a, the coarsened TiO2 nanobelts exhibit an absorption edge at about 400 nm, indicating that the light absorption of TiO2 is limited to the UV range.39 BiOI shows strong capability of light absorption in both the UV and visible light regions of about 400–650 nm, in addition to the intrinsic absorption from the band gap transition, which will lead to a good visible light response. The UV-Vis spectra of the BiOI/TiO2 NHs and BiOI/TiO2 CNHs also display almost the same absorption band as BiOI. Compared with the coarsened TiO2 nanobelts, there is an obvious wavelength shift to about 650 nm for the BiOI/TiO2 NHs and BiOI/TiO2 CNHs due to the presence of BiOI. It has been reported that the inhibited recombination between photoelectrons and holes could result in a strong response in the visible region.40The band gaps can be evaluated from the DRS using the following equation (eqn (1)):41
 | (1) |
In order to explore the fate of photoinduced electron–hole pairs in the semiconductors, photoluminescence (PL) spectra of the coarsened TiO2 nanobelts and BiOI/TiO2 CNHs were studied, as shown in Fig. 7c. The PL emission peak appears when the photogenerated free carriers recombine again. The higher peaks indicate a higher recombination of carriers, while the lower peaks suggest a lower recombination.43 Fig. 7c shows the PL of the coarsened TiO2 nanobelts and BiOI/TiO2 CNHs. The two strong emission peaks of 470 nm and 525 nm might belong to the band gap transition of the pure coarsened TiO2 nanobelts.44 It is obvious that the PL intensities of the BiOI/TiO2 CNHs have decreased compared with the coarsened TiO2 nanobelts. This kind of situation should be caused by fewer photons reaching the TiO2 because of the BiOI on the surface of the coarsened TiO2 nanobelts. The BiOI could be easily stimulated, with induction of carriers after absorbing photons, and the heterojunctions formed between the BiOI and coarsened TiO2 are appropriate for the transfer of the photogenerated carriers to the TiO2, as in the subsequent discussion of the mechanism. The transfer of electrons from BiOI to coarsened TiO2 is beneficial for the separation of photoinduced electrons and holes in coarsened TiO2 and thus inhibits the recombination of the photogenerated charge carriers; hence, the photocatalytic activity of the BiOI/TiO2 CNHs is enhanced.
Photocatalytic activities analysis
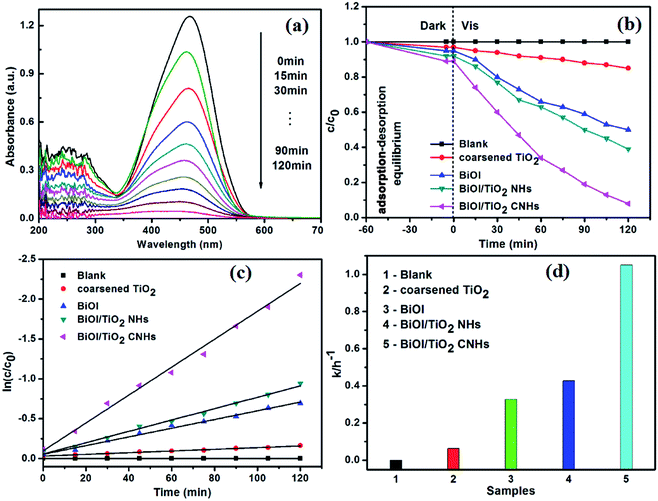
The photocatalytic degradation capabilities of the coarsened TiO2 nanobelts, BiOI, BiOI/TiO2 NHs, and BiOI/TiO2 CNHs were measured by decomposition of aqueous MO solution under visible light irradiation, as shown in Fig. 8. All measurements were carried out under the same conditions for accurate comparison. Before the light irradiation, the photocatalysts and MO were stirred for 60 min in the dark to achieve the balance of the adsorption/desorption equilibrium. Fig. 8a shows a temporal evolution of the spectral changes during the photodegradation of the MO solution in the presence of BiOI/TiO2 (mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) CNHs during the photocatalytic process. It can be seen that the typical absorption peak of MO decreases along with the increase in photocatalytic time, and almost disappears after 120 min, illustrating that the BiOI/TiO2 (mole ratio 1
1) CNHs during the photocatalytic process. It can be seen that the typical absorption peak of MO decreases along with the increase in photocatalytic time, and almost disappears after 120 min, illustrating that the BiOI/TiO2 (mole ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) CNHs possess good photocatalytic activity for MO decomposition under visible light. As shown in Fig. 8b, it is clear that the concentration of the MO solution without catalyst is undiminished after 60 min in the dark, while the decrease with coarsened TiO2 is only 3%, and that with BiOI is 5%, which results from the higher adsorption ability of BiOI than coarsened TiO2. Furthermore, the BiOI/TiO2 CNHs exhibit a slightly stronger adsorption ability toward the MO with an adsorption value of 11% in the dark, while that of BiOI/TiO2 NHs is about 8%. This may be due to the large specific surface area of the BiOI/TiO2 CNHs, with more BiOI nanosheets in the BiOI/TiO2 CNHs, as mentioned in Fig. 3 and 6. This kind of attractive nanomaterial with an adsorption capacity will have important applications in water treatment to remove organic pollutants and even some toxic heavy metal ions.45 After visible light irradiation, it can be seen from Fig. 8b that the coarsened TiO2 nanobelts exhibit a low 15% photodegradation rate of MO because of their low absorption of visible light. On the contrary, BiOI shows a little higher photodegradation rate of 50% of MO after 120 min of visible light irradiation, due to its low band gap (1.80 eV), which can be stimulated by visible light (less than 2.95 eV). With the same illumination time, the efficiency for MO degradation of the BiOI/TiO2 NHs reaches 61%, which is higher than that of BiOI, possibly due to the visible light response of BiOI and the formation of heterostructures, which inhibited the recombination of photogenerated electron–hole pairs in the interface between the BiOI nanosheets and TiO2 nanobelts. It is noteworthy that almost 98.2% of MO is degraded by the BiOI/TiO2 CNHs, which is much higher than that of BiOI/TiO2 NHs. In general, the photocatalytic activity of a composite photocatalyst is connected to its content and components, surface area, absorbance ability, heterojunction interface, band structure matching, etc. However, no remarkable difference in the absorbance ability is found between the BiOI/TiO2 NHs and BiOI/TiO2 CNHs (Fig. 6), which indicates that the major influences for the enhanced photocatalytic activity of the BiOI/TiO2 CNHs are the large specific surface area and the greater formation of a heterostructure interface with matched energy bands between the surface-coarsened TiO2 nanobelts and BiOI nanosheets.
1) CNHs possess good photocatalytic activity for MO decomposition under visible light. As shown in Fig. 8b, it is clear that the concentration of the MO solution without catalyst is undiminished after 60 min in the dark, while the decrease with coarsened TiO2 is only 3%, and that with BiOI is 5%, which results from the higher adsorption ability of BiOI than coarsened TiO2. Furthermore, the BiOI/TiO2 CNHs exhibit a slightly stronger adsorption ability toward the MO with an adsorption value of 11% in the dark, while that of BiOI/TiO2 NHs is about 8%. This may be due to the large specific surface area of the BiOI/TiO2 CNHs, with more BiOI nanosheets in the BiOI/TiO2 CNHs, as mentioned in Fig. 3 and 6. This kind of attractive nanomaterial with an adsorption capacity will have important applications in water treatment to remove organic pollutants and even some toxic heavy metal ions.45 After visible light irradiation, it can be seen from Fig. 8b that the coarsened TiO2 nanobelts exhibit a low 15% photodegradation rate of MO because of their low absorption of visible light. On the contrary, BiOI shows a little higher photodegradation rate of 50% of MO after 120 min of visible light irradiation, due to its low band gap (1.80 eV), which can be stimulated by visible light (less than 2.95 eV). With the same illumination time, the efficiency for MO degradation of the BiOI/TiO2 NHs reaches 61%, which is higher than that of BiOI, possibly due to the visible light response of BiOI and the formation of heterostructures, which inhibited the recombination of photogenerated electron–hole pairs in the interface between the BiOI nanosheets and TiO2 nanobelts. It is noteworthy that almost 98.2% of MO is degraded by the BiOI/TiO2 CNHs, which is much higher than that of BiOI/TiO2 NHs. In general, the photocatalytic activity of a composite photocatalyst is connected to its content and components, surface area, absorbance ability, heterojunction interface, band structure matching, etc. However, no remarkable difference in the absorbance ability is found between the BiOI/TiO2 NHs and BiOI/TiO2 CNHs (Fig. 6), which indicates that the major influences for the enhanced photocatalytic activity of the BiOI/TiO2 CNHs are the large specific surface area and the greater formation of a heterostructure interface with matched energy bands between the surface-coarsened TiO2 nanobelts and BiOI nanosheets.
Hence, it is necessary to analyze the degradation data using the pseudo-first-order reaction kinetics model as expressed in eqn (2), which is generally used to express the photocatalytic degradation process:46
| ln(c0/c) = kt | (2) |
Further studies were necessary to probe the influence of the mole ratio of BiOI to TiO2 on the photocatalytic ability of the BiOI/TiO2 CNHs. It can be seen from Fig. 9a that with an increasing mole ratio of BiOI/TiO2, the photocatalytic ability of the BiOI/TiO2 CNHs first strengthens and then decreases. The maximum value of the BiOI/TiO2 CNHs is reached when BiOI/TiO2 is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Here are the reasons for this phenomenon. Firstly, the visible light photocatalytic activity of the BiOI/TiO2 CNHs increases with increasing fraction of BiOI due to the good absorption of visible light by BiOI and the high photocatalytic activity under visible light. Secondly, when the molar ratio is lower than 1
1. Here are the reasons for this phenomenon. Firstly, the visible light photocatalytic activity of the BiOI/TiO2 CNHs increases with increasing fraction of BiOI due to the good absorption of visible light by BiOI and the high photocatalytic activity under visible light. Secondly, when the molar ratio is lower than 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the formed heterojunctions between the BiOI and surface-coarsened TiO2 nanobelts can promote the photocatalytic activity by facilitating the separation of photogenerated electron–hole pairs, which results in higher visible photocatalytic activity of the BiOI/TiO2 CNHs. However, for the sample with a mole ratio beyond 1
1, the formed heterojunctions between the BiOI and surface-coarsened TiO2 nanobelts can promote the photocatalytic activity by facilitating the separation of photogenerated electron–hole pairs, which results in higher visible photocatalytic activity of the BiOI/TiO2 CNHs. However, for the sample with a mole ratio beyond 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, excessive BiOI nanosheets cover the surface of the coarsened TiO2 nanobelts, which is disadvantageous to the formation of heterojunctions and impedes the transmission of the photoinduced electrons on the heterostructure interface of the BiOI/TiO2 CNHs and hence results in a lower photocatalytic activity.
1, excessive BiOI nanosheets cover the surface of the coarsened TiO2 nanobelts, which is disadvantageous to the formation of heterojunctions and impedes the transmission of the photoinduced electrons on the heterostructure interface of the BiOI/TiO2 CNHs and hence results in a lower photocatalytic activity.
Although the as-synthesized BiOI/TiO2 CNHs show good photocatalytic activity, they will has no practical value if the photocatalyst is not stable under repeated operations. The reusability and photocatalytic stability of the BiOI/TiO2 CNHs were investigated by reuse of the BiOI/TiO2 CNHs in the decomposition of MO under visible-light. As shown in Fig. 9b, after four cycles the degradation rate only decreased by about 8.1%, which indicates an excellent photocatalytic stability of the BiOI/TiO2 CNHs under visible light irradiation.
The efficient mineralization of organic dyes and pollutants is very important for preventing secondary pollution. TOC analysis was used to determine the degree of mineralization of the MO. The original TOC concentration of the MO solution with the BiOI/TiO2 CNHs is 20 mg L−1. After visible light irradiation for 100 min, the TOC concentration of the MO solution decreases by about 62% (Fig. 9c), which indicates that the BiOI/TiO2 CNHs exhibit an excellent performance in the mineralization of MO.
Mechanism of photocatalytic activity enhancement
It is known that the photocatalytic activity is determined by the energy band structure and the separation efficiency of photogenerated electron–hole pairs. The photogenerated electrons and holes could react with the adsorbed reactants on the surface of the catalyst or recombine again.In particular, BiOI is a p-type semiconductor,26,47,48 and TiO2 is an n-type semiconductor,26,48,49 which was further confirmed by the Mott–Schottky plots. According to the Mott–Schottky equation, a linear relationship of 1/C2 versus applied potential can be obtained, with the negative and positive slopes corresponding to p- and n-type conductivities,50,51 respectively. As shown in Fig. 10, the TiO2 and BiOI/TiO2 CNHs show positive slopes, as expected for an n-type semiconductor, while the BiOI shows a negative slope, indicating that BiOI is a p-type semiconductor.
The band edge positions of BiOI and TiO2 were predicted theoretically from the absolute electronegativity,52 and the conduction and valence band positions were evaluated using the following equation (eqn (3)):53
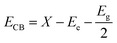 | (3) |
As reported earlier, the flat band potential represents the apparent Fermi level (EF) of a semiconductor in equilibrium with a redox couple,54,55 and the flat band position of semiconductors can be determined by means of the extrapolation of the Mott–Schottky plot. Therefore, we can propose the change in energy band structure of the two semiconductors before and after the contact, as shown in Fig. 11a and b. The EF values of TiO2 and BiOI are 0.03 and 2.18 V (vs. AgCl), respectively. As an n-type semiconductor, the Fermi level of TiO2 lies close to the CB, while the p-type BiOI has an EF close to its VB.56,57 The above analysis indicates that the results of the energy band positions obtained through the band gap values combined with either the conduction or valence band positions are reasonable.
In Fig. 11a, it can be seen that the CB of TiO2 is higher than that of BiOI before contact, and the Fermi level of TiO2 is also higher than that of BiOI. In addition, BiOI could be easily stimulated by visible-light (energy less than 2.95 eV) due to its narrow band gap of 1.80 eV, which induces the photogenerated electron–hole pairs. However, the photocatalytic activity of BiOI is low (Fig. 8b) due to recombination of the photogenerated electrons and holes, which is caused by its narrow band gap. On the contrary, TiO2 nanobelts cannot be stimulated by visible-light because of the wide band gap of 3.0 eV in this work.
After contact (Fig. 11b), the Fermi level of TiO2 is moved down and BiOI is moved up until reaching an equilibrium.58 Meanwhile, the energy bands of TiO2 shift downward along with the Fermi energy level, whereas those of BiOI shift upward in the process, and the CB edge of TiO2 is lower than that of BiOI. Meanwhile, an electric field is formed in the interface under the equilibrium state, thus TiO2 has a positive charge, while a negative charge exists in the BiOI region. The flat band potential of the BiOI/TiO2 CNHs was determined to verify the above deduction. As can be seen from Fig. 11a, the Fermi level position of the BiOI/TiO2 CNHs was 0.15 V (vs. AgCl), which indicates that the Fermi level position of TiO2 shifts down and that of BiOI shifts up after the formation of the p–n junction. In the visible light region, the photogenerated electrons in the VB of BiOI could be excited up to the CB of BiOI, and photogenerated holes remain in the VB of BiOI. The photogenerated electrons transfer to the CB of TiO2, crossing the interface field which will promote the transmission of the photo-generated electron–hole pairs. On the contrary, the holes in the VB of TiO2 transfer to the VB of BiOI. Therefore, the photogenerated electron–hole pairs could be effectively separated by the heterojunction at the p-BiOI/n-TiO2 interface. As a result, more effective photoinduced electrons and holes participate in the photodegradation process, with enhanced photocatalytic activity. Therefore, the separation of photoinduced electron–hole pairs is the key factor enhancing the photocatalytic activity of the BiOI/TiO2 CNHs. According to the band gap structure of BiOI/TiO2, the possible path for the photocatalytic degradation of MO or other dye molecules by the BiOI/TiO2 CNHs is proposed in the following equation (eqn (4)):
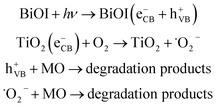 | (4) |
Conclusions
In summary, novel BiOI/TiO2 CNHs were successfully prepared via an efficient hydrothermal method. A dense mass of thick BiOI nanosheets was attached on the surface of coarsened TiO2 nanobelts. Importantly, the BiOI/TiO2 CNHs exhibited excellent decomposition efficiency in the photocatalytic degradation of MO compared to BiOI/TiO2 NHs and pure coarsened TiO2 nanobelts under visible-light irradiation. The photocatalytic activity of the BiOI/TiO2 CNHs varies with the BiOI to TiO2 mole ratios and reaches a maximum value at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In addition, the recycling testing of the BiOI/TiO2 CNHs for MO degradation suggests good stability of the BiOI/TiO2 CNHs. The enhanced photocatalytic activity can be attributed to the well-matched energy bands of the BiOI/TiO2 heterostructure, high specific surface area and suppressed photoelectron–hole recombination. Furthermore, the novel BiOI/TiO2 CNHs will provide a promising platform for potential applications in degradation of organic pollutants and nanotechnology.
1. In addition, the recycling testing of the BiOI/TiO2 CNHs for MO degradation suggests good stability of the BiOI/TiO2 CNHs. The enhanced photocatalytic activity can be attributed to the well-matched energy bands of the BiOI/TiO2 heterostructure, high specific surface area and suppressed photoelectron–hole recombination. Furthermore, the novel BiOI/TiO2 CNHs will provide a promising platform for potential applications in degradation of organic pollutants and nanotechnology.
Acknowledgements
The authors thank the Key Laboratory of Pulp and Paper Science & Technology of Ministry of Education of China (Grant no. 0308031378) for financial support. They also thank Shandong Provincial Key Laboratory of Processing and Testing Technology of Glass and Functional Ceramics for technological support.Notes and references
- Q. W. Tang, L. Lin, X. Zhao, K. Huang and J. H. Wu, Langmuir, 2012, 28, 3972–3978 CrossRef CAS PubMed.
- T. X. Fan, S. K. Chow and D. Zhang, Prog. Mater. Sci., 2009, 54, 542–659 CrossRef CAS.
- J. Y. He, W. M. Wang, F. Long, Z. G. Zou, Z. Y. Fu and Z. Xu, Mater. Sci. Eng., B, 2012, 177, 967–974 CrossRef CAS.
- I. Tacchini, A. Anson-Casaos, Y. Yu, M. T. Martinez and M. Lira-Cantu, Mater. Sci. Eng., B, 2012, 177, 19–26 CrossRef CAS.
- X. Wang, S. Q. Jin, H. Y. An, X. L. Wang, Z. C. Feng and C. Li, J. Phys. Chem. C, 2015, 119, 22460–22464 CAS.
- X. Wang, S. Shen, S. Q. Jin, J. X. Yang, M. R. Li, X. L. Wang, H. X. Han and C. Li, Phys. Chem. Chem. Phys., 2013, 15, 19380–19386 RSC.
- A. Wolcott, W. A. Smith, T. R. Kuykendall, Y. P. Zhao and J. Z. Zhang, Small, 2009, 5, 104–111 CrossRef CAS PubMed.
- X. Wang, Q. Xu, F. T. Fan, X. L. Wang, M. R. Li, Z. C. Feng and C. Li, Chem.–Asian J., 2013, 8, 2189–2195 CrossRef CAS PubMed.
- X. Wang, Q. Xu, M. R. Li, S. Shen, X. L. Wang, Y. C. Wang, Z. C. Feng, J. Y. Shi, H. X. Han and C. Li, Angew. Chem., Int. Ed., 2012, 51, 13089–13092 CrossRef CAS PubMed.
- L. Gomathi Devi and R. Kavitha, RSC Adv., 2014, 4, 28265–28299 RSC.
- X. Lin, M. Li, Y. J. Li and W. Chen, RSC Adv., 2015, 5, 105227–105238 RSC.
- J. M. Macak, M. Zlamal, J. Krysa and P. Schmuki, Small, 2007, 3, 300–304 CrossRef CAS PubMed.
- W. L. Wang, H. Lin, J. B. Li and N. Wang, J. Am. Chem. Soc., 2008, 91, 628–631 CAS.
- K. Zhu, N. R. Neale, A. Miedaner and A. J. Frank, Nano Lett., 2007, 7, 69–74 CrossRef CAS PubMed.
- P. G. Hu, G. J. Du, W. J. Zhou, J. J. Cui, J. J. Lin, H. Liu, D. Liu, J. Y. Wang and S. W. Chen, ACS Appl. Mater. Interfaces, 2010, 2, 3263–3269 CAS.
- Y. M. Wang, G. J. Du, H. Liu, D. Liu, S. B. Qin, N. Wang, C. G. Hu, X. Tao, J. Jiao, J. Wang and Z. L. Wang, Adv. Funct. Mater., 2008, 18, 1131–1137 CrossRef CAS.
- Y. F. Wang, M. Y. Wu and W. F. Zhang, Electrochim. Acta, 2008, 53, 7863–7868 CrossRef CAS.
- G. F. Ortiz, I. Hanzu, P. Lavela, J. L. Tirado, P. Knauthac and T. Djenizian, J. Mater. Chem., 2010, 20, 4041–4046 RSC.
- A. Kubacka, M. Fernandez-García and G. Colon, Chem. Rev., 2012, 112, 1555–1614 CrossRef CAS PubMed.
- J. Tian, P. Hao, N. Wei, H. Z. Cui and H. Liu, ACS Catal., 2015, 5, 4530–4536 CrossRef CAS.
- A. Tiwari, I. Mondala and U. Pal, RSC Adv., 2015, 5, 31415–31421 RSC.
- L. L. Lai and J. M. Wu, J. Mater. Chem. A, 2015, 3, 15863–15868 CAS.
- X. B. Chen and C. Burda, J. Am. Chem. Soc., 2008, 130, 5018–5019 CrossRef CAS PubMed.
- R. Liu, P. Wang, X. F. Wang, H. G. Yu and J. G. Yu, J. Phys. Chem. C, 2012, 116, 17721–17728 CAS.
- J. G. Yu, G. P. Dai and B. B. Huang, J. Phys. Chem. C, 2009, 113, 16394–16401 CAS.
- B. Chai, Q. Xu, J. Li and K. Dai, Sci. Adv. Mater., 2014, 6, 1806–1813 CrossRef CAS.
- W. J. Zhou, H. Liu, J. Y. Wang, D. Liu, G. J. Du and J. J. Cui, ACS Appl. Mater. Interfaces, 2010, 2, 2385–2392 CAS.
- Z. Y. Liu, D. D. Sun, P. Guo and J. O. Leckie, Nano Lett., 2007, 7, 1081–1085 CrossRef CAS PubMed.
- X. Zhang, L. Z. Zhang, T. F. Xie and D. J. Wang, J. Phys. Chem. C, 2009, 113, 7371–7378 CAS.
- X. J. Guo, M. M. Zhen, H. J. Liu and L. Liu, RSC Adv., 2015, 5, 24777–24782 RSC.
- D. Yu, J. Bai, H. O. Liang, J. Z. Wang and C. P. Lia, RSC Adv., 2015, 5, 91457–91465 RSC.
- T. B. Li, G. Chen, C. Zhou, Z. Y. Shen, R. C. Jin and J. X. Sun, Dalton Trans., 2011, 40, 6751–6758 RSC.
- E. H. Wang, S. W. Liu, Q. F. Lu, Z. L. Xiu, T. G. Li and L. J. Song, J. Sol–Gel Sci. Technol., 2011, 58, 705–710 CrossRef CAS.
- L. Ye, X. Liu, Q. Zhao, H. Xie and L. Zan, J. Mater. Chem. A, 2013, 1, 8978–8983 CAS.
- D. Hou, X. Hu, P. Hu, W. Zhang, M. Zhang and Y. Huang, Nanoscale, 2013, 5, 9764–9772 RSC.
- K. Gurunathan, Int. J. Hydrogen Energy, 2004, 29, 933–940 CrossRef CAS.
- D. V. Bavykin, V. N. Parmon, A. A. Lapkin and F. C. Walsh, J. Mater. Chem., 2004, 14, 3370–3377 RSC.
- C. X. Xu, X. Wei, Z. H. Ren, Y. Wang, G. Xu, G. Shen and G. R. Han, Mater. Lett., 2009, 63, 2194–2197 CrossRef CAS.
- K. X. Ren, K. Zhang, J. Liu, H. D. Luo, Y. B. Huang and X. B. Yu, CrystEngComm, 2012, 14, 4384–4390 RSC.
- T. Yan, H. Y. Liu, M. Sun, X. D. Wang, M. M. Li, Q. Yan, W. G. Xu and B. Du, RSC Adv., 2015, 5, 10688–10696 RSC.
- X. Zhang, Z. H. Ai, F. L. Jia and L. Z. Zhang, J. Phys. Chem. C, 2008, 112, 747–753 CAS.
- S. Sun, W. Wang, L. Zhang, L. Zhou, W. Yin and M. Shang, Environ. Sci. Technol., 2009, 43, 2005–2010 CrossRef CAS PubMed.
- M. Miyauchi, Y. Nukui, D. Atarashi and E. Sakai, ACS Appl. Mater. Interfaces, 2013, 5, 9770–9776 CAS.
- J. Tian, Y. H. Sang, Z. H. Zhao, W. J. Zhou, D. Z. Wang, X. L. Kang, H. Liu, J. Y. Wang, S. W. Chen, H. Q. Cai and H. Huang, Small, 2013, 9, 3864–3872 CrossRef CAS PubMed.
- W. J. Zhou, Z. Y. Yin, Y. P. Du, X. Huang, Z. Y. Zeng, Z. X. Fan, H. Liu, J. Y. Wang and H. Zhang, Small, 2013, 9, 140–147 CrossRef CAS PubMed.
- X. Zhang, L. Z. Zhang, T. F. Xie and D. J. Wang, J. Phys. Chem. C, 2009, 113, 7371–7378 CAS.
- J. Jiang, X. Zhang, P. B. Sun and L. Z. Zhang, J. Phys. Chem. C, 2011, 115, 20555–20564 CAS.
- G. P. Dai, J. G. Yu and G. Liu, J. Phys. Chem. C, 2011, 115, 7339–7346 CAS.
- J. Q. Liu, L. L. Ruan, S. B. Adeloju and Y. C. Wu, Dalton Trans., 2014, 43, 1706–1715 RSC.
- G. G. Liu, T. Wang, S. X. Ouyang, L. Q. Liu, H. Y. Jiang, Q. Yu, T. Kako and J. H. Ye, J. Mater. Chem. A, 2015, 3, 8123–8132 CAS.
- Z. Q. He, D. Wang, H. Y. Fang, J. M. Chen and S. Song, Nanoscale, 2014, 6, 10540–10544 RSC.
- M. C. Long, W. M. Cai, J. Cai, B. X. Zhou, X. Y. Chai and Y. H. Wu, J. Phys. Chem. B, 2006, 110, 20211–20216 CrossRef CAS PubMed.
- A. H. Nethercot Jr, Phys. Rev. Lett., 1974, 33, 1088–1091 CrossRef.
- Y. Zhang, Q. Pei, J. C. Liang, T. Feng, X. Zhou, H. Mao, W. Zhang, Y. Hisaeda and X. M. Song, Langmuir, 2015, 31, 10279–10284 CrossRef CAS PubMed.
- S. M. Sun, W. Z. Wang, D. Z. Li, L. Zhang and D. Jiang, ACS Catal., 2014, 4, 3498–3503 CrossRef CAS.
- S. J. Hong, S. Lee, J. S. Jang and J. S. Lee, Energy Environ. Sci., 2011, 4, 1781–1787 CAS.
- T. W. Kim and K. S. Choi, Science, 2014, 343, 990–994 CrossRef CAS PubMed.
- J. G. Yu, W. G. Wang and B. Cheng, Chem.–Asian J., 2010, 5, 2499–2506 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |