One-pot fabrication of multifunctional superparamagnetic attapulgite/Fe3O4/polyaniline nanocomposites served as an adsorbent and catalyst support†
Bin
Mu
and
Aiqin
Wang
*
Center of Eco-materials and Green Chemistry, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, Lanzhou 730000, China. E-mail: aqwang@licp.cas.cn; Fax: +86 931 8277088; Tel: +86 931 4968118
First published on 4th November 2014
Abstract
Multifunctional superparamagnetic attapulgite/Fe3O4/polyaniline (APT/Fe3O4/PANI) nanocomposites have been fabricated by a one-pot process using Fe(III) as the oxidant for aniline and the precursor of Fe3O4 in the presence of attapulgite. The introduction of attapulgite can effectively induce the uniform encapsulation of polyaniline and Fe3O4 nanoparticles on the surface of attapulgite to form the Anthurium andraeanum-like structure, preventing the formation of free aggregates of polyaniline and Fe3O4 nanoparticles. The morphologies and the magnetic properties of the as-prepared nanocomposites can be facilely controlled by adjusting the molar ratio of aniline to Fe(III). The APT/Fe3O4/PANI nanocomposites can be used as an adsorbent for the removal of dyes and the enrichment of Au(III) from the solution. In addition, the adsorbed Au(III) could be reduced to elemental gold to obtain APT/Fe3O4/PANI supported Au nanocomposites due to the good redox activities of PANI, which exhibited excellent catalytic activity toward the catalytic reduction of 4-nitrophenol in the presence of NaBH4.
Introduction
Superparamagnetic iron oxide nanoparticles such as magnetite (Fe3O4) and its oxidized form maghemite (γ-Fe2O3) have received increasing attention because of their inherent superparamagnetic properties, which make them desirable for medical imaging, magnetic field assisted transport, and separation.1,2 In order to develop new applications of magnetic materials, the functionalization of magnetic particles by constructing the organic/inorganic hybridization has become the research focus of various fields.3–5 Among many conducting polymers, polyaniline (PANI) has attracted much attention due to the advantages of easy synthesis, low cost, good environmental stability, and large amounts of amine and imine functional groups.6,7 Therefore, magnetic PANI composites composed of Fe3O4 and PANI have been extensively designed and synthesized for the removal of heavy metal ions and dye molecules,8,9 solid phase extraction,10 absorption of electromagnetic waves and microwaves,11–13 electromagnetic shielding,14etc.At present, two synthetic strategies have been employed to prepare the hybrids of PANI coupled with Fe3O4 particles. The first strategy involves the in situ formation of Fe3O4 nanoparticles in the presence of PANI upon chemical reactions, such as hydrolysis of Fe(II) and Fe(III) ions,15 the other method is based on the polymerization of aniline in the presence of magnetic Fe3O4 particles.16,17 By contrast, the latter can realize the effective control of the morphology, magnetic properties and the relevant application of the resultant hybrid materials. For example, Li et al. synthesized the flexible Fe3O4 nanocrystal-embedded PANI hybrids with a well-defined cluster-like morphology through macromolecule-induced self-assembly.18 Likhar et al. reported a high surface Fe3O4@mesoporous PANI core–shell nanocomposite with blackberry nanostructures as a magnetically separable catalyst for the cross-coupling of aryl chlorides and phenols.19 Electromagnetic functionalized Fe3O4/PANI composites with egg-like aggregates were prepared by two-step oxidative polymerization using Fe3+ and ammonium persulfate as the oxidants in each step.20 In addition, PANI shell cross-linked magnetic nanoparticles were also prepared by in situ polymerization of aniline on the surface of carboxyl PEGylated Fe3O4 nanoparticles for heat activated killing of cancer cells.21 However, these methods require extensive labour and time. In addition, it is difficult to prevent magnetic particles and PANI from the formation of aggregates in the preparation of composites, which is a decisive factor for their relevant application. Therefore, it remains a great challenge to explore a simple and mild approach to generate well-defined nanostructured composites composed of Fe3O4 and PANI.
Attapulgite (APT), a kind of one-dimensional hydrous layer-chain magnesium silicate mineral with rob-like morphology, has been widely used to fabricate novel organic/inorganic composites due to its large specific surface area, adjustable surface chemistry, non-toxicity, low cost and abundance in nature.22–24 Our group recently has prepared APT oriented carbon/PANI hybrid nanocomposites by in situ chemical oxidative polymerization of aniline after hydrothermal carbonization of glucose on the surface of APT.25 The results indicated that the introduction of APT can effectively induce the heterogeneous deposition of carbonaceous species on the surface of APT and prevent PANI from the free self-aggregation. Therefore, the superparamagnetic attapulgite/Fe3O4/polyaniline (APT/Fe3O4/PANI) nanocomposites were synthesized by a facile one-pot process combining the chemical oxidative polymerization and coprecipitation method in the presence of APT. It is worth noting that the inexpensive Fe(III) can have both of these roles, in the oxidative polymerization of aniline and providing the single iron source of Fe3O4 by the redox reaction between aniline and Fe(III). The morphologies and the magnetic properties of the as-prepared nanocomposites can facilely be controlled by adjusting the molar ratio of aniline to Fe(III). Due to the synergistic effect of the components, the superparamagnetic hierarchical APT/Fe3O4/PANI nanocomposite may be exploited for multiple purposes as illustrated in Scheme 1. It could be used as adsorbents for the removal of dyes and for the enrichment of Au(III) from the solution, and the adsorbed Au(III) was then in situ reduced to elemental gold to obtain a nanostructured catalyst taking into account the good redox activities of PANI toward precious metal ions. In addition, the catalytic activity toward the catalytic reduction of 4-nitrophenol was also investigated. The results revealed that the APT/Fe3O4/PANI nanocomposite might be a promising multifunctional nanomaterial not only for the adsorption and separation of dye molecules and precious metal ions, but also for the magnetically driven recovery of catalysts.
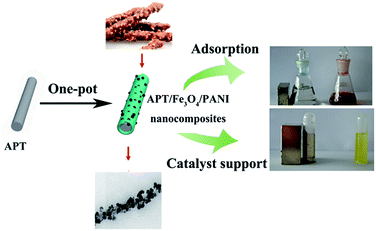 | ||
| Scheme 1 Superparamagnetic APT/Fe3O4/PANI nanocomposites with an Anthurium andraeanum-like or peanut chocolate bar structure used as an adsorbent and catalyst support. | ||
Experimental
Materials
ATP supplied from Jiuchuan Clay Technology Co., Jiangsu, China, is composed of CaO (1.29%), Al2O3 (10.47%), Na2O (1.52%), MgO (20.41%), SiO2 (64.31%), K2O (0.13%) and Fe2O3 (0.87%) determined by X-ray fluorescence. Chloroauric acid (HAuCl4·4H2O) was purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). Methylene blue (MB) and Congo red (CR) were purchased from Alfa Aesar Co. Ltd. FeCl3·6H2O, aniline, 4-nitrophenol (4-NP), and other reagents were all of analytical reagent grade from Tianjin Chemical Co., China, and used without further purification. Ultrapure water (18.25 MΩ cm) was used throughout.Preparation of Fe3O4/PANI/APT nanocomposites
In a typical procedure, 3.552 g of FeCl3·6H2O was dissolved in 60 mL of water, and 0.6 g of ATP treated with hydrochloric acid were ultrasonically dispersed into above solution for 1 h. Then 5 mL of ethanol containing 1.2 mL of aniline was added dropwise into the above yellow suspension, and the reaction was stirred for 8 h at room temperature. As the solution was heated to 70 °C, 10 mL of NH3·H2O (14 wt%) was added dropwise to the above mixture under vigorous stirring for 1 h to generate the magnetic nanoparticles, and then the temperature was increased to 85 °C to vapor the residual NH3. The obtained black products were washed with 0.1 M HCl, deionized water and ethanol in sequence after being cooled to room temperature, and then dried in a vacuum at 60 °C for 24 h. To investigate the effect of molar ratio of aniline to Fe(III) on the morphologies and the properties of the nanocomposites, the Fe3O4/PANI/APT nanocomposites with different contents of PANI and magnetic nanoparticles were also prepared; the conditions of the sample preparation are presented in Table 1.| Samples | FeCl3·6H2O/g | APT/g | Aniline/mL | NH3·H2O/mL | n aniline/nFe(III) |
|---|---|---|---|---|---|
| APT/Fe3O4/PANI1 | 3.552 | 0.6 | 0.6 | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| APT/Fe3O4/PANI2 | 3.552 | 0.6 | 1.2 | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| APT/Fe3O4/PANI3 | 3.552 | 0.6 | 2.4 | 5 | 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| APT/Fe3O4/PANI4 | 3.552 | 0.6 | 3.6 | 5 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| APT/PANI | 3.552 | 0.6 | 1.2 | 0 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| Fe3O4/PANI | 3.552 | 0 | 1.2 | 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
As a control experiment, the products without APT or magnetic nanoparticles were also prepared using the same procedure without the addition of APT or NH3·H2O, the conditions of the sample preparation are also shown in Table 1.
Adsorption of dyes
The adsorption of APT/Fe3O4/PANI2 nanocomposites toward different dyes of MB and CR was performed in a series of conical flasks containing 25 mg of APT/Fe3O4/PANI2 nanocomposites and 25 mL of aqueous solution containing 100 mg L−1 of MB or CR at different pH values, respectively. The mixtures were shaken in a thermostatic shaker at 25 °C for 60 min, and then APT/Fe3O4/PANI2 nanocomposites were separated by a magnetic separation technique. The dye concentration in the solution was analyzed using an ultraviolet (UV) spectrophotometer by monitoring the adsorption behavior at a wavelength of maximum absorbance (664 nm and 498 nm for MB and CR, respectively). The adsorption rate toward dye molecules of APT/Fe3O4/PANI2 nanocomposites was calculated from the dye concentrations in solutions before and after adsorption.Enrichment of Au(III) and catalytic reduction of 4-NP
First, 0.05 g of APT/Fe3O4/PANI2 nanocomposites was added to 100 mL of HAuCl4 aqueous solution (10 ppm) at room temperature for 6 h. The adsorbed Au(III) was then in situ reduced to elemental gold to obtain APT/Fe3O4/PANI2 supported Au (APT/Fe3O4/PANI2/Au) nanocomposites due to the good redox activities of the PANI.26 Finally, the products were washed with water and then dried in a vacuum at 60 °C for 24 h.To study the catalytic activity, the as-prepared APT/Fe3O4/PANI2/Au nanocomposites were used as the catalyst for the catalytic reduction of 4-NP using NaBH4 as a reductant. In the typical procedure, 1 mL of 4-NP solution (0.1 mM) was mixed with 2 mL of fresh NaBH4 solution (0.1 M), and then 2.0 mg of APT/Fe3O4/PANI2/Au nanocomposites were added. A digital camera and UV-vis spectrometer were employed to monitor the progress of the reduction reaction. After the reaction was completed, the catalysts were magnetically separated from the mixture and washed with water for recycling in the next cycle, and this process was repeated eight times.
Characterization
A Bruker IFS 66 v/s IR spectrometer (Bruker, Karlsruhe, Germany) was used for the Fourier transform infrared spectroscopy analysis in the range of 400–4000 cm−1 with a resolution of 4 cm−1. The morphology of the samples was characterized with a JEM-1200 EX/S transmission electron microscope (TEM) (JEOL, Tokyo, Japan). The samples were dispersed in ethanol and vibrated for 10 min and then deposited on a copper grid covered with a perforated carbon film. The surface morphology of APT/PANI and APT/Fe3O4/PANI2 nanocomposites was observed using a scanning electron microscope (SEM, JSM-6701F, JEOL, Ltd). The samples were fixed on the copper stubs and then coated with gold for the SEM observation. The elemental compositions of APT/Fe3O4/PANI2/Au nanocomposites were determined using a Kevex energy dispersive spectrometer (EDS). Thermogravimetric analysis (TGA) was performed on a Perkin Elmer STA6000 thermogravimetric analyzer under an oxygen atmosphere at a heating rate of 10 °C min. The X-ray diffraction (XRD) analysis was conducted with an X-ray powder diffractometer with a Cu anode (PAN analytical Co. X'pert PRO), operating at 40 kV and 30 mA. The magnetic properties of samples were detected by using a vibrating sample magnetometer (Lakeshore 7304). UV-vis absorption spectra were recorded at room temperature by using a Lambda 35 UV-vis spectrometer (Perkin-Elmer, U.S.A.).Results and discussion
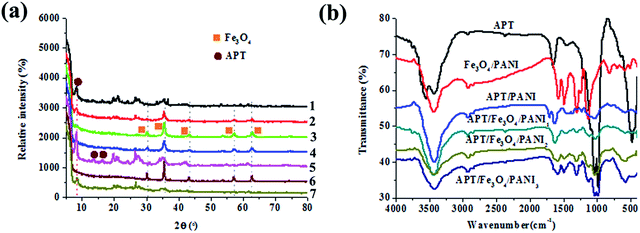
The XRD patterns of APT, Fe3O4/PANI, APT/PANI, and the as-prepared APT/Fe3O4/PANI nanocomposites are shown in Fig. 1. For the XRD pattern of APT, the characteristic peaks of APT at 2θ = 8.4°, 13.7° and 16.3° can be found, which are assigned to the reflection of (110), (200) and (040) crystallographic planes.27 The presence of diffraction peaks at 2θ = 26.6° indicates the existence of a small amount of quartz in the samples.28 As depicted in the XRD patterns of APT/Fe3O4/PANI nanocomposites and Fe3O4/PANI nanocomposites, five characteristic diffraction peaks of Fe3O4 located at 30.2°, 35.8°, 43.3°, 57.2°, and 62.6° are assigned to the planes of (220), (311), (400), (511) and (440), respectively.29 It indicates a cubic phase, which matches well with the standard XRD pattern of Fe3O4 (PDF 74-0748). In particular, it is worth noting that the relative intensity of these diffraction peaks first increases while the addition of aniline increases from 0.6 mL to 1.2 mL, and then decreases with a further increase in the addition of aniline. This phenomenon is attributed to the fact that Fe(III) as an oxidant is reduced to Fe(II) with the oxidative polymerization of aniline,30–32 and the contents of Fe(III) and the generated Fe(II) are optimal for the formation of Fe3O4 as the molar ratio of aniline to Fe(III) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The amount of Fe(III) decreases with the increase in the addition of aniline, and the content of Fe3O4 in the as-prepared composites also decreases, thus the relative intensity of the characteristic diffraction peaks of Fe3O4 decreases. In the case of the samples containing PANI, the crystallization peaks of PANI are not observed, which might be ascribed to the amorphous phase of the as-prepared PANI. In addition, the XRD patterns of the samples containing APT demonstrate a (110) characteristic diffraction peak at 8.4° of APT, revealing that the structure of APT is maintained very well in the preparation process. However, the relative intensity of this diffraction peak decreases to some extent due to the introduction of PANI and magnetic nanoparticles.
1. The amount of Fe(III) decreases with the increase in the addition of aniline, and the content of Fe3O4 in the as-prepared composites also decreases, thus the relative intensity of the characteristic diffraction peaks of Fe3O4 decreases. In the case of the samples containing PANI, the crystallization peaks of PANI are not observed, which might be ascribed to the amorphous phase of the as-prepared PANI. In addition, the XRD patterns of the samples containing APT demonstrate a (110) characteristic diffraction peak at 8.4° of APT, revealing that the structure of APT is maintained very well in the preparation process. However, the relative intensity of this diffraction peak decreases to some extent due to the introduction of PANI and magnetic nanoparticles.
To further confirm the existence of PANI and Fe3O4, FTIR spectroscopy is employed to investigate the molecular structure of the obtained products. In the case of the FTIR spectrum of APT (Fig. 1b), the absorption bands at 3561 cm−1 and 3430 cm−1 are assigned to the O–H stretching vibrations of structural water and other water molecules (zeolitic water and superficially adsorbed water in APT).33 The absorption bands located at 1036 cm−1 and 467 cm−1 are attributed to the stretching vibration of Si–O–Si and δSi–O.34 As presented in the spectra of Fe3O4/PANI, APT/PANI, and APT/Fe3O4/PANI nanocomposites, the characteristic adsorption bands of PANI can be observed at 1570 cm−1, 1483 cm−1, 1309 cm−1, 1135 cm−1, 820 cm−1, which correspond to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (quinoid, Q) stretching vibration, C
C (quinoid, Q) stretching vibration, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibration mode of the benzenoid ring, the aromatic C–N stretching vibration, and N
C stretching vibration mode of the benzenoid ring, the aromatic C–N stretching vibration, and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Q
Q![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration, respectively.35 Furthermore, the peak at 820 cm−1 is owing to the aromatic C–H bending in the plane and out of the plane for the 1,4-disubstituted aromatic ring. These absorption bands indicate the presence of PANI, which are also consistent with those of leucoemeraldine.36 The characteristic absorption band of Fe–O at 578 cm−1 can be observed, indicating the presence of Fe3O4 in the corresponding samples.
N stretching vibration, respectively.35 Furthermore, the peak at 820 cm−1 is owing to the aromatic C–H bending in the plane and out of the plane for the 1,4-disubstituted aromatic ring. These absorption bands indicate the presence of PANI, which are also consistent with those of leucoemeraldine.36 The characteristic absorption band of Fe–O at 578 cm−1 can be observed, indicating the presence of Fe3O4 in the corresponding samples.
The surface electrical characteristic of APT and APT/Fe3O4/PANI2 suspensions at different pH values (1 mg mL−1) is also investigated by the zeta potential, as depicted in Fig. 2. It can be seen that the isoelectric point (pH of zero point charge, pHpzc) of APT and APT/Fe3O4/PANI2 nanocomposites is about 2.5 and 9.2, respectively. At pH < pHpzc, APT and APT/Fe3O4/PANI2 nanocomposites possess a net positive surface charge, and then the surface charge transforms to a negative charge at pH > pHpzc. This reversal of charge properties may be attributed to the different degree of ionization of functional groups at different pH values. Furthermore, the increase in surface charge in the measured pH range and the shifting of the isoelectric point toward a higher pH value of APT/Fe3O4/PANI2 nanocomposites are mainly ascribed to the protonation of amino groups of the introduced PANI. This phenomenon also suggests that PANI has been successfully bonded on the surface of APT.
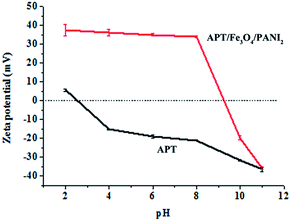 | ||
| Fig. 2 Variation in the zeta potential of APT and APT/Fe3O4/PANI2 suspensions at different pH values (1 mg mL−1). | ||
Fig. 3 illustrates the typical TEM images of APT, Fe3O4/PANI, APT/PANI, and APT/Fe3O4/PANI nanocomposites. It can be found that APT is rod-like in shape with a length of 0.4–2 μm and a diameter of 10–40 nm (Fig. 3a). Without the addition of APT, the aggregates composed of PANI and magnetic nanoparticles can be found, as shown in Fig. 3b. Due to the permanent negative charges and the exchangeable cations, APT can adsorb the protonated aniline, which can be polymerized to form a uniform polymer shell on the surface of APT.37Fig. 3c clearly exhibits the well-defined core–shell structure of APT/PANI composites without magnetic nanoparticles, and the thickness of the PANI shell layer is about 20 nm. It can also be confirmed by the EDS spectrum and the N elemental mapping of APT/PANI nanocomposites, as shown in Fig. S1.† Compared with the smooth surface of APT (Fig. S2†), APT/PANI composites show a coarse surface (Fig. 3d). In this process, Fe(III) was employed as the oxidant for aniline, which was reduced to be Fe(II) with the polymerization of aniline, and the excess of Fe(III) could be transformed into Fe3O4 nanoparticles with the generated Fe(II) via a co-precipitation method under alkaline conditions. As shown in the TEM image of APT/Fe3O4/PANI1, it can be seen that small amounts of Fe3O4 nanoparticles are anchored on the surface of APT (Fig. 3e). The loading content of magnetic nanoparticles anchored on the surface of APT greatly increases while the amount of aniline increases to 1.2 mL, as shown in Fig. 3f. This phenomenon is ascribed to the increase in the number of Fe(II) in the system with the oxidative polymerization of aniline. In addition, it can be revealed that the presence of APT can effectively induce the uniform encapsulation of PANI and Fe3O4 nanoparticles on the surface of APT (Fig. 3g), preventing the formation of the free aggregations of PANI and Fe3O4 nanoparticles. This is also consistent with the results of the SEM image of APT/Fe3O4/PANI2; it can been seen that the magnetic Fe3O4 is well anchored on the surface of rods, forming a peanut chocolate bar or Anthurium andraeanum-like structure (Fig. 3h). However, the loading contents of Fe3O4 nanoparticles of APT/Fe3O4/PANI nanocomposites gradually decrease with a further increase in the addition of aniline due to the decrease in the content of Fe(III), as shown in Fig. S3.† This is also confirmed by the results of XRD, indicating that the content of Fe3O4 can be well controlled by adjusting the molar ratio of aniline to Fe(III).
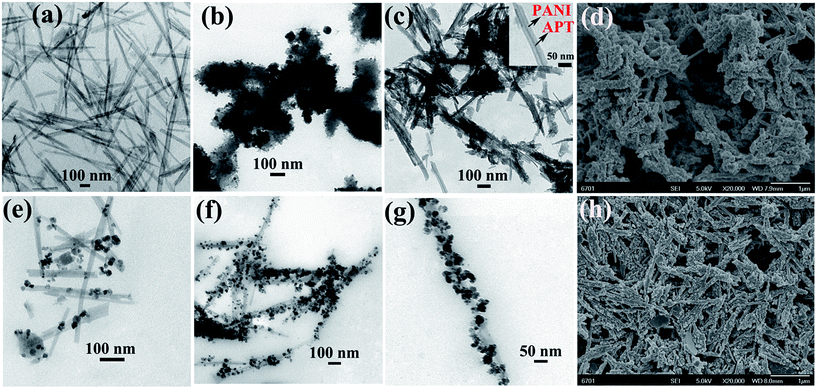 | ||
| Fig. 3 TEM images of (a) APT, (b) Fe3O4/PANI, (c) APT/PANI, (e) APT/Fe3O4/PANI1, (f) and (g) APT/Fe3O4/PANI2 nanocomposites. SEM images of (d) APT/PANI and (h) APT/Fe3O4/PANI2. | ||
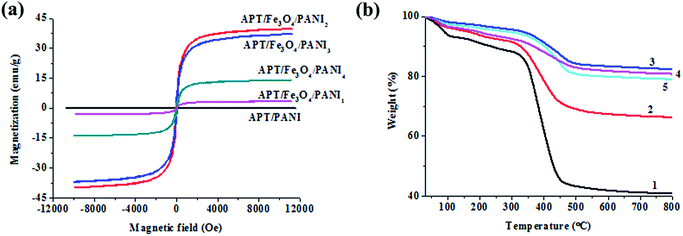
In order to assess the potential of APT/Fe3O4/PANI nanocomposites in magnetic separation, the magnetic properties of APT/PANI and APT/Fe3O4/PANI nanocomposites are characterized by VSM at 300 K, and the magnetic hysteresis loops are illustrated in Fig. 4a. It can be found that the saturation magnetization of APT/PANI is almost zero due to no magnetism. All of APT/Fe3O4/PANI samples exhibit superparamagnetic behavior without remanence and coercivity.38 The saturation magnetization of APT/Fe3O4/PANI1, APT/Fe3O4/PANI2, APT/Fe3O4/PANI3, and APT/Fe3O4/PANI4 is 3.23 emu g−1, 39.79 emu g−1, 37.05 emu g−1 and 13.95 emu g−1, respectively. It can be observed that the saturation magnetization of APT/Fe3O4/PANI nanocomposites increases with the increase in the molar ratio of aniline to Fe(III) in the range of 0–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then decreases with the increase in the addition of aniline. The initial increase in the saturation magnetization is ascribed to the increase in the content of Fe3O4 nanoparticles, and the increment of magnetic content is greater than that of PANI. With the increase in the molar ratio of aniline to Fe(III) (>1
1, and then decreases with the increase in the addition of aniline. The initial increase in the saturation magnetization is ascribed to the increase in the content of Fe3O4 nanoparticles, and the increment of magnetic content is greater than that of PANI. With the increase in the molar ratio of aniline to Fe(III) (>1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the decrease in the magnetization of APT/Fe3O4/PANI3 and APT/Fe3O4/PANI4 can be attributed to the decrease in the effective mass of magnetite due to the increase in the content of PANI with the increasing addition of aniline. Furthermore, the decrease in the magnetization of samples compared with bulk Fe3O4 (92 emu g−1) can be attributed to the combined effect of nano-sized Fe3O4 particles (large surface to volume ratio), robust coating of nonmagnetic PANI on their surface (quenching of magnetic moment by electron exchange between coating and surface atoms), and the introduction of the inorganic clay mineral of APT.39 However, the magnetism of the APT/Fe3O4/PANI nanocomposite is still strong enough to be easily separated using a magnet.
1), the decrease in the magnetization of APT/Fe3O4/PANI3 and APT/Fe3O4/PANI4 can be attributed to the decrease in the effective mass of magnetite due to the increase in the content of PANI with the increasing addition of aniline. Furthermore, the decrease in the magnetization of samples compared with bulk Fe3O4 (92 emu g−1) can be attributed to the combined effect of nano-sized Fe3O4 particles (large surface to volume ratio), robust coating of nonmagnetic PANI on their surface (quenching of magnetic moment by electron exchange between coating and surface atoms), and the introduction of the inorganic clay mineral of APT.39 However, the magnetism of the APT/Fe3O4/PANI nanocomposite is still strong enough to be easily separated using a magnet.
The TGA curve of ATP (Fig. S4†) presents a weight loss of about 15% in the temperature range of 30 °C to 280 °C, which can be assigned to the removal of the physically adsorbed water and zeolitic water located in the channels.40 In the following temperature range, the weight loss refers to the removal of the coordinated water and the structural water released from the clay framework.41Fig. 4b shows the TGA curves of APT/PANI and APT/Fe3O4/PANI nanocomposites in an oxygen atmosphere. For all the samples, the weight losses observed at 100 °C and 280 °C are attributed to the moisture entrapped inside the polymer moiety and the removal of zeolitic water of APT, respectively. In the case of APT/PANI nanocomposites, the TGA curve exhibits a major weight loss in the range of 300–500 °C due to the thermal degradation of PANI, and the content of PANI can be calculated to be 45%. After introducing the magnetic nanoparticles, it can be seen that the thermal stability of APT/Fe3O4/PANI nanocomposites is improved compared with that of APT and APT/PANI nanocomposites. It suggests that the components of APT/Fe3O4/PANI nanocomposites form strong interactions, such as electrostatic interactions between APT and PANI, coordination between Fe3O4 and PANI, and hydrogen bonds between hydroxyl groups and amino groups. The major weight loss located at 300–500 °C is attributed to the decomposition of molecular chains of PANI. The degradation of PANI backbone chains refers to the formation of small aromatic fragments, substituted aromatic fragments, the extended aromatic fragments,42 and eventually transforms into CO2 in an oxygen atmosphere. Therefore, the content of PANI of APT/Fe3O4/PANI1, APT/Fe3O4/PANI2, APT/Fe3O4/PANI3, and APT/Fe3O4/PANI4 can be calculated to be 25.0%, 12.7%, 15.3%, and 16.6%, respectively. And the residuals remaining at 800 °C are composed of inorganic silicate and Fe2O3 transformed by Fe3O4 in an oxygen atmosphere. It can be inferred that the content of Fe3O4 nanoparticles of APT/Fe3O4/PANI2 is higher than other APT/Fe3O4/PANI nanocomposites in the same case of the APT content, which is also consistent with the results of XRD and VSM.
Due to the numerous amine and imine functional groups of PANI, the as-prepared magnetic APT/Fe3O4/PANI nanocomposites can be used as an adsorbent to realize their application in the water treatment, such as the removal of dye molecules and the enrichment of precious metal ions, as shown in Fig. 5. Based on above experimental results, APT/Fe3O4/PANI2 nanocomposites were selected as the typical adsorbent to investigate the adsorption properties toward dye molecules from water using two common dyes MB and CR as the model at different pH values (pH = 3, 7, and 11) in this study. As shown in Table 2, the adsorption ratio of APT and APT/Fe3O4/PANI2 nanocomposites toward two dyes exhibits an obvious pH-dependent behavior due to the difference in the surface charge between adsorbent and dye molecules. APT is often applied as an adsorbent to remove cation dyes (such as MB) from water due to the abundant silicon hydroxyls, the negative surface charges and the exchangeable cations of APT.43 Thus the adsorption ratio of APT toward MB is higher than that toward CR due to the different electrostatic interaction, and it can reach 76.3% from the MB solution with a pH value of 11. However, the adsorption ratio of APT/Fe3O4/PANI2 nanocomposites toward CR at a pH of 7 significantly increases to 96.0% compared with APT with the introduction of PANI, which is mainly ascribed to the electrostatic interaction, hydrogen bond and the π–π stacking interaction between PANI and MB. In addition, the adsorption ratio was higher under the neutral medium compared with the alkaline one, which is related to the transformation of the surface charges with the protonation and deprotonation of amine and imine functional groups of PANI in the corresponding pH medium.
 | ||
| Fig. 5 Digital photograph of the solution of (a) 100 mg L−1 of CR and (b) 10 ppm of HAuCl4 before and after addition of APT/Fe3O4/PANI2 nanocomposites. | ||
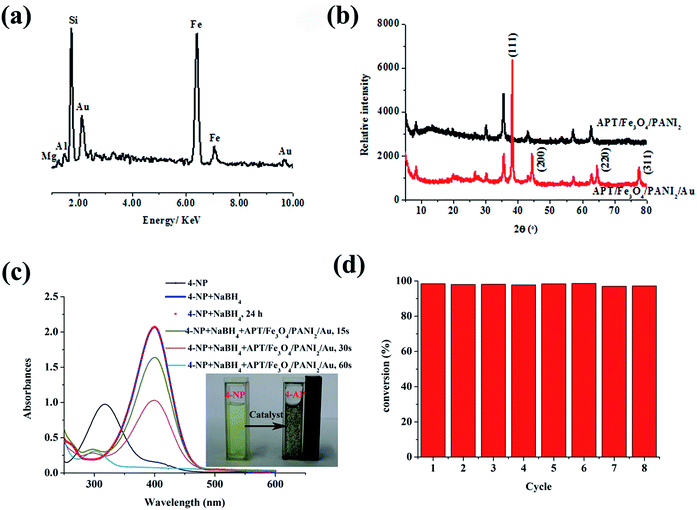
Furthermore, it is well-known that a metal ion with a higher reduction potential than that of a conducting polymer can be directly reduced by the conducting polymer to form metal elements.44 Thus the composites composed of PANI and precious metal nanoparticles have been successfully fabricated via simply mixing PANI and noble metal salt together taking into account the good redox activities of PANI and its derivatives toward precious metal ions.45 As illustrated in Fig. 5b, the color of the HAuCl4 solution fades after adding APT/Fe3O4/PANI2 nanocomposites, indicating that AuCl4 has been adsorbed and reduced to elemental gold to form APT/Fe3O4/PANI2 supported Au (APT/Fe3O4/PANI2/Au) nanocomposites. This also can be confirmed by EDS and XRD patterns of APT/Fe3O4/PANI2/Au nanocomposites, as shown in Fig. 6. The EDS spectrum of APT/Fe3O4/PANI2/Au nanocomposites (Fig. 6a) shows the presence of Si, Fe and Au elements in the nanocomposites with the content of 11.78%, 43.8% and 24.45%, respectively. Besides the characteristic diffraction peaks of APT and Fe3O4, four obvious main peaks can also be observed which are indexed to the (111), (200), (220), and (311) planes of face-centred cubic (fcc) gold from the XRD pattern of APT/Fe3O4/PANI2/Au nanocomposites (Fig. 6b),46 suggesting the presence of elemental gold in APT/Fe3O4/PANI2/Au nanocomposites.
In addition, the catalytic reduction of 4-NP by NaBH4 to 4-aminophenol (4-AP) was chosen as a model reaction to evaluate the catalytic properties of the as-prepared APT/Fe3O4/PANI2/Au nanocomposites as shown in Fig. 6c. The aqueous solution of 4-NP has a maximum absorption at 317 nm, and then immediately red shifted to 400 nm with the addition of fresh NaBH4 solution (Fig. 6c), which can be assigned to the formation of 4-nitrophenolate ions under alkaline conditions. The reduction reaction did not proceed in the absence of APT/Fe3O4/PANI2/Au nanocomposites confirmed by a constant absorption peak at 400 nm after 24 h. However, the absorption at 400 nm quickly decreased while the absorption at 298 nm increased with the introduction of the APT/Fe3O4/PANI2/Au catalyst. The peak at 298 nm can be indexed to the characteristic absorbance peak of 4-AP. It can be revealed that 4-NP is reduced to 4-AP, and the reduction of 4-NP into 4-AP is completed within about 1 min. The complete conversion of 4-NP also can be visualized with the discoloration of the characteristic yellow-green color of 4-nitrophenolate ions (see inset Fig. 6c). The peak assigned to the nitro compound at 400 nm disappears after the catalytic reduction, indicating that the catalytic reduction of 4-NP is completed. The isosbestic point is not observed, which might be attributed to the following factors. On the one hand, the generated H2 gas bubbles can result in a minor degree of scattering.47 On the other hand, the multiple intermediates are feasible, such as 4-nitrosophenol, hydroxylamine, etc.48 In addition, the as-prepared APT/Fe3O4/PANI2/Au catalyst can rapidly gather on the inner wall of a cuvette using a magnet. It suggests that the APT/Fe3O4/PANI2/Au catalyst with good catalytic performance for the reduction of 4-NP can be easily manipulated by an external magnetic field.
It is well-known that the reusability is the main advantage of using a heterogeneous catalyst rather than a homogeneous catalyst. Therefore, the recyclability of the APT/Fe3O4/PANI2/Au catalyst was also investigated by repeating the reduction reaction with the same catalyst eight times (Fig. 6d). The catalysts are recovered by simple magnetic separation after completion of the reduction reaction, and then washed with water for reusing in the next cycle. It can be found that the APT/Fe3O4/PANI2/Au catalyst shows high catalytic activity after eight successive cycles of reactions with a conversion of about 99% within 1 min. This phenomenon might be attributed to the fact that the presence of the PANI shell can effectively protect the generated gold nanoparticles due to the interaction between functional groups of PANI and gold nanoparticles. Furthermore, the as-prepared catalyst with strong magnetism can be recovered efficiently from the reaction system by using external magnetic fields for the following cycles without significant losses.
Conclusions
In summary, we have developed a facile synthetic route for the fabrication of APT/Fe3O4/PANI nanocomposites with an Anthurium andraeanum-like structure. The results demonstrated that the morphologies and magnetic properties of the composites could be well regulated by controlling the molar ratio of aniline to Fe(III). The APT/Fe3O4/PANI nanocomposites can be used as an adsorbent for the removal of dyes and for the enrichment of Au(III) from the solution. The adsorbed Au(III) could be reduced to elemental gold through a simple and green method without the addition of the reductant, and the resulting hybrid heterogeneous catalyst exhibited high catalytic properties and good reusability for the reduction of 4-NP. This facile one-pot method is expected to provide a simple and versatile approach to realize the functionalization of magnetic particles and the multiple applications in relevant fields.Acknowledgements
The authors gratefully acknowledge the support of the “863” Project of the Ministry of Science and Technology, P. R. China (no. 2013AA032003).Notes and references
- C. Sun, K. Du, C. Fang, N. Bhattarai, O. Veiseh, F. Kievit, Z. Stephen, D. Lee, R. G. Ellenbogen, B. Ratner and M. Zhang, ACS Nano, 2010, 4, 2402 CrossRef CAS PubMed.
- L. Zhang, W. F. Dong and H. B. Sun, Nanoscale, 2013, 5, 7664 RSC.
- A. H. Latham and M. E. Williams, Acc. Chem. Res., 2008, 41, 411 CrossRef CAS PubMed.
- V. Sokolova and M. Epple, Angew. Chem., Int. Ed., 2008, 47, 1382 CrossRef CAS PubMed.
- T. Okada, Y. Takeda, N. Watanabe, T. Haeiwa, T. Sakai and S. Mishima, J. Mater. Chem. A, 2014, 2, 5751 CAS.
- H. L. Yu, T. S. Wang, B. Wen, M. M. Lu, Z. Xu, C. L. Zhu, Y. J. Chen, X. Y. Xue, C. W. Sun and M. S. Cao, J. Mater. Chem., 2012, 22, 21679 RSC.
- M. S. Cao, J. Yang, W. L. Song, D. Q. Zhang, B. Wen and H. B. Jin, ACS Appl. Mater. Interfaces, 2012, 4, 6949 CAS.
- X. Y. Han, L. G. Gai, H. H. Jiang, L. C. Zhao, H. Liu and W. Zhang, Synth. Met., 2013, 171, 1 CrossRef CAS PubMed.
- H. B. Gu, S. B. Rapole, J. Sharma, Y. D. Huang, D. M. Cao, H. A. Colorado, Z. P. Luo, N. Haldolaarachchige, D. P. Young, B. Walters, S. Y. Wei and Z. H. Guo, RSC Adv., 2012, 2, 11007 RSC.
- A. Mehdinia, F. Roohi and A. Jabbari, J. Chromatogr. A, 2011, 1218, 4269 CrossRef CAS PubMed.
- Y. P. Sun, F. Xiao, X. G. Liu, C. Feng and C. G. Jin, RSC Adv., 2013, 3, 22554 RSC.
- L. B. Sun, L. X. Zhan, Y. C. Shi, L. Y. Chu, G. L. Ge and Z. P. He, Synth. Met., 2014, 187, 102 CrossRef CAS PubMed.
- B. Zhang, Y. C. Du, P. Zhang, H. T. Zhao, L. L. Kang, X. J. Han and P. Xu, J. Appl. Polym. Sci., 2013, 130, 1099 Search PubMed.
- K. Singh, A. Ohlan, V. H. Pham, R. Balasubramaniyan, S. Varshney, J. Jang, S. H. Hur, W. M. Choi, M. Kumar, S. K. Dhawan, B. S. Kong and J. S. Chung, Nanoscale, 2013, 5, 2411 RSC.
- K. Basavaiah, Y. Pavan Kumar and A. V. Prasada Rao, Appl. Nanosci., 2013, 3, 409 CrossRef CAS.
- C. K. Cui, Y. C. Du, T. H. Li, X. Y. Zheng, X. H. Wang and X. J. Han, J. Phys. Chem. B, 2012, 116, 9523 CrossRef CAS PubMed.
- S. S. Umare, B. H. Shambharkar and R. S. Ningthoujam, Synth. Met., 2010, 160, 1815 CrossRef CAS PubMed.
- J. Huang, Q. Li, D. N. Li, Y. Wang, L. J. Dong, H. A. Xie, J. Wang and C. X. Xiong, Langmuir, 2013, 29, 10223 CrossRef CAS PubMed.
- R. Arundhathi, D. Damodara, P. R. Likhar, M. L. Kantam, P. Saravanan, T. Magdaleno and S. H. Kwon, Adv. Synth. Catal., 2011, 353, 1591 CrossRef CAS.
- C. K. Cui, Y. C. Du, T. H. Li, X. Y. Zheng, X. H. Wang, X. J. Han and P. Xu, J. Phys. Chem. B, 2012, 116, 9523 CrossRef CAS PubMed.
- S. Rana, N. V. Jadhav, K. C. Barick, B. N. Pandey and P. A. Hassan, Dalton Trans., 2014, 12263 RSC.
- D. J. Huang, W. B. Wang, J. X. Xu and A. Q. Wang, Chem. Eng. J., 2012, 210, 166 CrossRef CAS PubMed.
- B. Mu, Q. Wang and A. Q. Wang, J. Mater. Chem. A, 2013, 1, 7083 CAS.
- L. P. Jiang and P. Liu, ACS Sustainable Chem. Eng., 2014, 2, 1785 CrossRef CAS.
- W. B. Zhang, B. Mu, A. Q. Wang and S. J. Shao, Synth. Met., 2014, 192, 87 CrossRef CAS PubMed.
- J. Han, L. Y. Li and R. Guo, Macromolecules, 2010, 43, 10636 CrossRef CAS.
- W. F. Bradley, Am. Mineral., 1940, 25, 405 CAS.
- J. X. Xu, W. B. Wang and A. Q. Wang, Powder Technol., 2013, 235, 652 CrossRef CAS PubMed.
- Q. L. Ma, W. S. Yu, X. T. Dong, J. X. Wang and G. X. Liu, Nanoscale, 2014, 6, 2945 RSC.
- G. D. Nestorovi, K. B. Jeremi and S. M. Jovanovi, J. Serb. Chem. Soc., 2006, 1, 895 CrossRef.
- A. Yasuda and T. Shimidzu, Synth. Met., 1993, 61, 239 CrossRef CAS.
- M. M. Ayad, W. A. Amer and M. Whdan, J. Appl. Polym. Sci., 2012, 125, 2695 CrossRef CAS.
- M. Suárez and E. García-Romero, Appl. Clay Sci., 2006, 31, 154 CrossRef PubMed.
- B. Mu, Y. R. Kang and A. Q. Wang, J. Mater. Chem. A, 2013, 1, 4804 CAS.
- E. Marie, R. Rothe, M. Antonietti and K. Landfester, Macromolecules, 2003, 36, 3967 CrossRef CAS.
- M. Pousti, M. Abbaszadeh, M. S. lashkenari and M. Ghorbani, Synth. Met., 2013, 183, 63 CrossRef CAS PubMed.
- Y. S. Liu, P. Liu and Z. X. Su, Synth. Met., 2007, 157, 585 CrossRef CAS PubMed.
- H. C. Liu, W. Chen, C. Liu, Y. Liu and C. L. Dong, Microporous Mesoporous Mater., 2014, 194, 72 CrossRef CAS PubMed.
- M. Mikhaylova, D. Y. Kim, N. Bobrysheva, M. Osmolowsky, V. Semenov, T. Tsakalakos and M. Muhammed, Langmuir, 2004, 20, 2472 CrossRef CAS.
- L. Boudriche, R. Calvet, B. Hamdib and H. Balard, Colloids Surf., A, 2012, 399, 1 CrossRef CAS PubMed.
- S. Ovarlez, F. Giulieri, F. Delamare, N. Sbirrazzuoli and A. M. Chaze, Microporous Mesoporous Mater., 2011, 142, 371 CrossRef CAS PubMed.
- E. C. Gomes and M. A. S. Oliveira, Am. J. Polym. Sci., 2012, 2, 7 CrossRef PubMed.
- G. H. Zhao, J. Z. Wang, Y. F. Li, X. Chen and Y. P. Liu, J. Phys. Chem. C, 2011, 115, 6350 CAS.
- Y. P. Ting, K. G. Neoh, E. T. Kang and K. L. Tan, J. Chem. Technol. Biotechnol., 1994, 59, 31 CrossRef CAS.
- J. Han, Y. Liu and R. Guo, Adv. Funct. Mater., 2009, 19, 1112 CrossRef CAS.
- X. S. Wang, D. P. Yang, P. Huang, M. Li, C. Li, D. Chen and D. X. Cui, Nanoscale, 2012, 4, 7766 RSC.
- Z. V. Feng, J. L. Lyon, J. S. Croley, R. M. Crooks, D. A. Vanden Bout and K. J. Stevenson, J. Chem. Educ., 2009, 86, 368 CrossRef CAS.
- R. Bhandari and M. R. Knecht, ACS Catal., 2011, 1, 89 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: EDX spectrum and N elemental mapping pattern of APT/PANI nanocomposites, SEM images of APT, TEM images of APT/Fe3O4/PANI3 and APT/Fe3O4/PANI4, and TGA curve of APT. See DOI: 10.1039/c4ta05367b |
| This journal is © The Royal Society of Chemistry 2015 |