Thienoisoindigo-based copolymer with fused thieno[3,2-b]thiophene as a donor in thin film transistor applications with high performance†
Chi-Min
Chen
a,
Sunil
Sharma
a,
Yi-Lun
Li
a,
Jey-Jau
Lee
b and
Show-An
Chen
*a
aChemical Engineering Department and Frontier Research Center on Fundamental and Applied Sciences of Matters, National Tsing-Hua University, Hsinchu 30013, Taiwan, Republic of China. E-mail: sachen@che.nthu.edu.tw
bNational Synchrotron Radiation Research Center, No. 101, Hsin-Ann Road, Hsinchu Science Park, Hsinchu 30076, Taiwan, Republic of China. E-mail: jjlee@nsrrc.org.tw
First published on 5th November 2014
Abstract
A new thienoisoindigo (TIG)-based copolymer with thieno[3,2-b]thiophene as a donor is synthesized. The copolymer with a short π–π stacking distance of 3.43 Å exhibits an excellent hole mobility up to 0.69 cm2 V−1 s−1 under optimum conditions. This performance is the record value among reported TIG-based transistors.
Recently, donor–acceptor (D–A) alternating copolymers have been investigated for polymer thin film transistor (PTFT) applications due to their excellent field-effect mobilities.1 Using the D–A structural approach, charge delocalization in the polymer backbone can be improved by the formation of a quinoidal structure, which is driven by the intramolecular charge transfer (ICT) between the D–A moieties, facilitating intrachain charge transport.2 Besides, the intermolecular π–π stacking distance can be shortened by the polar interaction of the intermolecular D–A moieties, which is advantageous for interchain charge transport.3 Hence D–A copolymers manifest a relatively high mobility compared to conventional thiophene-based polymers.4 Isoindigo (IG) is an acceptor molecule consisting of two indolin-2-one units with strong electron-withdrawing characteristics. It has a hole mobility (μh) in the range of 10−3 to 1.0 cm2 V−1 s−1 when incorporated into copolymers.5 However, the structural repulsion between the protons on the phenyl rings and the oxygen atoms of the ketopyrrole moieties in IG not only results in a reduction of coplanarity but also interferes in the stacking interaction of neighboring polymer chains, which is unfavourable for charge transport.6 To alleviate this structural drawback, a novel thienoisoindigo (TIG) acceptor has been proposed where the outer phenyl rings of IG were replaced by thiophene units.7 Due to the reduction in steric hindrance between the sulfur atoms in the thiophene units and the carbonyl oxygen atoms, the structural planarity of TIG can be improved.6–8 In addition, the interchain packing can also be promoted by the π-conjugated backbone and the polar functionality, resulting in better charge carrier transport.7,8 However, the mobilities of TIG-based copolymers only reach the range of 10−3 to 0.20 cm2 V−1 s−1, but with a lower on/off current ratio (Ion/Ioff < 103) and a higher threshold voltage (Vth > 5.9 V).7,8
In recent reports, fused thieno[3,2-b]thiophene (TT) with a more coplanar structure and more electron-donating properties has been employed as a donor in D–A copolymers to enhance the charge carrier mobilities of PTFTs, where a mobility up to 1.9 cm2 V−1 s−1 has been achieved.5,9 This high mobility can be ascribed to the enhanced interchain π orbital overlap due to the extension of the π-conjugation in the backbone and the shortened π–π stacking distance caused by the strengthened interchain interaction of the D–A moieties.9 This result suggests that TT is a promising donor for D–A type copolymer semiconductors. Therefore, in this work, fused TT is introduced as a donor moiety in the synthesis of a new TIG-based copolymer (PTIG-co-TT) to further enhance the mobility in TIG-based transistors. Furthermore, thiophene molecules are used as end-cappers for the copolymer to eliminate the influence of end group defects in the performance of the transistor, such as disturbance of the chain packing, charge carrier traps and a rise in the off current.10
The synthetic procedure for PTIG-co-TT was via a Stille coupling polycondensation reaction between (E)-2,2′-dibromo-4,4′-bis(2-octyldodecyl)-[6,6′-bithieno[3,2-b]pyrrolylidene]-5,5′(4H,4′H)-dione and 2,5-bis(trimethylstannyl)-thieno[3,2-b]thiophene, and then the reaction was terminated by thiophene molecules (Scheme 1). The monomer was synthesized according to the reported procedure7 and the details are described in the ESI.† The copolymer, with a number average molecular weight (Mn) of 90.6 kD and a corresponding polydispersity index (PDI) of 2.92, was evaluated by gel permeation chromatography (GPC) using THF as the eluent (Fig. S1 in the ESI†). The copolymer showed excellent thermal stability with a decomposition temperature over 437 °C (Fig. S2 in the ESI†).
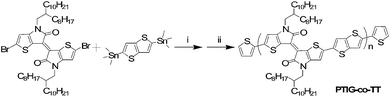 | ||
| Scheme 1 Synthetic route for the PTIG-co-TT copolymer. (i) Pd2(dba)3, P(o-Tol)3, toluene, 110 °C; (ii) 2-tributylstannylthiophene, then 2-bromothiophene. | ||
The UV-vis-NIR absorption spectra of PTIG-co-TT in 1,2,4-trichlorobenzene (TCB) solution and as a thin film annealed at 200 °C display dual absorption band characteristics at high and low energy regions (Fig. 1), which are similar to those of TIG-based copolymers reported in the literature.7,8 In TCB solution, the maximum absorption peak (λmax) is at 952 nm. Compared to the solution spectrum, the spin-cast film shows a slight red-shift of 35 nm in λmax (987 nm), but exhibits a significant red shift of 70 nm in the absorption onset (from 1230 nm to 1300 nm). This implies that the copolymer has a more ordered molecular organization upon film formation. The optical band gap (Eoptg) of PTIG-co-TT, calculated from the absorption onset (1300 nm) of the thin film, is 0.95 eV, which is lower than the TT-containing copolymer with an IG acceptor (ref. 5, Eoptg = 1.55 eV) for 0.6 eV. The smaller optical band gap of PTIG-co-TT illustrates that the TIG acceptor is able to form more effective π-conjugation compared to IG in a copolymer. The highest occupied molecular orbital (HOMO) level of the PTIG-co-TT film was measured by ultraviolet photoelectron spectroscopy (UPS) and was estimated to be −5.15 eV (Fig. S3 in the ESI†). This indicates that it is beneficial for charge injection from gold electrodes to form a p-channel transport, relative to −5.70 eV for the analogous IG-based copolymer.5 Using the HOMO level and the optical band gap, the lowest unoccupied molecular orbital (LUMO) level of PTIG-co-TT was calculated to be −4.20 eV.
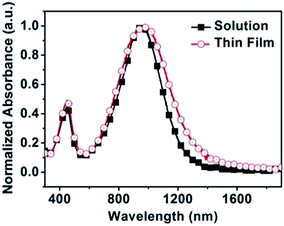 | ||
| Fig. 1 Normalized absorption spectra of PTIG-co-TT in 1,2,4-trichlorobenzene solution and as a thin film annealed at 200 °C. | ||
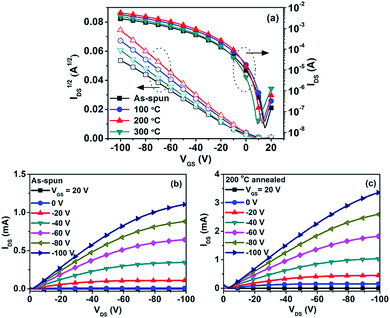
Bottom-gate/bottom-contact (BG/BC) transistors were fabricated for investigating the charge transport behaviour of PTIG-co-TT. Thin films of PTIG-co-TT were deposited on top of n-octadecyltrimethoxysilane (OTS) treated Si/SiO2 substrates by spin-coating the polymer solution in TCB. Subsequently, the devices were annealed and measured under nitrogen. Fig. 2(a) presents the transfer characteristics of the PTIG-co-TT-based devices at a drain voltage (VDS) of −100 V before and after heat treatment. All devices exhibited typical p-channel transfer characteristics with a linear trend in the square root of the drain current (IDS1/2) versus the gate voltage (VGS) plot above the Vth, indicating that the mobilities are independent of the VGS bias in operation. This implies that charge traps from electronic defects are absent in the channel.11 The field-effect mobilities were extracted by the linear fitting of IDS1/2versus VGS in the saturation regime. All results are summarized in Table 1. The device with the as-spun film has a mobility of 0.12 cm2 V−1 s−1 with an Ion/Ioff value of 1.7 × 105 and a Vth value of −0.6 V. After thermal treatment, the mobilities were improved compared to that of the pristine film. The annealed device at 200 °C yielded the highest mobility, with a value of 0.23 cm2 V−1 s−1, which is about 2 times better than that of the as-spun device. Up to 0.69 cm2 V−1 s−1 with a current density over 0.01 A cm−1, an Ion/Ioff value over 104 and a Vth value of 1.5 V was obtained for the device with a channel length (L) of 20 μm and a channel width (W) of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μm (Fig S4 in the ESI†). When the annealing temperature was further raised to 300 °C, the mobility decreased to 0.14 cm2 V−1 s−1, which can be attributed to a change in the structural order of the polymer chains as discussed below. In addition, all the devices had close Ion/Ioff values and Vth values regardless of whether the film was or was not thermally treated, suggesting that PTIG-co-TT possesses an outstanding thermal stability in devices without rise in the off current and Vth shift in comparison with some other D–A copolymers.7,8,12Fig. 2(b) and (c) show the output characteristic curves of the PTIG-co-TT-based devices with the as-spun film and the film annealed at 200 °C. In the device annealed at 200 °C, the saturation drain current goes up to 3.33 mA at a VGS value of −100 V. This is more than a 3 times improvement over the as-spun device (1.1 mA), implying that a better performance can be obtained after thermal annealing.
000 μm (Fig S4 in the ESI†). When the annealing temperature was further raised to 300 °C, the mobility decreased to 0.14 cm2 V−1 s−1, which can be attributed to a change in the structural order of the polymer chains as discussed below. In addition, all the devices had close Ion/Ioff values and Vth values regardless of whether the film was or was not thermally treated, suggesting that PTIG-co-TT possesses an outstanding thermal stability in devices without rise in the off current and Vth shift in comparison with some other D–A copolymers.7,8,12Fig. 2(b) and (c) show the output characteristic curves of the PTIG-co-TT-based devices with the as-spun film and the film annealed at 200 °C. In the device annealed at 200 °C, the saturation drain current goes up to 3.33 mA at a VGS value of −100 V. This is more than a 3 times improvement over the as-spun device (1.1 mA), implying that a better performance can be obtained after thermal annealing.
| Heat treatment temp. [°C] | μ h [cm2 V−1 s−1] | I on/Ioff | V th [V] | |
|---|---|---|---|---|
| Maximum | Average | |||
a Maximum and average hole mobilities of the PTFTs. The average value was calculated from at least three devices (L = 10 μm and W = 5000 μm).
b Different device geometry (L = 20 μm and W = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μm) and dielectric thickness (200 nm). 000 μm) and dielectric thickness (200 nm).
|
||||
| As-spun | 0.12 | 0.10 | 1.7 × 105 | −0.6 |
| 100 | 0.18 | 0.14 | 1.3 × 105 | 0.1 |
| 200 | 0.23 | 0.21 | 2.0 × 105 | −0.5 |
| 200b | 0.69 | 0.59 | 6.4 × 104 | 1.5 |
| 300 | 0.14 | 0.13 | 2.0 × 105 | −1.6 |
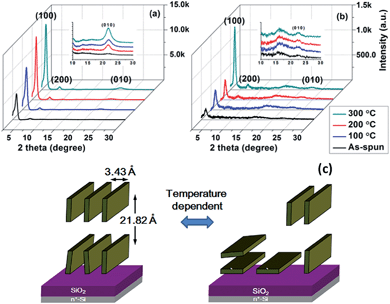
To investigate the effects of thermal treatment on the charge carrier mobility, PTIG-co-TT thin films were characterized by grazing incidence X-ray diffraction (GI-XRD) analysis to explore the chain packing and molecular orientation (Fig. 3 and 2D patterns in Fig. S5 in the ESI†). A primary diffraction peak (100) at a 2θ value of 3.50° and a weak diffraction peak (010) at a 2θ value of 22.40° were observed in the out-of-plane direction and the in-plane direction, respectively, corresponding to aligned PTIG-co-TT chains with a d-spacing of 21.82 Å and a π–π stacking distance of 3.43 Å. The annealed films exhibited more intense diffraction peaks, indicating a more ordered crystalline structure in the annealed PTIG-co-TT film device for efficient charge carrier transport. The results above clearly demonstrate that PTIG-co-TT chains take on a predominantly ordered lamellar edge-on packing structure relative to the substrate. This orientation is conducive to achieving a high carrier mobility because of the direction of the charge carrier transport through the π–π stacking, which is parallel to the channel between source and drain electrodes.13 The remarkably small π–π stacking distance (3.43 Å) of PTIG-co-TT is the shortest distance among reported TIG-based copolymers and consequently this results in a higher mobility compared to other reported TIG-based copolymers.7,8 However, when the annealing temperature was increased to 300 °C, both the intensity of the diffraction peak (010) in the out-of-plane direction (Fig. 3(a)) and the intensity of the diffraction peak (100) in the in-plane direction (Fig. 3(b)) obviously increased. This implies that PTIG-co-TT at this annealing temperature has a critical alignment transition to form more PTIG-co-TT chains with a face-on configuration as shown in Fig 3(c), leading to a decrease in the mobility (0.14 cm2 V−1 s−1) relative to the highest mobility (0.23 cm2 V−1 s−1) with an optimal packing structure at 200 °C for PTFT performance.14 Additionally, the thin films of PTIG-co-TT were also characterized by atomic force microscopy (AFM) to better understand the relationships between the morphology and device performance. As shown in Fig. 4(a), the as-spun PTIG-co-TT film has highly independent granules on the OTS-modified substrate. In contrast to the as-spun film, the annealed film at 200 °C consists of uniformed polymer nanofibrils with interconnected networks (Fig. 4(b)), resulting in an enhanced mobility due to more efficient pathways for charge carrier transport.10
 | ||
| Fig. 4 AFM tomography images of PTIG-co-TT on OTS-treated Si/SiO2 substrates with (a) the as-spun film and (b) the annealed film at 200 °C. | ||
Conclusions
In conclusion, we have presented the synthesis of a new TIG-based copolymer, PTIG-co-TT, with fused thieno[3,2-b]thiophene as a donor and thiophene molecules as end-cappers for use in PTFTs. The shallow HOMO level of −5.15 eV is ideal for the injection of hole carriers from gold electrodes. The polymer chains of PTIG-co-TT form an ordered lamellar edge-on orientation with the shortest π–π stacking distance (3.43 Å) among reported TIG-based copolymers. However, the copolymer has an alignment transition of chain packing at a higher post-annealing temperature (300 °C). The annealed PTIG-co-TT film possesses a reticular structure consisting of intertwined nanofibrils compared to a nodular structure in the as-spun film. Using optimal heat treatment and a proper device geometry for the best chain packing and morphology, an excellent hole mobility up to 0.69 cm2 V−1 s−1 can be achieved. Besides, PTIG-co-TT shows an outstanding thermal stability in device performance, providing further progress towards the use of TIG-based copolymers in PTFT applications.Acknowledgements
This work was supported by the Ministry of Education and the Ministry of Science and Technology with financial aid through projects NSC-101-2120-M-007-004, NSC-102-2633-M-007-002 and NSC-102-2221-E-007-131.Notes and references
- L. Biniek, B. C. Schroeder, C. B. Nielsen and I. McCulloch, J. Mater. Chem., 2012, 22, 14803 RSC.
- J. D. Yuen, J. Fan, J. Seifter, B. Lim, R. Hufschmid, A. J. Heeger and F. Wudl, J. Am. Chem. Soc., 2011, 133, 20799 CrossRef CAS PubMed.
- H. Sirringhaus, M. Bird, T. Richards and N. Zhao, Adv. Mater., 2010, 22, 3893 CrossRef CAS PubMed.
- B. S. Ong, Y. Wu, Y. Li, P. Liu and H. Pan, Chem.–Eur. J., 2008, 14, 4766 CrossRef CAS PubMed.
- T. Lei, Y. Cao, X. Zhou, Y. Peng, J. Bian and J. Pei, Chem. Mater., 2012, 24, 1762 CrossRef CAS.
- P. Deng and Q. Zhang, Polym. Chem., 2014, 5, 3298 RSC.
- R. S. Ashraf, A. J. Kronemeijer, D. James, H. Sirringhaus and I. McCulloch, Chem. Commun., 2012, 48, 3939 RSC.
- G. K. Dutta, A.-R. Han, J. Lee, Y. Kim, J. H. Oh and C. Yang, Adv. Funct. Mater., 2013, 23, 5317 CrossRef CAS; Y. Koizumi, M. Ide, A. Saeki, C. Vijayakumar, B. Balan, M. Kawamotoa and S. Seki, Polym. Chem., 2013, 4, 484 RSC; G. W. P. Van Pruissen, F. Gholamrezaie, M. M. Wienk and R. A. J. Janssen, J. Mater. Chem., 2012, 22, 20387 RSC.
- C. B. Nielsen, M. Turbiez and I. McCulloch, Adv. Mater., 2013, 25, 1859 CrossRef CAS PubMed; Z. Chen, M. J. Lee, R. S. Ashraf, Y. Gu, S. Albert-Seifried, M. M. Nielsen, B. Schroeder, T. D. Anthopoulos, M. Heeney, I. McCulloch and H. Sirringhaus, Adv. Mater., 2012, 24, 647 CrossRef PubMed; H. Bronstein, Z. Chen, R. S. Ashraf, W. Zhang, J. Du, J. R. Durrant, P. S. Tuladhar, K. Song, S. E. Watkins, Y. Geerts, M. M. Wienk, R. A. J. Janssen, T. Anthopoulos, H. Sirringhaus, M. Heeney and I. McCulloch, J. Am. Chem. Soc., 2011, 133, 3272 CrossRef PubMed; Y. Li, S. P. Singh and P. Sonar, Adv. Mater., 2010, 22, 4862 CrossRef PubMed.
- J.-F. Chang, J. Clark, N. Zhao, H. Sirringhaus, D. W. Breiby, J. W. Andreasen, M. M. Nielsen, M. Giles, M. Heeney and I. McCulloch, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 115318 CrossRef CAS; Y. Kim, S. Cook, J. Kirkpatrick, J. Nelson, J. R. Durrant, D. D. C. Bradley, M. Giles, M. Heeney, R. Hamilton and I. McCulloch, J. Phys. Chem. C, 2007, 111, 8137 Search PubMed.
- J.-F. Chang, B. Sun, D. W. Breiby, M. M. Nielsen, T. I. Sölling, M. Giles, I. McCulloch and H. Sirringhaus, Chem. Mater., 2004, 16, 4772 CrossRef CAS.
- Z. Chen, M. J. Lee, R. S. Ashraf, Y. Gu, S. Albert-Seifried, M. M. Nielsen, B. Schroeder, T. D. Anthopoulos, M. Heeney, I. McCulloch and H. Sirringhaus, Adv. Mater., 2012, 24, 647 CrossRef CAS PubMed; A. J. Kronemeijer, E. Gili, M. Shahid, J. Rivnay, A. Salleo, M. Heeney and H. Sirringhaus, Adv. Mater., 2012, 24, 1558 CrossRef PubMed.
- H. Sirringhaus, P. J. Brown, R. H. Friend, M. M. Nielsen, K. Bechgaard, B. M. W. Langeveld-Voss, A. J. H. Spiering, R. A. J. Janssen, E. W. Meijer, P. Herwig and D. M. de Leeuw, Nature, 1999, 401, 685 CrossRef CAS PubMed.
- D. H. Kim, Y. D. Park, Y. Jang, H. Yang, Y. H. Kim, J. I. Han, D. G. Moon, S. Park, T. Chang, C. Chang, M. Joo, C. Y. Ryu and K. Cho, Adv. Funct. Mater., 2005, 15, 77 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, TFT fabrication, UPS, AFM and GI-XRD. See DOI: 10.1039/c4tc02355b |
| This journal is © The Royal Society of Chemistry 2015 |