Antibiotic nanoparticles embedded into the Parylene C layer as a new method to prevent medical device-associated infections
Olga
Grinberg
a,
Michal
Natan
b,
Anat
Lipovsky
a,
Alexander
Varvak
c,
Herbert
Keppner
d,
Aharon
Gedanken
*ae and
Ehud
Banin
*b
aDepartment of Chemistry, Kanbar Laboratory for Nanomaterials, Nanotechnology Research Center, Institute of Nanotechnology and Advanced Materials, Bar-Ilan University, Ramat-Gan, 52900, Israel. E-mail: gedanken@mail.biu.ac.il; Olga.Grinberg@biu.ac.il; ecoli2310@yahoo.com
bThe Biofilm Research Laboratory, The Bar-Ilan Institute of Nanotechnology and Advanced Materials, The Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel. E-mail: natan.michal@gmail.com; ehud.banin@biu.ac.il
cChromatography Unit, Scientific Equipment Center, The Mina and Everard Goodman Faculty of Life Sciences, Bar-Ilan University, Ramat-Gan, Israel. E-mail: Alexander.Varvak@mail.biu.ac.il
dHES-SO Arc, Institut des Microtechnologies Appliquées, Eplatures-Grises, 1 7, 2300 La Chaux-de Fonds, Switzerland. E-mail: Herbert.Kepner@he-arc.ch
eDepartment of Materials Science & Engineering, National Cheng Kung University, Tainan, 70101, Taiwan
First published on 1st October 2014
Abstract
Tetracycline nanoparticles (NPs) were synthesized and simultaneously deposited on Parylene-C coated glass slides using ultrasound irradiation. The optimization of the process conditions, the specific reagent ratio and the precursor concentration resulted in the formation of uniform NPs with an average size of ∼50 nm. These novel tetracycline NP coated-surfaces were tested against two common bacterial pathogens, Escherichia coli and Staphylococcus aureus, and were found to be extremely potent against both bacteria, suggesting that these antibiotic NPs provide the Parylene surface with self-sterilizing properties. Finally, the mechanism describing the formation of tetracycline NPs and their subsequent deposition on the Parylene C surface is presented.
Introduction
Over the past several years Parylene C (poly(chloro-para-xylylene)) has been widely used in numerous applications. Parylene C provides a range of advantages, such as low water permeability,1 durability and biocompatibility.2 This polymer has received considerable attention as a biomaterial from a number of research groups worldwide.3,4 Parylene C is used for coating printed circuit boards (PCBs) and medical devices. There are numerous other applications as Parylene is an excellent moisture barrier. It is the most bio-accepted coating for stents, defibrillators, pacemakers and other devices permanently implanted into the body. It is easily deposited at room-temperature,5 yet it can be autoclaved or sterilized using ethylene oxide or c-irradiation sterilization techniques.6 These properties meet critical requirements for use in different medical devices. The Parylene C coated substrates or devices are stable, and show little or no change in their response characteristics. When coated with Parylene C, the device or substrate is chemically isolated and compatible with its environment. Typical applications of Parylene C include the coating of catheters, guide wires, stents, sensors, transducers and probes, which empower the surfaces with the required properties. All of these devices have been used or implanted and found to be compatible with body tissues.7 A major concern with many medical implants is the colonization of the implant by bacteria (i.e. biofilm formation) and subsequent infection of the patient. Thus preventing biofilm development on medical devices and implants is of great importance in an attempt to reduce infections and complications. Traditional techniques currently used to minimize device-associated infections include practicing good aseptic techniques and systemic administration of antibiotics. However, these techniques do not show satisfactory results.8 For example, in the case of catheter infections, systemic antibiotic (vancomycin and gentamicin) administration alone without catheter removal was only effective in treating 22–37% of catheter-associated bacteremia cases.9 The reason why these traditional practices are ineffective is usually the formation of biofilms on the medical device and the existence of antibiotic-resistant bacteria.Antibiotic coating on the surface of medical devices is an alternative strategy developed in an attempt to minimize the risk of infection. To be effective, polymer-associated antibiotics need to fulfill several prerequisites. First, they must be effective against the most common organisms found in infections of the particular prosthesis, as well as maintain potency when bound to the polymer. Second, the device must provide effective antibacterial concentrations in situ during the period when it is most susceptible to colonization/infection. Finally, the issue of adverse reactions to the antibiotic formulations used needs to be considered. The development of side effects can have disastrous consequences for the patient. Although no antibiotic can presently meet all these criteria, various antibiotics have been tested. Schierholz et al.10 looked at drug-release rates, bacterial colonization, and morphological features of antibiotic-incorporated polyurethanes in an attempt to determine the antimicrobial activity of these delivery systems. Bacterial colonization was inhibited effectively by preparation showing slower but more sustained antimicrobial delivery, as seen with gentamicin-base and flucloxacillin, whereas polymers loaded with ciprofloxacillin and fosfomycin showed a granular structure of crystallized drug leading to a less predictable rate of release.8 It was concluded that high homogeneity is required for a sustained and prolonged release over time and effective inhibition of bacterial colonization. The efficacy of different combinations of antibiotic coating on catheters was also investigated.11 Raad et al. evaluated the activities of central venous catheters coated with minocycline and rifampin, and with chlorhexidine gluconate and silver sulfadiazine both in vitro, as well as in a rabbit model.12 It was found that the combination of minocycline and rifampin is unique and highly effective in preventing the colonization of catheters with slime-producing staphylococci and that it also displays a broad-spectrum inhibitory activity against Gram-negative bacteria and yeast cells. A similar approach was also adopted in bladder catheters.13
Antibacterial nanomaterials with intrinsic bactericidal properties are some of the emerging trends today. Among these nanomaterials are metals,14 metallic oxide NPs,15–17 carbon nanotubes,18 fullerenes,19 nanosized metal fluorides,20 and chitosan.21 Each material has its own advantages toward certain types of bacteria even though the mechanisms of how these materials affect the bacteria remain elusive. Furthermore, different strategies used to combine antibacterial materials to achieve synergistic effects are highly desirable. Recent studies on composite nanomaterials of silver–chitosan,22 chitosan–arginine,23 zinc–iron oxide,24 and polymer–antibiotics25 have shown great potential in inhibiting bacterial growth and look promising as future therapeutic agents.
Sonochemistry has proved to be a very effective technique for the fabrication of antibacterial nanomaterials.26,27 Nevertheless, many of the fabricated NPs were not soluble in water. Recently, we have reported on the synthesis of water soluble salts such as NaCl, KI, KBr, and CuSO4.28 As already reported, this technique was also applied to organic soluble materials,29 and we have succeeded, for example, in the fabrication of NPs of the water-soluble thermophilic amylase enzyme, using a one-step sonochemical process. This sonication has led to the creation of enzymatic NPs. To prevent the redissolution of the water soluble NPs in water, we subsequently incorporated the NPs into polyethylene films. The same principle was applied in the current study, where NPs of the antibiotic tetracycline were prepared and subsequently embedded into the Parylene C layer of glass coupons. The obtained composite combines the antibacterial nanomaterial (tetracycline) with the known biocompatible polymer (Parylene C) allowing the generation of surfaces (e.g., medical devices) with antibacterial activity. The synthesis and deposition of the antibiotic nanoparticles on the surface was carried out using sonochemistry. This process involves in situ generation of tetracycline NPs under ultrasound irradiation and their subsequent deposition on Parylene C layer in a one-step reaction. To the best of our knowledge, this is the first report on the synthesis and deposition of antibiotic NPs in their pure form and not as drug molecules adsorbed or chemically attached to carrier NPs. The obtained product was shown to be extremely effective against bacteria. Taken together, this study highlights the potential use of tetracycline NP-coated surfaces for various biomedical applications.
Results and discussion
Characterization of the particles and morphology of coating
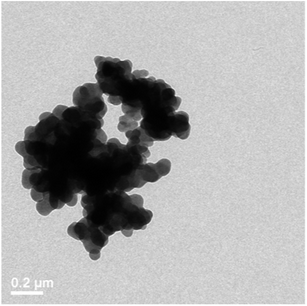
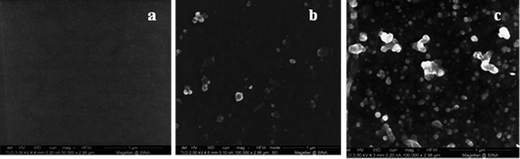
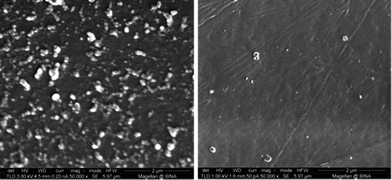
In order to prove that sonochemical irradiation leads to the formation of antibiotic NPs and to obtain the best deposition of tetracycline NPs on Parylene C coated surfaces, various reaction conditions were tested. First, an aqueous solution of tetracycline (0.5 mg ml−1) was ultrasonically irradiated in the presence of Parylene C-coated glass slides. The irradiated solution itself was examined by TEM (Fig. 1) and the Parylene C surface, embedded with the NPs or without, was investigated using HRSEM (Fig. 2a and b). NPs in the 50–70 nm size range (Fig. 1) and microparticles (data not shown) were observed in the TEM measurements. The HRSEM characterization of the surface showed the presence of a small amount of NPs (45–70 nm) and small agglomerates deposited in a non-homogeneous manner on the Parylene C layer. No evidence for the existence of macroparticles was found on the surface of the Parylene C (Fig. 2b). It is worth pointing out that the sonochemical irradiation of the control solution (distilled water without tetracycline) did not result in any visible particles in the sonicated solution (data not shown) and on the surface of Parylene C (Fig. 2a).In order to reduce the water solubility of the obtained tetracycline NPs and in an attempt to improve the synthesis/yield of NPs, the organic solvent dodecane was added to the reaction vessel. The HRSEM results showed that this change in reaction conditions led to a significant improvement in the amount of tetracycline NPs deposited on the surfaces (Fig. 2c). The sonication of water and dodecane without the addition of tetracycline did not lead to the production of any particles on the surface of the Parylene C glass slides (Fig. 3a).
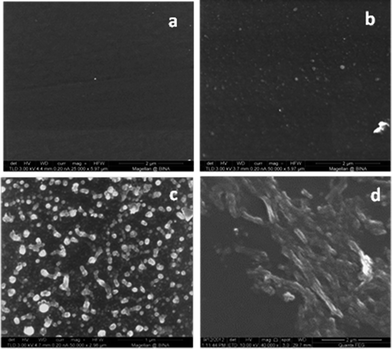
We also examined how changing the tetracycline concentration in the aqueous solution affects the surface deposition (Fig. 3). We utilized three different concentrations: high: 0.75–3 mg ml−1, medium: 0.5–0.75 mg ml−1, and low: 0.01–0.5 mg ml−1. Our results revealed that the best deposition was obtained in the medium concentration range, i.e. 0.5–0.75 mg ml−1 (Fig. 3c). Using the low concentration of antibiotics led to a smaller number of particles found on the Parylene C surface (Fig. 3b), while increasing the tetracycline concentration to 0.75–3 mg ml−1 resulted in the deposition of a non-continuous film composed of large particles (Fig. 3d). Based on these results we continued to work at the medium concentration for the rest of the study.
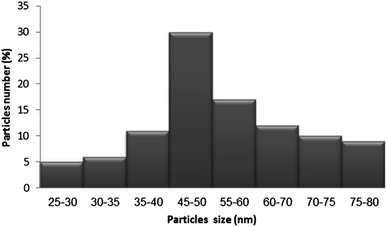
The size distribution of the particles deposited on the Parylene C layer (using the starting concentration 0.66 mg ml−1) was evaluated using the “Scion image” program (Fig. 4). The histogram was calculated based on the HRSEM picture shown in Fig. 3c. The histogram is built on measuring the sizes of 100 particles. The results indicate that approximately 70% of the particles were in the range of 40–70 nm.
Finally we aimed at testing the drug amount on the surface of Parylene C, and this was performed by dissolving the deposited tetracycline in double distilled water. The coated slides (1 cm2) were immersed in water for one week to ensure complete solubility of the drug. The concentration of Tetracycline was found to be 108 ± 15 ng cm−2 (as determined by HPLC, see the Method section).
Mechanism of the sonochemical deposition
The sonochemical mechanism by which the NPs were deposited on the substrates has been discussed previously and is related to the creation of microjets and shock waves that are generated as the acoustic bubble collapses.30 Recently, the formation of water soluble NPs of ionic salts and their subsequent deposition on different surfaces in a one-step sonochemical reaction were described.25 The mechanism of the tetracycline NP deposition process is supposed to be similar to that of the water-soluble inorganic salts, already described. When ultrasonic irradiation is applied to the aqueous solution of an antibiotic drug, its molecules are adsorbed on the surface or near the formed acoustic bubbles.31 When cavity collapse occurs, the adsorbed molecules are exposed to extreme localized conditions of temperature and pressure and, as a result, the individual molecules are coming closer to each other and van-der Waals interactions help to create the antibiotic nanoparticle. The explanation for the greater yield of NPs achieved by adding the organic solvent (i.e. dodecane) to the reaction vessel is most likely due to a decrease in the solubility of the tetracycline NPs in water which leads to an increase in the efficiency of the process. In addition, the existence of cavitation in the liquid near the solid surface and not only in the liquid bulk also increases the nucleation rate.32 The high-speed jets of the liquid formed after the collapse of the bubble throw the generated NPs at high speeds to the surfaces. When the tetracycline NPs impinge on the surface, they are embedded into the polymer because of the local melting of the Parylene C layer. It is worth noting that at the same time, the Parylene film was not damaged and its transparency following the NP deposition did not change drastically (2–3%). The morphology of the Parylene-glass layer before and after the deposition of tetracycline by the ultrasound-assisted method was checked by HRSEM (Fig. 2 and 3), and no changes were observed in the texture of the Parylene substrate within the limits of the resolution of the instrument.Leaching studies
To determine the stability of the NPs deposited on the Parylene C substrate we conducted leaching experiments, by placing the coated slides in double distilled water at 37 °C under constant stirring. The leaching was carried out for 7 days. The content of tetracycline in the solution was determined by HPLC (Fig. 5). The average release was 22–26 ng ml−1 of tetracycline per day measured during the first 72 hours. After 72 hours the amount of released drug started to decrease and there was almost no change in the tetracycline concentration after 5–6 days. The total amount of the tetracycline released from the surface was around 100 ng cm−2. If we assume that approximately 100 ng cm−2 is the total amount of tetracycline embedded in Parylene, than after 24 h only about 20% of the amount is released due to the solubility of the antibiotic in water. This is not surprising since no chemical bonds are formed between the tetracycline NPs and the Parylene surface. The tetracycline undergoes physical adsorption due to the melting of the surface, so the gradual release of the drug to the solvent is expected. In addition, the DLS and TEM measurements revealed that no tetracycline NPs leached out from the surface (data not shown). It is also important to note that the low levels of soluble tetracycline detected in the solution are below the minimal inhibitory concentration values of this antibiotic thus the antimicrobial activity is most likely due to the interaction with the nanoparticles found on the surface.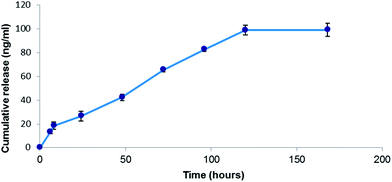 | ||
| Fig. 5 The release profile of tetracycline NPs from the Parylene C surface. The content of the antibiotic in the solution was determined by HPLC. | ||
To further corroborate the leaching study results the morphology of the slides following leaching was evaluated by HRSEM (Fig. 6). As clearly visible in the obtained pictures, a very few nanoparticles are visible on the Parylene C surface after 7 days of leaching further indicating that the antibiotic nanoparticles dissolved over time.
Antibacterial activity of tetracycline NP-coated Parylene C surfaces
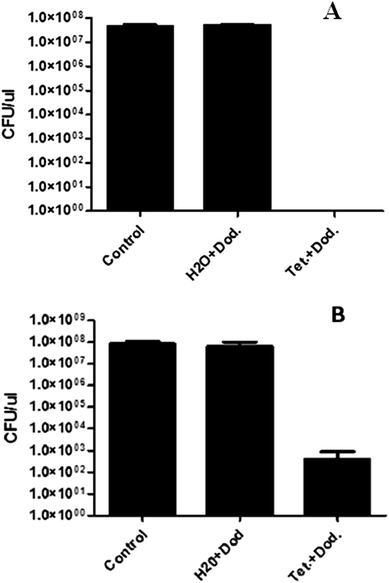
The antibacterial properties of tetracycline NP coated surfaces were tested against E. coli and S. aureus, two common bacterial pathogens. As shown in Fig. 7A, tetracycline NP coated Parylene samples that were incubated for 24 h with E. coli managed to kill all the tested bacteria as opposed to uncoated Parylene C coupons or to ones that were sonicated with dodecane alone, suggesting that the potent antimicrobial activity derives from the tetracycline NPs, thus providing the Parylene surface with antibacterial activity. As shown in Fig. 7B, S. aureus bacteria were significantly attenuated (i.e. a decay of almost 6 orders of magnitude) though not completely, implying that they are less susceptible to tetracycline NPs than E. coli.These results show that sonochemistry is a very simple method to deposit antimicrobial agents on surfaces and functionalize the surface with antimicrobial activity. We speculate that the NPs interact with the bacteria and this brings a high-payload of antibiotics to the cell surface. However, it is also possible that the NPs dissolve into the solution which allows Tetracyclin molecules to enter the cell and inhibit growth.
Experimental
Materials
All of the chemical reagents were purchased from Sigma Aldrich, and were of analytical chemical purity and used without further purification. The Parylene C coated microscopic glasses were received from COMELEC SA (Switzerland) and their preparation has been described previously.33 The thickness of the Parylene C layer was around 4.5 μm. Each glass slide was cut into 15 × 15 mm so it could be inserted into the reaction vessel.Deposition procedure
Typically, tetracycline was dissolved in double distilled water to obtain a concentration range of 0.03–3 mg ml−1. The three different concentrations were utilized: high: 0.75–3 mg ml−1, medium: 0.5–0.75 mg ml−1, and low: 0.01–0.5 mg ml−1. At low concentrations the experiments were performed at 0.01 mg ml−1, 0.05 mg ml−1, 0.1 mg ml−1, 0.2 mg ml−1, 0.3 mg ml−1, 0.4 mg ml−1 and 0.5 mg ml−1. At medium concentrations the experiments were performed at 0.5 mg ml−1, 0.55 mg ml−1, 0.6 mg ml−1, 0.65 mg ml−1, 0.7 mg ml−1 and 0.75 mg ml−1. At high concentrations the 0.8 mg ml−1, 1 mg ml−1, 1.5 mg ml−1, 2.5 mg ml−1 and 3 mg ml−1 were tested. Each experiment was conducted 3 times in replicates.To improve the solubility of tetracycline in water, the antibiotic solution was slightly heated to 40–50 °C for 15 min. Two sets of experiments were performed. In the first set, 40 ml of tetracycline solution were poured into the 50 ml beaker and this solution was ultrasonically irradiated in the presence of 2–4 Parylene C-coated glasses. In the second set of experiments, dodecane (20 ml) was added to the reaction vessel while the rest of the conditions were kept as above. All reactions were performed by immersing the high intensity Ti-horn operated with a booster (20 kHz, 750 W at 22% efficiency, Sonics & Materials VCX600 Sonofier) into the reaction vessel. The ultrasonic intensity used in these experiments is 45 W cm−2. The reaction time varied from 1 to 10 minutes. All the experiments were repeated three times.
Characterization
The morphologies and structures of the tetracycline NPs were characterized with a Transmission Electron Microscope (TEM) Model JEM-1200EX, working at an acceleration voltage of 120 keV. Samples for TEM analyses were prepared by placing a drop of the solution immediately after the end of the sonochemical reaction on a copper grid, and then the samples were left to dry in the air. The overall morphology of Parylene C layers with embedded NPs was investigated by high-resolution scanning electron microscopy (HR-SEM, JSM, 7000 F). The samples were coated with carbon before the HRSEM measurements.Leaching studies
Leaching studies were performed by placing the coated glass surfaces in 1 ml of double distilled water at 36–37 °C for different periods of time (from 6 hours to 7 days) under continuous stirring. A sample of 400 microliters was removed at different time intervals and this volume was replaced by doubly distilled water. The concentration of tetracycline that leached out of the surface into the surrounding solution was measured using an HPLC (La-Chrom Elite® system from VWR Hitachi, FL Detector L-2485) as described previously.34 The column was a Phenomenex Luna, C18, 150 × 4.6 mm, 5 μm. The mobile phase consisted of methanol and a 0.1 M sodium acetate buffer containing 25 mM EDTA and 35 mM CaCI2 dihydrate (pH 6.5) (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60, v/v) at a flow rate of 1.0 ml min−1. The injection volume was 50 μl and the run time was 15 min. The fluorescence detector had an excitation wavelength of 390 nm and an emission wavelength of 512 nm.
60, v/v) at a flow rate of 1.0 ml min−1. The injection volume was 50 μl and the run time was 15 min. The fluorescence detector had an excitation wavelength of 390 nm and an emission wavelength of 512 nm.
Antibacterial test
The antimicrobial activity of tetracycline NP-coated Parylene surfaces was evaluated and compared to uncoated surfaces using Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 29213 as the experimental models. Both E. coli and S. aureus bacteria were grown overnight in Nutrient Broth (NB, Sigma) media under shaking (250 rpm) at 37 °C. On the following day, the overnight cultures were each diluted in a fresh NB medium to obtain stock solutions with a working concentration of 105 colony forming units (CFU) per ml. 100 μl from each of the stock solutions (corresponding to 104 CFU) were removed and laid on the Parylene surfaces. The surfaces were then incubated at 37 °C for 24 hours. On the following day, in order to determine the CFU in each treatment, serial dilutions were carried out and the cells were spotted onto NB agar plates. The NB plates were incubated at 37 °C for 20 hours. Cell growth was monitored and determined by viable cell count.Conclusion
Tetracycline NPs were deposited on the surface of Parylene C coated glass slides by ultrasound irradiation. The size of the observed NPs and their distribution on the substrate are both affected by the concentration of the starting solution, the sonication time and the addition of the organic solvent as one of the reaction components. The best deposition is obtained at the medium concentration range of 0.5–0.75 mg ml−1. The particle-size distribution study showed that around 70% of the particles were in the range of 40–70 nm. The physical and chemical analyses have shown that tetracycline NPs, 50 nm in size, are homogenously dispersed onto the surface of Parylene C without damage to the structure of the polymer. The leaching experiments detected the release of 100 ng ml−1 of tetracycline during the 7 days. The mechanism of tetracycline NP formation and adhesion to the surface is based on the local melting of the substrate due to the high rate and temperature of tetracycline NPs thrown at the solid surface by sonochemical microjets. The excellent bactericidal effect of tetracycline NP-coated Parylene C surfaces against two common bacterial pathogens, E. coli and S. aureus was also demonstrated. These results highlight the potential use of tetracycline NP-coated surfaces for various biomedical applications.Acknowledgements
This research was carried out as part of the activities of the PARYLENES Consortium Contract no. NMP-2009-1.1-1. PARYLENES is an IP Project of the 7th EC Program.References
- J. J. Licari, Coating Materials for Electronic Applications: Polymers, Processes, Reliability, Testing, ed. W. Andrew, Noyes Publication, Norwich, NY, 2003, p. 159 Search PubMed.
- N. Stark, Medical Plastics and Biomaterials Magazine, 1996, 3, 30 Search PubMed.
- C. P. Unsworth, E. Delivopoulosb, T. Gillespiec and A. F. Murrayd, Biomaterials, 2011, 32, 2566 CrossRef CAS PubMed.
- C. P. Unsworth, H. Holloway, E. Delivopoulos, A. F. Murray, M. C. Simpson, M. E. Dickinson and E. S. Graham, Biomaterials, 2011, 32, 6541 CrossRef CAS PubMed.
- J. B. Fortin and T. M. Lu, in Chemical Vapor Deposition Polymerization: The Growth and Properties of Parylene Thin Films, Kluwer Academic Publishers, New York, 2004, p. 4 Search PubMed.
- Specialty Coatings Systems, SCS Parylene Properties, Specialty Coatings Systems, Indianapolis, 2008, p. 5 Search PubMed.
- http://www.paryleneinc.com//pdf/Parylene-for-Medical.pdf .
- D. G. Maki and P. A. Tambyah, Emerging Infect. Dis., 2001, 7, 342 CrossRef CAS.
- Z. Krishnasami, D. Carlton, L. Bimbo, M. E. Taylor, D. F. Balkovetz, J. Barker and M. Allon, Kidney Int., 2002, 61, 1136 CrossRef PubMed.
- J. M. Schierholz, H. Steinhauser, A. F. Rump, R. Berkels and G. Pulverer, Biomaterials, 1997, 18, 839 CrossRef CAS.
- I. Raad, R. Darouiche, R. Hachem, M. Sacilowski and G. P. Bodey, Antimicrob. Agents Chemother., 1995, 39, 2397 CrossRef CAS.
- I. Raad, R. Darouiche, R. Hachem, M. Mansouri and G. P. Bodey, J. Infect. Dis., 1996, 173, 418 CrossRef CAS PubMed.
- R. O. Darouiche, O. Z. Hampel, T. B. Boone and I. I. Raad, Int. J. Antimicrob. Agents, 1997, 8, 243 CrossRef CAS.
- Z. Huang, X. Jiang, D. Guo and N. Gu, J. Nanosci. Nanotechnol., 2011, 11, 9395 CrossRef CAS PubMed.
- I. Perelshtein, G. Applerot, N. Perkas, E. Wehrschetz-Sigl, A. Hasmann, G. M. Guebitz and A. Gedanken, ACS Appl. Mater. Interfaces, 2009, 2, 361 Search PubMed.
- A. Simon-Deckers, S. Loo, M. Mayne-L'Hermite, N. Herlin-Boime, N. Menguy, C. Reynaud, B. Gouget and M. Carriere, Environ. Sci. Technol., 2009, 43, 8423 CrossRef CAS PubMed.
- S. Makhluf, R. Dror, Y. Nitzan, Y. Abramovich, R. Jelinek and A. Gedanken, Adv. Funct. Mater., 2005, 15, 1708 CrossRef CAS.
- A. S. Brady-Estvez, S. Kang and M. Elimelech, Small, 2008, 4, 481 CrossRef PubMed.
- D. Y. Lyon, L. K. Adams, J. C. Falkner and P. J. Alvarez, Environ. Sci. Technol., 2006, 40, 4360 CrossRef CAS.
- J. Lellouche, A. Friedman, J.-P. Lellouche, A. Gedanken and E. Banin, Nanomed.: Nanotechnol., Biol. Med., 2012, 8, 702 CrossRef CAS PubMed.
- W. Ye, J. H. Xin, P. Li, K. L. D. Lee and T. L. Kwong, J. Appl. Polym. Sci., 2006, 102, 1787 CrossRef CAS.
- L. Huang, T. Dai, Y. Xuan, G. P. Tegos and M. R. Hamblin, Antimicrob. Agents Chemother., 2011, 55, 3432 CrossRef CAS PubMed.
- H. Tang, P. Zhang, T. L. Kieft, S. J. Ryan, S. M. Baker, W. P. Wiesmann and S. Rogelj, Acta Biomater., 2010, 6, 2562 CrossRef CAS PubMed.
- T. Gordon, B. Perlshtein, O. Houbara, I. Felner, E. Banin and S. Margel, Colloids Surf., A, 2010, 374, 1 CrossRef PubMed.
- S. Balakrishnan and R. Anand, Acta Biomater., 2013, 5, 6511 Search PubMed.
- A. Gedanken, Ultrason. Sonochem., 2004, 11, 47 CrossRef CAS PubMed.
- J. H. Bang and K. S. Suslick, Adv. Mater., 2010, 22, 1039 CrossRef CAS PubMed.
- S. Kiel, O. Grinberg, N. Perkas, J. Charmet, H. Kepner and A. Gedanken, Beilstein J. Nanotechnol., 2012, 3, 267 CrossRef PubMed.
- D. Meridor and A. Gedanken, Ultrason. Sonochem., 2013, 20, 425 CrossRef CAS PubMed.
- A. Gedanken, Ultrason. Sonochem., 2007, 14, 418 CrossRef CAS PubMed.
- D. Sunartio, K. Yasui, T. Tuziuti, T. Kozuka, Y. Iida, M. Ashokkumar and F. Grieser, ChemPhysChem, 2007, 8, 2331 CrossRef CAS PubMed.
- A. Mersmann, Crystallization Technology Handbook, Marcel Dekker, Inc., 2nd edn, 2001, p. 58 Search PubMed.
- W. F. Gorham, J. Polym. Sci., 1966, 4, 3027 CrossRef CAS.
- D. S. Vienneau and C. G. Kindherg, J. Pharm. Biomed. Anal., 1997, 16, 111 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |