Amorphous monodispersed hard carbon micro-spherules derived from biomass as a high performance negative electrode material for sodium-ion batteries†
Yunming
Li‡
,
Shuyin
Xu‡
,
Xiaoyan
Wu
,
Juezhi
Yu
,
Yuesheng
Wang
,
Yong-Sheng
Hu
*,
Hong
Li
,
Liquan
Chen
and
Xuejie
Huang
Key Laboratory for Renewable Energy, Beijing Key Laboratory for New Energy Materials and Devices, National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing 100190, China. E-mail: yshu@aphy.iphy.ac.cn
First published on 23rd October 2014
Abstract
Sodium-ion batteries (SIBs) are expected to be a promising commercial alternative to lithium-ion batteries (LIBs) for large-scale and low-cost electrical energy storage applications in the near future. Despite this, the absence of a suitable negative electrode material hinders their development. In this contribution, we synthesized monodispersed hard carbon spherules (HCS) from an abundant biomass of sucrose, and investigated the influence of the carbonization temperature on the microstructure and electrochemical performance. The initial coulombic efficiency of the HCS was increased to 83% by coating its surface with soft carbon through the pyrolysis of toluene. Interestingly, the plateau capacity at the low potential region increased with increasing carbonization temperature. The HCS carbonized at 1600 °C showed the highest plateau capacity (220 mA h g−1) and excellent cycling performance with a capacity retention of 93% after 100 cycles. When coupled with an air-stable P2-Na2/3Ni1/3Mn2/3O2 positive electrode, the full cell exhibited a high initial coulombic efficiency of 76%, a mean operating voltage of 3.5 V and excellent cycling performance. The theoretical energy density of this system was estimated to be 200 W h kg−1. These promising properties are believed to be close to the level required for practical applications.
Introduction
Lithium-based batteries have been developed successfully as a power source for portable electronics owing to their outstanding energy and power density since their first commercialization in 1991.1 They are also a promising alternative energy source for electric vehicles.2,3 On the other hand, the limited lithium sources may hinder their applications in large-scale energy storage for smart grid and solar/wind energy.4–6Sodium is the second alkali metal next to lithium and has similar physical and chemical properties.7 In addition, sodium is distributed in the ocean at almost unlimited levels.8 Therefore, the low cost of sodium salts would pave the way for large-scale applications. Sodium-ion batteries have attracted considerable attention as a kind of energy storage technology in recent years.9 Thus far, there have been some positive electrode materials for SIBs,10–14 but the major obstacle in realizing SIBs is the absence of suitable negative electrodes. Probably due to the unique features of large and highly ionized Na+ ions or thermodynamic issues, graphite materials, which are widely used as a negative electrode material for LIBs, shows poor sodium storage performance in SIBs.15,16 The proposed negative electrode materials mainly include amorphous carbon materials, alloys, Ti-based oxides and organic compounds.17 Na alloy negative electrode materials have high reversible capacities. Nanocomposites of Sb/C18 and Sn/C19 exhibit a high capacity of 610 mA h g−1 and 295 mA h g−1, respectively. Recently, a composite electrode of SnSb/C was reported to deliver a large reversible capacity of 540 mA h g−1 with 80% capacity retention over 50 cycles.20 The main problem of alloys lies in the structural destruction caused by the large volume expansion in the reaction with Na.21 Ti-based oxides mainly include Na2Ti3O7,22 Li4Ti5O12 (ref. 23 and 24) and Na0.66[Li0.22Ti0.78]O2,25 but the storage capacity is not high. Organic compounds, such as disodium terephthalate (Na2C8H4O4), can deliver a large reversible capacity with good capacity retention in SIBs.26,27 Nevertheless, the low initial coulombic efficiency and insufficient electronic conductivity limit the applications of organic compounds. Recently, expanded graphite was fabricated as an anode material for SIBs, but the first coulombic efficiency reported was only 49.53%.28 Hard carbon is likely to be the most promising alternative to graphite electrodes29–31 because of its relatively high capacity, natural abundance and renewability. Reversible Na insertion/extraction in hard-carbon was first reported by Stevens and Dahn in 2000.32 The hard-carbon electrodes delivered ca. 300 mA h g−1 of reversible capacity, but the cycle performance was insufficient. Komaba et al. improved the cycle performance of a hard carbon electrode significantly by optimizing the electrolyte, and fabricated hard-carbon/NaNi0.5Mn0.5O2 cells.33 Unfortunately, the hard carbon electrode only showed a low capacity of 250 mA h g−1, and hard-carbon/NaNi0.5Mn0.5O2 full cells only exhibited a capacity of 200 mA h g−1 with moderate cycling performance. Until now, numerous carbon materials with various morphologies have been proposed for SIBs,34–37 but their initial coulombic efficiency and cyclic stability cannot satisfy the requirements of practical applications.
Sucrose is an abundant and renewable raw material for producing hard carbon from biomass. Here, we prepared hard carbon spherules with a perfect spherical morphology and smooth surface through the hydrothermal process and the pyrolysis of sucrose. The initial coulombic efficiency could be improved further by coating soft carbon on its surface through the pyrolysis of toluene. Interestingly, a higher carbonization temperature leads to a higher sodium storage capacity. In particular, the plateau capacity at the low potential region increases significantly, which is desirable for achieving a higher energy density in a full cell.
Experimental
Materials synthesis
HCS was prepared by the pyrolysis of sucrose in two steps, namely dewatering at low temperatures and carbonization at high temperatures. In the first step, a 1 M sugar solution was placed in a stainless steel autoclave with a fill rate of 90%, and treated hydrothermally at 190 °C to obtain a black powder according to a previous study.38–40 In the second step, the obtained black powder was carbonized for 2 hours in a tube furnace under an Ar and toluene flow, and all samples were then cooled naturally to room temperature. The carbonization temperatures were 1000 °C, 1300 °C and 1600 °C.Materials characterization
The structure was characterized using an X'Pert Pro MPD X-ray diffractometer (XRD) (Philips, Netherlands) with Cu-Kα radiation (1.5405 Å) and Raman spectroscopy (JY-HR 800). The morphologies of the samples were investigated by scanning electron microscopy (SEM) (Hitachi S-4800). Nitrogen adsorption and desorption isotherms were determined by nitrogen physisorption on a Micromeritics ASAP 2020 analyzer.Electrochemical measurements
All the electrochemical tests were conducted using coin cells (CR2032). The working electrode was prepared by spreading the mixed slurry of active material and PVDF binder in N-methyl-2-pyrrolidone (NMP) solvent at a weight ratio of 9.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 onto Cu foil, followed by drying at 100 °C in vacuum for 10 hours. The loading mass of hard carbon electrode was controlled to between 1.5–2.5 mg cm−2. The electrolyte was a solution of 1 M NaClO4 in ethylene (EC) and diethyl carbonate (DEC) (1
0.5 onto Cu foil, followed by drying at 100 °C in vacuum for 10 hours. The loading mass of hard carbon electrode was controlled to between 1.5–2.5 mg cm−2. The electrolyte was a solution of 1 M NaClO4 in ethylene (EC) and diethyl carbonate (DEC) (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in volume). Sodium foil was used as the counter electrode and glass fiber was used as the separator. All the operations were performed in an argon-filled glove box. The discharge and charge tests were carried out using a Land BT2000 battery test system (Wuhan, China) over a voltage range of 0–3 V at various C-rates at room temperature. A sodium-ion full cell was constructed using HCS1600 as the negative electrode and Na2/3Ni1/3Mn2/3O2 as the positive electrode in a CR2032 coin-type cell. The synthesis method of the Na2/3Ni1/3Mn2/3O2 material is reported elsewhere.43 The weight ratio of the two electrodes (negative/positive) was 1
1 in volume). Sodium foil was used as the counter electrode and glass fiber was used as the separator. All the operations were performed in an argon-filled glove box. The discharge and charge tests were carried out using a Land BT2000 battery test system (Wuhan, China) over a voltage range of 0–3 V at various C-rates at room temperature. A sodium-ion full cell was constructed using HCS1600 as the negative electrode and Na2/3Ni1/3Mn2/3O2 as the positive electrode in a CR2032 coin-type cell. The synthesis method of the Na2/3Ni1/3Mn2/3O2 material is reported elsewhere.43 The weight ratio of the two electrodes (negative/positive) was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4. The full cells were charged and discharged over the voltage range, 0–3.9 V, at 0.1 C and 0.2 C current rates.
4. The full cells were charged and discharged over the voltage range, 0–3.9 V, at 0.1 C and 0.2 C current rates.
Results and discussion
Structural and morphological characterizations
Fig. 1a shows a typical SEM image of HCS. HCS has an almost spherical shape with a uniform particle size and smooth surfaces. The diameter of the obtained carbon spherules was about 1 μm. The size of HCS is determined mainly by the dewatering procedure at low temperatures rather than the following carbonization process, thus HCS carbonized at different temperatures has the same size.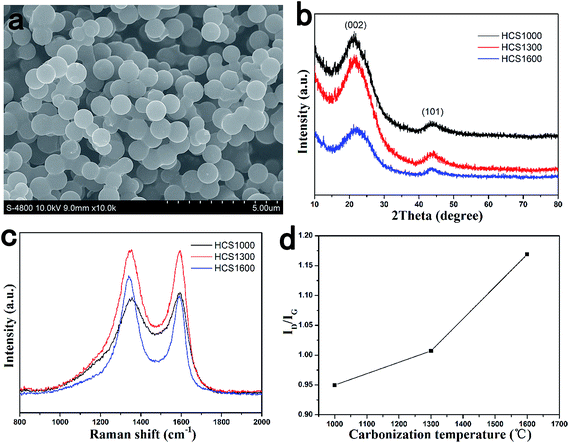 | ||
| Fig. 1 Structure of HCS carbonized at different temperatures. (a) SEM image; (b) XRD patterns; (c) Raman spectra; (d) relation curve of ID/IG and carbonization temperature. | ||
The structure of HCS with different carbonization temperatures was investigated by XRD and Raman spectroscopy, and the results are shown in Fig. 1b and c. All XRD patterns showed the broad peaks of (002) and (101), which confirm their amorphous structure. The Raman spectra exhibited two broad bands of the D-band peak at ∼1343 cm−1 (the defect-induced band) and G-band peak at ∼1589 cm−1 (the crystalline graphite band).41 As shown in Fig. 1d, the intensity of the ID/IG ratios were 0.950, 1.007 and 1.169 for HCS carbonized at 1000 °C (HCS1000), 1300 °C (HCS1300) and 1600 °C (HCS1600), respectively, showing an increase with increasing carbonization temperature. This indicates that the disorder degree increases with increasing carbonization temperature,42 which is in contrast to the general expectations. The mechanism is still unclear at the moment.
Brunauer–Emmett–Teller (BET) measurements were used to examine the specific surface area of HCS1000 before and after coating with soft carbon, as shown in Fig. S1.† The results showed a much larger specific surface area for the HCS1000 sample without coating, while the coated HCS1000 sample only had a small surface area. This suggests that coating soft carbon can effectively reduce the exposed surface area.
Electrochemical performances of HCS
The electrochemical performance of HCS was first evaluated in half cells with Na as the counter electrode, as shown in Fig. 2. Fig. 2a shows the 1st, 2nd and 10th discharge–charge profiles of the HCS1000 electrode at 0.1 C (30 mA g−1). The initial discharge and charge capacities were 335 mA h g−1 and 279 mA h g−1, respectively. The profile can be divided into two regions with a slope above 0.2 V and a plateau close to 0 V. The observed charge capacity of the plateau region was 150 mA h g−1, which is approximately 53% of the total capacity. The initial coulombic efficiency was 83%, while the initial coulombic efficiency of the HCS1000 sample without coating soft carbon was only 54% (Fig. S2†). The high initial coulombic efficiency can be attributed to the soft carbon coated on the surface of HCS from the pyrolysis of toluene, which significantly reduces the contact area with the electrolyte and the formation of solid electrolyte interphase (SEI). In addition, the polarization between discharge and charge was also reduced, the plateau capacity can be utilized completely above 0 V. Therefore, the coated HCS1000 sample has higher capacity than the sample without the coating. After the tenth cycle, the coulombic efficiency increased to 99%, which suggests that the coating is an effective way of improving the coulombic efficiency and reducing the polarization of hard carbon.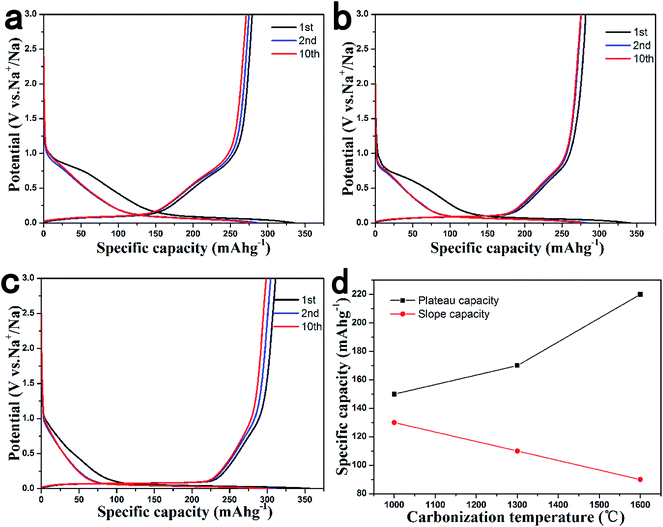 | ||
| Fig. 2 Discharge and charge curves for the 1st, 2nd and 10th cycles of (a) HCS1000, (b) HCS1300 and (c) HCS1600; (d) relation curves of the plateau and slope capacity and carbonization temperature. | ||
Fig. 2b and c show the 1st, 2nd and 10th discharge–charge profiles of the HCS1300 and HCS1600 electrodes, respectively. Compared to the HCS1000 electrode, the carbonization temperature mainly affected the percentage of the plateau region among the charge capacity, whereas it had little influence on coulombic efficiency. The observed plateau capacity of HCS1300 was 170 mA h g−1, which is approximately 60% of the total capacity. For HCS1600, the plateau capacity increased to 220 mA h g−1, accounting to 70% of the whole capacity. As shown in Fig. 2d, the percentage of the plateau capacity increases with increasing carbonization temperature, whereas the sloping capacity decreases. As the plateau at the low potential region may correspond to Na adsorption–desorption in the nanopores of carbon particles,33,44–46 it indicates that the quantity of the nanopores increases with increasing carbonization temperature.
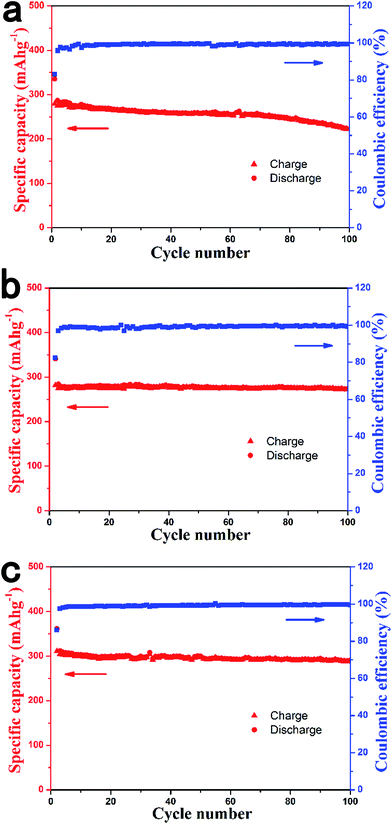
Fig. 3 shows the cycling performance of the HCS1000, HCS1300 and HCS1600 electrodes at 0.1 C for 100 cycles. After 100 cycles, the HCS1000 sample retained a capacity of 222 mA h g−1, corresponding to a capacity retention of 80%. In contrast, the HCS1300 and HCS1600 electrodes exhibited much lower capacity decay; about 272 mA h g−1 and 290 mA h g−1 remained after 100 cycles, respectively, corresponding to capacity retention of 97% and 93%.
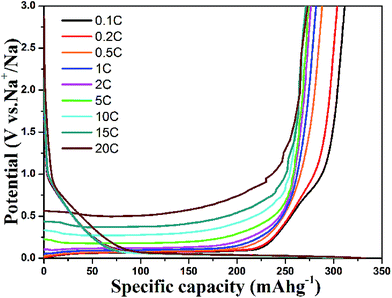
The rate performance of the HCS electrodes is shown in Fig. 4. The cells were discharged and charged at various rates from 0.1 C to 2 C for five cycles. The HCS1000 sample delivered a specific capacity of 201 mA h g−1 at 0.5 C, but HCS1300 and HCS1600 only delivered a specific capacity of 110 mA h g−1 and 98 mA h g−1 at 0.5 C. When the current rate was reduced, the capacity could return to the previous value, suggesting the good stability of HCS under a wide current range. Overall, HCS shows poor rate capability, as reported for other hard carbon materials, and the rate performance deteriorates with increasing carbonization temperature (Fig. S3†). Interestingly, the total capacity decay at a high rate is mainly due to the rapid plateau capacity decay, as shown in Fig. 4d. This suggests that Na adsorption–desorption in the nanopores at the low potential plateau region has poorer kinetic properties than Na adsorption–desorption between the graphene layers at the slope region. An asymmetric current rate test was performed to determine if the insertion step or extraction step limits the rate performance. The HCS1600 electrode was discharged (Na insertion) at a constant current rate of 0.1 C and charged (Na extraction) at different rates. The results are shown in Fig. 5. Even at a very high charging rate of 20 C (3 min charge), a reversible charge capacity of 270 mA h g−1 was achieved. This suggests that Na extraction from hard carbon is quite fast and Na insertion into hard carbon is the limiting step.
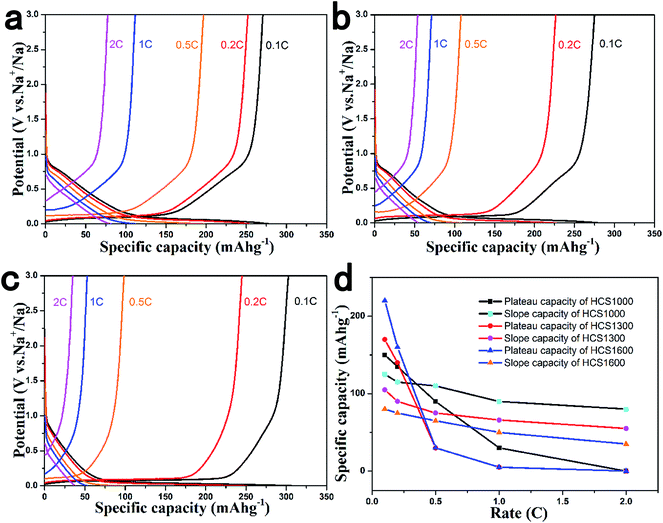 | ||
| Fig. 4 Rate performance of (a) HCS1000, (b) HCS1300 and (c) HCS1600 samples; (d) retained capacity from plateau and slope contributions under different current rates. | ||
Full cell performance of sodium-ion batteries
To test the actual performance of HCS in full cells, we selected the air-stable P2-Na2/3Ni1/3Mn2/3O2 material as the positive electrode to assemble a full cell. The P2-Na2/3Ni1/3Mn2/3O2 cathode material is of particular interest because of the high operation voltage with the Ni2+/Ni3+/Ni4+ redox couple and the stability in cycling.43 Unlike other layered oxides, this material is quite stable in air and even in water, as shown in Fig. S4.† Herein, we tested the electrochemical performance of the Na2/3Ni1/3Mn2/3O2 electrode over the voltage range of 2.5–3.9 V to avoid the P2-O2 phase transition. Stable cycle performance was achieved with a reversible capacity of 80 mA h g−1 in a Na/Na2/3Ni1/3Mn2/3O2 half cell (Fig. S5†).We fabricated coin-type full sodium-ion batteries with the Na2/3Ni1/3Mn2/3O2 positive electrode and HCS1600 negative electrode. The cell was cycled at 0.1 C for the first 20 cycles, then at 0.2 C for the subsequent cycles. Fig. 6a shows the charge and discharge curves of a Na2/3Ni1/3Mn2/3O2/HCS1600 cell at room temperature. The Na2/3Ni1/3Mn2/3O2/HCS1600 full cell exhibits a high average operating voltage of 3.5 V, a high capacity more than 300 mA h g−1 (based on negative electrode) and a high initial coulombic efficiency of 76%. The theoretical energy density of this system was estimated to be 200 W h kg−1 owing to the low average storage voltage of HCS1600. The Na2/3Ni1/3Mn2/3O2/HCS1600 full cell also exhibited superior cycle performance, as shown in Fig. 6b with a capacity retention of 73% after 150 cycles. To the best of our knowledge, this is the first report to demonstrate a sodium-ion full cell with a relatively high operating voltage and long cycling performance. These promising properties are close to the level required for practical applications.
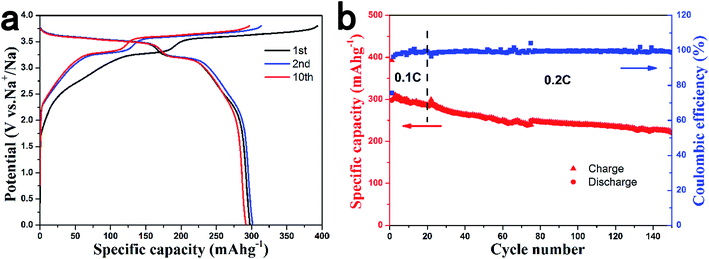 | ||
| Fig. 6 Performance of a coin-type HCS1600/Na2/3Ni1/3Mn2/3O2 full cell. (a) Charge and discharge curves for the 1st, 2nd and 10th cycles and (b) cycling performance. | ||
Conclusions
In summary, we fabricated monodispersed hard carbon spherules coated with soft carbon, and found that the coating can improve the initial coulombic efficiency significantly from 54% to 83%. We then investigated the influence of the carbonization temperature on the microstructure and electrochemistry performance of HCS in detail. Interestingly, the plateau capacity at the low potential region increases with increasing carbonization temperature. When the carbonization temperature was increased from 1000 °C to 1600 °C, the plateau capacities increased from 150 mA h g−1 to 220 mA h g−1 at 0.1 C. HCS also displays excellent cycling performance with capacity retention of 93% of HCS1600 after 100 cycles. The rate performance of HCS is limited by the Na insertion process. When the HCS1600 electrode was discharged at a low current rate and the charging rate was up to 20 C, a reversible capacity of 270 mA h g−1 was achieved. Finally, the application of HCS was also demonstrated in full cells with an air-stable P2-Na2/3Ni1/3Mn2/3O2 positive electrode. The full cell exhibited a high average operating voltage of 3.5 V, a high capacity more than 300 mA h g−1, a high initial coulombic efficiency of 76%, and superior cycling performance. This outstanding performance highlights the potential of this system for practical applications.Acknowledgements
This work was supported by funding from the NSFC (51222210, 11234013), “973” Projects (2010CB833102, 2012CB932900), and One Hundred Talent Project of the Chinese Academy of Sciences.Notes and references
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed
.
- L. M. Suo, Y.-S. Hu, H. Li, M. Armand and L. Q. Chen, Nat. Commun., 2013, 4, 1481 CrossRef PubMed
.
- H. L. Pan, Y.-S. Hu and L. Q. Chen, Energy Environ. Sci., 2013, 6, 2338–2360 CAS
.
- J. M. Tarascon, Nat. Chem., 2010, 2, 510 CrossRef CAS PubMed
.
- S. W. Kim, D. H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS
.
- M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater., 2013, 23, 947–958 CrossRef CAS
.
- V. Palomares, M. C. Cabanas, E. C. Martínez, M. H. Hanb and T. Rojo, Energy Environ. Sci., 2012, 5, 5884–5901 CAS
.
- M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater., 2013, 23, 947–958 CrossRef CAS
.
- N. Yabuuchi, M. Kajiyama, J. Iwatate, H. Nishikawa, S. Hitomi, R. Okuyama, R. Usui, Y. Yamada and S. Komaba, Nat. Mater., 2012, 11, 512–517 CrossRef CAS PubMed
.
- M. Sathiya, K. Hemalatha, K. Ramesha, J. M. Tarascon and A. S. Prakash, Chem. Mater., 2012, 24, 1846–1853 CrossRef CAS
.
- Z. L. Jian, L. Zhao, H. L. Pan, Y.-S. Hu, H. Li, W. Chen and L. Q. Chen, Electrochem. Commun., 2012, 14, 86–89 CrossRef CAS PubMed
.
- H. Wang, S. Yuan, D. Ma, X. Huang, F. Meng and X. Zhang, Adv. Energy Mater., 2014, 4, 1301651 Search PubMed
.
- H. Yoshida, N. Yabuuchi, K. Kubota, I. Ikeuchi, A. Garsuch, M. S. Dobrick and S. Komaba, Chem. Commun., 2014, 50, 3677–3680 RSC
.
- S. Y. Xu, X. Y. Wu, Y. M. Li, Y.-S. Hu and L. Q. Chen, Chin. Phys. B, 2014, 23, 118202 Search PubMed
.
- P. Ge and M. Fouletier, Solid State Ionics, 1988, 28, 1172–1175 CrossRef
.
- M. M. Doeff, Y. P. Ma, S. J. Visco and L. C. Dejonghe, J. Electrochem. Soc., 1993, 140, L169–L170 CrossRef CAS PubMed
.
- J. Zhao, L. W. Zhao, K. Chihara, S. Okada, J. Yamaki, S. Matsumoto, S. Kuze and K. Nakane, J. Power Sources, 2013, 244, 752–757 CrossRef CAS PubMed
.
- J. F. Qian, Y. Chen, L. Wu, Y. L. Cao, X. P. Ai and H. X. Yang, Chem. Commun., 2012, 48, 7070–7072 RSC
.
- Y. H. Xu, Y. J. Zhu, Y. H. Liu and C. S. Wang, Adv. Energy Mater., 2013, 3, 128–133 CrossRef CAS
.
- L. F. Xiao, Y. L. Cao, J. Xiao, W. Wang, L. Kovarik, Z. M. Nie and J. Liu, Chem. Commun., 2012, 48, 3321–3323 RSC
.
- V. L. Chevrier and G. Ceder, J. Electrochem. Soc., 2011, 15, A1011–A1014 CrossRef PubMed
.
- H. L. Pan, X. Lu, X. Q. Yu, Y.-S. Hu, H. Li, X. Q. Yang and L. Q. Chen, Adv. Energy Mater., 2013, 3, 1186–1194 CrossRef CAS
.
- L. Zhao, H. L. Pan, Y.-S. Hu, H. Li and L. Q. Chen, Chin. Phys. B, 2012, 21, 028201 CrossRef
.
- Y. Sun, L. Zhao, H. L. Pan, X. Lu, L. Gu, Y.-S. Hu, H. Li, M. Armand, Y. Ikuhara, L. Q. Chen and X. J. Huang, Nat. Commun., 2013, 4, 1870 CrossRef PubMed
.
- Y. S. Wang, X. Q. Yu, S. Y. Xu, J. M. Bai, R. J. Xiao, Y.-S. Hu, H. Li, X. Q. Yang, L. Q. Chen and X. J. Huang, Nat. Commun., 2013, 4, 2365 Search PubMed
.
- L. Zhao, J. M. Zhao, Y.-S. Hu, H. Li, Z. B. Zhou, M. Armand and L. Q. Chen, Adv. Energy Mater., 2012, 2, 962–965 CrossRef CAS
.
- Y. Park, D. S. Shin, S. H. Woo, N. S. Choi, K. H. Shin, S. M. Oh, K. T. Lee and S. Y. Hong, Adv. Mater., 2012, 24, 3562–3567 CrossRef CAS PubMed
.
- Y. Wen, K. He, Y. J. Zhu, F. D. Han, Y. H. Xu, I. Matsuda, Y. Ishii, J. Cumings and C. S. Wang, Nat. Commun., 2014, 5, 4033 Search PubMed
.
- P. Thomas and D. Billaud, Electrochim. Acta, 2002, 47, 3303–3307 CrossRef CAS
.
- A. Ponrouch, E. Marchante, M. Courty, J. M. Tarascon and M. RosaPalacín, Energy Environ. Sci., 2012, 5, 8572–8583 CAS
.
- J. Zhao, L. Zhao, K. Chihara, S. Okada, J. Yamaki, S. Matsumoto, S. Kuze and K. Nakane, J. Power Sources, 2013, 244, 752–757 CrossRef CAS PubMed
.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 1271–1273 CrossRef CAS PubMed
.
- S. Komaba, W. Murata, T. Ishikawa, N. Yabuuchi, T. Ozeki, T. Nakayama, A. Ogata, K. Gotoh and K. Fujiwara, Adv. Funct. Mater., 2011, 21, 3859–3867 CrossRef CAS
.
- S. Wenzel, T. Hara, J. Janek and P. Adelhelm, Energy Environ. Sci., 2011, 4, 3342–3345 CAS
.
- K. Tang, L. J. Fu, R. J. White, L. H. Yu, M. M. Titirici, M. Antonietti and J. Maier, Adv. Energy Mater., 2012, 2, 873–877 CrossRef CAS
.
- Y. L. Cao, L. F. Xiao, M. L. Sushko, W. Wang, B. Schwenzer, J. Xiao, Z. M. Nie, L. V. Saraf, Z. G. Yang and J. Liu, Nano Lett., 2012, 12, 3783–3787 CrossRef CAS PubMed
.
- H. G. Wang, Z. Wu, F. I. Meng, D. l. Ma, X. l. Huang, L. M. Wang and X. B. Zhang, ChemSusChem, 2013, 6, 56–60 CrossRef PubMed
.
- Q. Wang, H. Li, L. Q. Chen and X. J. Huang, Carbon, 2001, 39, 2211–2214 CrossRef CAS
.
- M. M. Titirici, M. Antonietti and N. Baccile, Green Chem., 2008, 10, 1204–1212 RSC
.
- B. Hu, K. Wang, L. Wu, S. H. Yu, M. Antonietti and M. M. Titirici, Adv. Mater., 2010, 22, 813–828 CrossRef CAS PubMed
.
- L. Qie, W. M. Chen, H. H. Xu, X. Q. Xiong, Y. Jiang, F. Zou, X. L. Hu, Y. Xin, Z. L. Zhang and Y. H. Huang, Energy Environ. Sci., 2013, 6, 2497–2505 Search PubMed
.
- M. M. Doeff, Y. Hu, F. M. Larnon and R. Kostecki, Electrochem. Solid-State Lett., 2003, 6, A207–A209 CrossRef CAS PubMed
.
- Z. H. Lu and J. R. Dahn, J. Electrochem. Soc., 2001, 148, A1225–A1229 CrossRef CAS PubMed
.
- A. Mabuchi, K. Tokumitsu, H. Fujimoto and T. Kasuh, J. Electrochem. Soc., 1995, 142, 1041–1046 CrossRef CAS PubMed
.
- D. A. Stevens and J. R. Dahn, J. Electrochem. Soc., 2000, 147, 4428–4431 CrossRef CAS PubMed
.
- S. M. Mamun, M. Herstedt, K. Oikawa, T. Gustafsson, T. Otomo, M. Furusaka, T. Kamiyama, H. Sakaebe and K. Edstrom, Appl. Phys. A: Mater. Sci. Process., 2002, 74, S1028–S1030 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ta05451b |
| ‡ Y. M. Li and S. Y. Xu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |