Synthesis of core–shell Au–Pt nanodendrites with high catalytic performance via overgrowth of platinum on in situ gold nanoparticles†
Yijing
Li
a,
Wenchao
Ding
a,
Mingrui
Li
a,
Haibing
Xia
*a,
Dayang
Wang
b and
Xutang
Tao
a
aState Key Laboratory of Crystal Materials, Shandong University, Jinan, 250100, P. R. China. E-mail: hbxia@sdu.edu.cn
bIan Wark Research Institute, University of South Australia, Adelaide, SA 5095, Australia
First published on 6th November 2014
Abstract
We present a simple and effective strategy for high yield synthesis of well-dispersed, core–shell Au–Pt nanodendrites (CS Au–Pt NDs) via overgrowth of platinum on in situ 5.5 nm gold nanoparticles in water at room temperature. The sizes of the resulting CS Au–Pt NDs are 14 nm, which should be the smallest so far to the best of our knowledge. The average dimensions of the small Pt branches on the Au nanoparticle surfaces are about 2.6 nm × 4.2 nm, which lead to a significantly increased electrochemically active surface area (up to 35.2 m2 g−1). It is found that the morphology of CS Au–Pt NDs is dependent on the reaction conditions such as the incubation time of citrate–HAuCl4 solution, the mixing time of citrate–HAuCl4–K2PtCl4 solution before AA addition, and Pt-to-Au and AA-to-Pt molar ratios. In comparison with commercial Pt black (0.12 A mgPd−1), the resulting Au–Pt5 NDs show a superior catalytic activity towards methanol oxidation (0.45 A mgPd−1) due to the electronic interaction between the Au cores and Pt branches in bimetallic Au–Pt NDs and the high fraction of atomic steps, kinks, and corner atoms on the surfaces of the Pt branches.
1 Introduction
Due to the high cost and scarcity of Pt resources, the current studies have focused on reduction of Pt mass loading and improvement of Pt utilization.1–3 These goals can be achieved by optimization of the structures of Pt and Pt-based catalysts.2–6 Typical parameters to tailor the catalytic performance are the composition, size, and morphology of Pt-based catalysts. The composition, including the constituent elements (Pt and other metals) and their ratios, determines the basic essence of Pt-based catalysts. The size determines the specific surface area and the ratio of surface to bulk atoms in the catalysts. The morphology (including shape and interface/surface engineering) controls the fractions of atoms at the corners and edges of the catalysts. It is known that the introduction of other metals to form bimetallic Pt-based catalysts is the most promising strategy to implement superior catalytic activity owing to electronic effects.7 For instance, foreign metals can alter the electronic properties of Pt and lower the adsorption energy of CO, thereby facilitating the oxidation of CO at lower potentials. In addition, Pt-based catalysts with core–shell structures can greatly reduce the Pt utilization as the majority of Pt atoms are distributed at the electrochemical reaction interface.8 The structure and properties of the Pt shells can be finely manipulated by the core materials, owing to structure-induced strain (geometry) and electronic effects (alloying). For example, the existence of subsurface Au atoms may provide an additional contribution to the durability enhancement of the Pt skin layer by modifying the well-known core-hindered place-exchange mechanism.8,9To date, high-quality Pt-based catalysts with different shapes, including nanodendrites (NDs),10 nanotubes,4,11 nanopolyhedra,12 nanocages,13 nanowires,14 and so on, have been synthesized. Among them, non-compact small spike structures can reduce the overlaps during deposition, thus enabling the catalytically active surface area to remain high and more high-index facets to be exposed and thus supplying more active sites.15–17 The Yamauchi group has prepared monometallic,15,16,18 bimetallic (Pd–Pt and Au–Pt),19–21 and trimetallic (Au–Pd–Pt)22 Pt particles with multiple nanobranches as highly active electrocatalysts. Recently core–shell (CS) NDs with Au cores decorated with Pt nanobranches atop9,23 gain increasing attention due to their higher chemical stability and durability stemming from the Au component. However, the current preparation of CS Au–Pt NDs is either implemented via a tedious two-step process or via a simple one-step process with rather broad size distribution.9,24 Furthermore, the sizes of the Au–Pt NDs obtained so far are still large due to the use of large Au nanoparticles as seeds. For example, Yang and co-workers reported the synthesis of bimetallic Au–Pt NDs with sizes of 22.6 nm via selective overgrowth of Pt on 11.4 nm multiply twinned Au seeds.25 The catalyst size determines the specific surface area and the ratio of surface to bulk atoms. Although dendritic CS Au–Pt nanomaterials were also prepared on small Au NPs by the two-step method, the electrocatalytic performance (about 0.204 A mgPd−1) is still to be improved for fuel cells due to dense branches on their surfaces.26 Thus, it is technically favorable to synthesize high quality CS Au–Pt NDs with sizes as small as possible in a facile and economic manner.
Recently, one has become increasingly aware of the effects of surfactants,27 used to stabilize nanoparticles and control their morphology, on the nanoparticle catalytic activity. Since these capping agents are essential for the preparation, tedious post-treatment processes have to be conducted to remove the surfactants from the surfaces of the catalysts before the use of these catalysts. Herein, we present a simple and efficient strategy for high yield synthesis of well-dispersed CS Au–Pt NDs via the overgrowth of platinum on in situ gold nanoparticles in aqueous media at room temperature. The as-prepared NDs are marked as CS Au–Ptm NDs, where m represents the Pt-to-Au molar ratio used for the ND synthesis. Instead of additional pre-formed ones, Au nanoparticles in situ formed in the reaction media were used as seeds for subsequent nucleation and growth of Pt nanobranches atop, resulting in CS Au–Pt5 NDs with sizes of about 14 nm. To the best of our knowledge, the resulting NDs should be the smallest compared to those reported in the literature. The average dimensions of the Pt nanobranches on the ND surfaces are about 2.6 nm × 4.2 nm. Due to the morphological features, the as-prepared CS Au–Pt5 NDs exhibit an electrochemically active surface area as large as 35.2 m2 g−1. In comparison with commercial Pt black (0.12 A mgPd−1), the resulting CS Au–Pt5 NDs have a superior catalytic activity towards methanol oxidation (0.45 A mgPd−1) due to the electronic interaction between the Au cores and Pt nanobranches in bimetallic Au–Pt5 NDs and the high fraction of atomic steps, kinks, and corner atoms on the surfaces of the Pt nanobranches.
2 Experimental section
2.1 Materials
Chloroauric acid tetrahydrate (HAuCl4·4H2O), trisodium citrate dihydrate (Na3C6H5O7·2H2O) and ascorbic acid (AA) were purchased from Sinopharm Chemical Reagent Co. Ltd. Potassium tetrachloroplatinate(II) (K2PtCl4, 99%) and commercial Pt black (nominally 10% on carbon black) were purchased from Alfa Aesar (Tianjin, China). All glassware and stirring bars were cleaned with aqua regia (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v HCl (37%)
1 v/v HCl (37%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 (65%) solutions) and then rinsed thoroughly with H2O before use. (Caution: aqua regia solutions are dangerous and should be used with extreme care; never store these solutions in closed containers.) Water used in all experiments was prepared in a three-stage Millipore Milli-Q plus 185 purification system and had a resistivity higher than 18.2 MΩ cm.
HNO3 (65%) solutions) and then rinsed thoroughly with H2O before use. (Caution: aqua regia solutions are dangerous and should be used with extreme care; never store these solutions in closed containers.) Water used in all experiments was prepared in a three-stage Millipore Milli-Q plus 185 purification system and had a resistivity higher than 18.2 MΩ cm.
2.2 Synthesis of CS Au–Ptm NDs
A typical procedure for synthesis of Au–Pt5 NDs is as follows. First, the aqueous solution of HAuCl4 (0.50 mL, 25 mM) was added into the aqueous solution of sodium citrate (1.5 mL, 34 mM) at room temperature under stirring. Water (0.50 mL) was added to bring the volume of the citrate–HAuCl4 premixture solution to 2.5 mL. After about 11 min incubation, the aqueous solution of K2PtCl4 (12.5 mL, 5.0 mM) was added into the citrate–HAuCl4 premixture solution under stirring, followed by a further 30 min incubation under stirring. After addition of the aqueous solution of ascorbic acid (AA) (2.0 mL, 0.25 M) into the citrate–HAuCl4–K2PtCl4 solution for 3 h, CS Au–Pt5 NDs were obtained. The final concentrations of K2PtCl4, HAuCl4, citrate, and AA were 3.6, 0.70, 3.0, and 29.4 mM, respectively. The molar ratios of Pt-to-Au and AA-to-Pt were 5.0 and 8.0, respectively. The Au–Pt5 NDs were separated and excess of AA was removed by repetition of centrifugation (at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rcf for 10 min), supernatant decanting, and water washing three times. Subsequently, the CS Au–Pt5 NDs were redispersed in water with the help of sonication to make a colloidal suspension for further characterization.
000 rcf for 10 min), supernatant decanting, and water washing three times. Subsequently, the CS Au–Pt5 NDs were redispersed in water with the help of sonication to make a colloidal suspension for further characterization.
2.3 Synthesis of spherical hyperbranched Pt particles
The spherical hyperbranched Pt particles were prepared according to a modified protocol reported in the literature.28 Typically, 2.5 mL of aqueous AA solution (0.10 M) was quickly added into 2.5 mL of aqueous K2PtCl4 solution (25 mM). The mixture solution was sonicated for 30 min. The hyperbranched Pt particles were separated and excessive AA was also removed by repetition of centrifugation (at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rcf for 10 min), supernatant decanting, and water washing three times. Finally, the resulting hyperbranched Pt particles were redispersed in water with the help of sonication to make a colloidal suspension for further characterization.
000 rcf for 10 min), supernatant decanting, and water washing three times. Finally, the resulting hyperbranched Pt particles were redispersed in water with the help of sonication to make a colloidal suspension for further characterization.
2.4 Characterization
Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were obtained with a JEOL JEM-2100F transmission electron microscope operating at an acceleration voltage of 200 kV. Elemental mapping images were acquired by energy dispersive X-ray spectroscopy (EDS) using a JEOL JEM-2100F electron microscope equipped with a STEM unit. X-Ray Photoelectron Spectroscopy (XPS) measurements were carried out on a Thermo Fisher Scientific Escalab 250 XPS spectrometer, using Al Ka X-ray radiation for excitation.2.5 Electrochemical measurements
Cyclic voltammetry (CV) and chronoamperometric (CA) experiments were performed in a standard three-electrode cell in a CHI660D workstation at room temperature. A glassy carbon electrode (GCE) modified by the as-prepared CS Au–Ptm NDs was employed as the working electrode while an Ag/AgCl electrode and Pt wire were used as the reference electrode and auxiliary electrode, respectively. The bare GCE was polished successively with 0.3 and 0.05 μm alumina slurries, followed by rinsing thoroughly with pure water and drying at room temperature.A typical procedure for the preparation of the GCE modified by the as-prepared CS Au–Ptm NDs is as follows. 12 μL of Au–Ptm ND solution was drop-coated on a freshly prepared bare GCE, followed by drying in air. 10 μL of the ethanol solution of Nafion (0.2 wt%) was cast on the surface of the GCE coated by Au–Pt5 NDs, followed by drying in air.
Cyclic voltammograms (CVs) used for the determination of the electrochemically active surface area (ECSA) of Pt-based catalysts were recorded between −0.2 V and 1.2 V in N2-saturated 0.5 M H2SO4 solution with a scan rate of 50 mV s−1. For methanol oxidation in acidic media, CVs were recorded between 0 V and 1.0 V in N2-saturated 0.5 M H2SO4 and 1.0 M methanol solution at a scan rate of 20 mV s−1. The ECSA was calculated by measuring the charge collected in the hydrogen adsorption/desorption region after double-layer correction and assuming a value of 0.21 mC cm−2 for the adsorption of a hydrogen monolayer.29,30 Their specific current densities and mass current densities were normalized by their ECSAs and the loaded Pt amounts of each catalyst.
CO stripping voltammetry was performed in 0.1 M H2SO4 solution. After purging the solution with ultrapure N2 for 30 min, ultrapure CO gas was bubbled for 10 min under a fixed potential of −0.2 V vs. Ag/AgCl, to promote the formation of a CO adlayer on the surface of the catalyst. Further, dissolved CO was flushed out by purging the solution with ultrapure N2 for 30 min. The CO stripping voltammetry patterns were recorded at a potential scan rate of 50 mV s−1.
3 Results and discussion
3.1 Morphology of as-prepared Au–Pt5 NDs
Fig. 1a shows the TEM image of CS Au–Pt5 NDs obtained under optimal reaction conditions. The nanoparticles with other shapes are hardly visible. These CS Au–Pt5 NDs have a uniform shape and narrow size distribution; the average size is about 14 nm. The average length and width of the small branches on the ND surfaces are about 4.2 nm and 2.6 nm, respectively. These small branches are spatially separated from each other, which render these Au–Pt5 NDs with a maximally accessible surface area.19 | ||
| Fig. 1 TEM (a) and HRTEM images (b) and (c) of Au–Pt5 NDs, The atomic steps, kinks, and corner atoms on one single branch in the Au–Pt5 ND are indicated by black arrows in the HRTEM image (c). The inset in Fig. 1c is the corresponding Fourier-transform (FT) pattern of the selected branch. HAADF-STEM-EDS mapping images (d–f) and cross-sectional compositional line profile (g) of one single Au–Pt5 ND. The concentrations of K2PtCl4, HAuCl4, citrate, and AA are 3.6, 0.70, 3.0, and 29.4 mM, respectively. The molar ratios of Pt-to-Au and AA-to-Pt are 5.0 and 8.0, respectively. | ||
The HRTEM image (Fig. 1b) of a single Au–Pt5 ND shows that the lattice distances of the branches are about 0.226 nm, which is the typical d spacing of the (111) plane of face centered cubic (fcc) Pt. The HRTEM image of a single branch of one Au–Pt5 ND (Fig. 1c) clearly indicates that the observed d spacings are the (111) and (200) planes of Pt, which is also in good agreement with the corresponding Fourier-transform (FT) pattern (Fig. 1c, inset). It can be seen that a number of defects (such as atomic steps, kinks, and corner atoms) are present on the surfaces of the Pt branches (Fig. 1c and S1, ESI†), which can act as highly active sites for catalysis.31–34 Moreover, the ring patterns with intense spots in the selected area electron diffraction (SAED) patterns (Fig. S2, ESI†) are assigned to (111), (200), (220), (311), and (400) planes of fcc Pt, respectively, indicating that the shells are composed of Pt. The HAADF-STEM-EDS mapping images (Fig. 1d–f) of one single Au–Pt5 ND demonstrate that the nanodendrite is composed of elemental Au and Pt. In addition, Pt is distributed throughout the entire particle (Fig. 1e) while Au is mainly concentrated in the core region (Fig. 1f), confirming the CS structure feature of the as-prepared Au–Pt5 NDs. The CS structure is further confirmed by the EDS mapping of a patch of Au–Pt5 NDs (Fig. S3, ESI†), which shows that each particle has an individual Au core and Pt shell. The cross-sectional compositional line profile of one single Au–Pt5 ND (Fig. 1g) reveals that different from that of conventional CS structures, the line profile of the Au–Pt5 ND shows a noticeable valley, which is attributed to the dendritic structure. Therefore, the CS structured Au–Pt NDs were successfully obtained. In addition, the Pt-to-Au atomic ratio in the resulting Au–Pt5 NDs is 4.6, which is rather close to the initial molar ratio of Pt-to-Au in the citrate–HAuCl4–K2PtCl4 solution.
3.2 Formation mechanism of CS Au–Pt5 NDs
According to our experimental procedure, as depicted in Scheme 1, the synthesis of CS Au–Ptm NDs involves three major steps: (a) mixing of HAuCl4 and sodium citrate in water at room temperature under stirring; (b) formation of Au seeds upon adding K2PtCl4 into the citrate–HAuCl4 mixture solution under stirring, and (c) seeded growth of Au–Ptm NDs upon AA addition.To shed light on the formation mechanism of CS Au–Pt5 NDs, a series of aliquots of the intermediate reaction solutions were extracted from the reaction mixtures at different reaction times for TEM characterization.
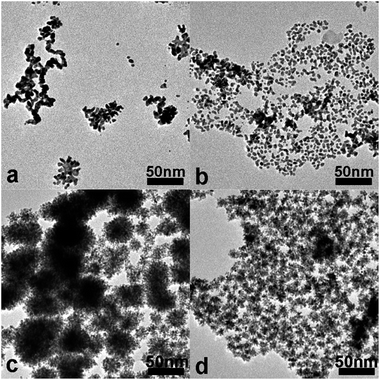
After 11 min incubation of the citrate–HAuCl4 premixture solution, the intermediate product is nanowire-like Au nanoparticles (Fig. 2a). After addition of K2PtCl4 into citrate–HAuCl4 mixture solutions, followed by 30 min incubation, nearly quasi-spherical Au nanoparticles of about 5.5 nm in size are gradually formed (Fig. 2b). These small Au nanoparticles tend to readily coalesce and fuse together during TEM characterization, indicating their high surface activity. The lattice distances of these nanoparticles in the HRTEM image (Fig. 2c) are 0.235 nm, which is in good agreement with the d spacing of the (111) plane of fcc Au. Fig. 3a shows that the Pt nanobranches immediately grow on the surfaces of the Au nanoparticles after AA addition (within 1 min); some Au nanoparticles with fewer branches can be clearly observed. The result indicates that Au nanoparticles act as seeds for the growth of CS Au–Pt NDs. Within 5 min after AA addition, all Au nanoparticles are covered by Pt branches of different lengths and diameters (Fig. 3b). With the reaction further proceeding, both the length and width of the Pt branches increase; the average diameter of Au–Pt NDs also increases from 6.7 nm to 14 nm (Fig. 3a–d). Due to lattice mismatch, Pt can hardly grow uniformly on the surface of Au seed particles. 2,3-Diketo-L-gulonic acid (DGA) from AA oxidation serves as a shape-directing agent to direct the branched growth of Pt on Au seeds.28
Moreover, the fast reduction rate of Pt by an autocatalytic process is also widely used to account for the formation of dendritic Pt branches10,35 Furthermore, the Au seeds should provide the nucleation sites for Pt branches to grow in a spatially well separated manner to avoid overlap and fusion, thus leading to an open dendritic structure.10
3.3 Effect of incubation time of citrate–HAuCl4 premixture
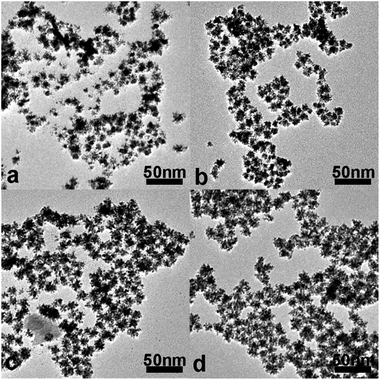
In our previous work,36–38 the Au nuclei can be formed during incubation of the citrate–HAuCl4 mixture at room temperature, which determines the amount of nuclei and the morphology. For synthesis of CS Au–Pt5 NDs, the incubation time was tuned in the range of 9 to 18 min (Fig. S4a and b, ESI†). During this period, enough Au nuclei should be formed by citrate reduction of HAuCl4.39 Before addition of K2PtCl4 solution, nanowire-like Au nanoparticles are formed (Fig. 2a), and however, nearly quasi-spherical Au nanoparticles are obtained after addition of K2PtCl4 solution (Fig. 2b). In the previous work reported by the Lee group, Au(0) atoms can be formed quickly by citrate reduction at high temperature, which leads to the transformation of wire-like to spherical morphology.40 Thus, fast formation of Au(0) atoms is also necessary at room temperature in our case. Note that this shape transformation did not occur at room temperature in the absence of K2PtCl4 or when the aqueous solution of K2PtCl6 was added into the citrate–HAuCl4 mixture solutions (Fig. S5, ESI†). This is because that, on the basis of their reduction potential, Pt(II) ions are oxidized by Au(III) ions into Pt(IV) ions (Table S1, ESI†), thus leading to fast formation of Au(0) atoms and in turn facilitating nucleation. In contrast, Pt(IV) ions cannot be oxidized by Au(III) ions. Thus, the fast formation rate of Au(0) atoms by citrate reduction at room temperature is achieved by the addition of K2PtCl4. As shown in Fig. 4, the morphology and dispersity of the Au seeds had a vital effect on the final morphology of Au–Pt products after AA addition. When the citrate–HAuCl4 mixtures are incubated for 38 min, nanowire-like Au nanoparticles cannot be totally transformed into nearly quasi-spherical Au nanoparticles after addition of K2PtCl4 solution (Fig. 4a) possibly due to fusion and growth of Au nuclei by the autocatalytic reaction.41 In addition to a small amount of Au–Pt NDs, short nanorod-like, hyperbranched Au–Pt products are obtained as byproducts as a result of the growth of Pt on larger Au aggregates (Fig. 4c). When the incubation time is further extended to 68 min, short-rod-like Au nanoparticles and big Au nanoparticles are obtained (Fig. 4b), thus leading to the formation of spherical, hyperbranched Au–Pt products, the sizes of which are in the range of 20 to 100 nm (Fig. 4d).3.4 Effect of the mixing time of citrate–HAuCl4–K2PtCl4 solution before AA addition
As mentioned above, the formation of nearly quasi-spherical Au seeds is mainly due to the reaction between Pt(II) ions and Au(III) ions. Thus, the mixing time before AA addition also has an effect on the synthesis of CS Au–Pt NDs. It is known that AA can quickly reduce Au(III) ions into Au(I) ions and further into Au atoms by the autocatalytic reaction. Thus, the nanowire-like and dendritic structured Au nanoparticles are formed (Fig. 5a) if AA is quickly added shortly after K2PtCl4 addition (e.g. 30 s). Accordingly, the short nanorod-like and spherical, hyperbranched Au–Pt products dominate (Fig. 5c). In order to form nearly quasi-spherical Au nanoparticles in the current work, AA solution should be added to citrate–HAuCl4–K2PtCl4 mixture solutions after the mixture solutions are incubated for 10 min (Fig. 5b), thus resulting in the fabrication of CS Au–Pt NDs with high quality (Fig. 5d).3.5 Effect of the Pt-to-Au molar ratio on the morphology of Au–Pt products
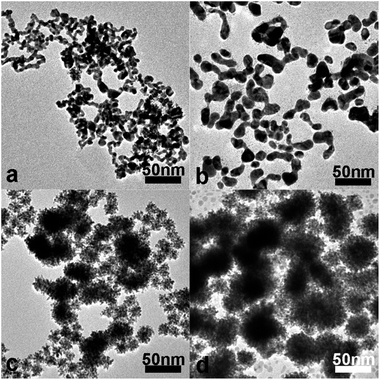
The effect of Au-to-Pt molar ratios on the morphology of the CS Au–Pt NDs and the corresponding electrocatalytic performance on methanol has been investigated in detail (Fig. 6 and 7). When the Pt-to-Au molar ratio is 1.0, the Au–Pt nanoparticles have irregular-shape and broad size distribution; the particle sizes are in the range of 3 to 11 nm (Fig. 6a). With the Pt-to-Au molar ratio increasing to about 2.5, smaller and fewer branches start to emerge on each Au–Pt nanoparticle; the particle sizes are in the range of 6 to 13 nm (Fig. 6b). When the Pt-to-Au molar ratio is slightly increased from 4.0 to 6.0, well-defined Au–Pt NDs with narrow size distribution were obtained and the average ND sizes increase from 11 to 14 and 16 nm (Fig. 6c, 1a and 6d). The size increase of Au–Pt NDs can be attributed to the increase of the Pt amount used. These results indicate that finely tuning the Pt-to-Au molar ratio can produce high-quality well-defined Au–Pt products.3.6 Electrochemical active surface area (ECSA) of Au–Pt products with different morphologies
Pt catalysts with dendritic structures are expected to show high electrocatalytic performance on methanol oxidation due to their large electrochemical active surface area (ECSA). Here we tested all the Au–Ptm products obtained and compared their performance in order to select the best catalyst. The ECSA values of Au–Pt1, Au–Pt2.5, Au–Pt4, Au–Pt5, and Au–Pt6 NDs are calculated to be 22.2, 25.3, 30.3, 35.2, and 34.3 m2 g−1, respectively, by measuring the charge collected in the hydrogen adsorption/desorption region after double-layer correction in 0.5 M H2SO4 solution at room temperature at a scan rate of 50 mV s−1 (Fig. 7A). Among the nanoparticles obtained, Au–Pt NDs have high ECSA values, which increase and then decrease with the Pt-to-Au molar ratio. As such, Au–Pt5 NDs show the highest ECSA value. Fig. 7B depicts the CVs of the GCE modified by Au–Pt1, Au–Pt2.5, Au–Pt4, Au–Pt5, and Au–Pt6 products. Similarly, the GCE modified by Au–Pt5 NDs exhibits the highest electrocatalytic activity by displaying the highest mass normalized current density under the same conditions (Fig. 7B). The peak of the mass-normalized current density of Au–Pt5 NDs (about 0.45 A mgPd−1) is about 3.6-fold larger than that of Au–Pt1 nanoparticles without dendritic structures (about 0.13 A mgPd−1) in the positive-going scan. The currents of these Au–Pt particles are also normalized with respect to the ECSA values to obtain their specific activity (Fig. 7C). The current density of the Au–Pt5 NDs is still the largest (1.27 mA cm−2), which is 2.2-fold larger that of Au–Pt1 products without dendritic structures (0.57 mA cm−2). All these results indicate that the Au–Pt5 NDs show the best electrocatalytic performance for methanol oxidation (Table S2, ESI†).3.7 Effect of the AA concentration on the morphology of Au–Pt5 NDs and their ECSAs
In general, an excess amount of AA is used to prepare Pt dendrites.28 However, we found that the AA concentration still had a slight effect on the morphology of Au–Pt5 NDs (Fig. 8 and Table S3, ESI†). After careful assessment of Au–Pt5 NDs in each TEM image, it is found that all Au–Pt5 NDs have similar sizes around 14 nm, while the length of the Pt nanobranches on the Au–Pt5 NDs increases with the AA-to-Pt molar ratio and the width (about 2.6 nm) changed little; the maximum average length is about 4.2 nm, obtained when the AA-to-Pt molar ratio is >8.0. As expected, these Au–Pt5 NDs obtained at a AA-to-Pt molar ratio of 8.0 show the highest ECSA (Fig. 8d).3.8 Electrocatalytic activity of Au–Pt5 NDs for methanol oxidation
On the basis of the aforementioned results, the Au–Pt5 NDs prepared at the AA-to-Pt molar ratio of 8.0 are good candidates for catalysis of methanol oxidation. To investigate their potential use as anodes for direct methanol fuel cells, the electrocatalytic performance of the Au–Pt5 NDs on methanol oxidation was studied in detail and compared with those of commercial Pt black and spherical hyperbranched Pt particles28 (Fig. S6, ESI†). The ECSA values of the Au–Pt5 NDs, commercial Pt black and spherical hyperbranched Pt particles are calculated by measuring the charge collected in the hydrogen adsorption/desorption region after double-layer correction (Fig. 9A). The ECSA of the GCE modified by Au–Pt5 NDs (35.2 m2 g−1) is about 2.35 times higher than that of hyperbranched Pt nanoparticles (15.0 m2 g−1) and 2.5 times higher than that of commercial Pt black electrodes (14.1 m2 g−1). Fig. 9B depicts the CVs of the GCE modified by commercial Pt black, spherical hyperbranched Pt nanoparticles and Au–Pt5 NDs. The mass-normalized current density of Au–Pt5 NDs (about 0.45 A mgPd−1) is about 2.3-fold and 3.8-fold higher than that of spherical hyperbranched Pt particles (0.20 A mgPd−1) and the commercial Pt black (0.12 A mgPd−1) in the positive-going scan. These results should be attributed to the multi-nanobranch-structure (2.6 nm × 4.2 nm) and the ultrasmall size (ca. 14 nm) of the as-prepared Au–Pt5 NDs. They also underline excellent electrochemical accessibility, which is of great importance for electrocatalytic reactions. In order to assess the specific activity of Au–Pt5 NDs, hyperbranched Pt particles and commercial Pt black, the currents of their modified GCEs were also normalized by the ECSA values (Fig. S7, ESI†). The current density of Au–Pt5 NPs (1.28 mA cm−2) is comparable to that of hyperbranched Pt particles (1.33 mA cm−2) as hyperbranched Pt particles bear a smaller ECSA. However, the current density of Au–Pt5 NPs is higher than that of commercial Pt black (0.84 mA cm−2) or commercial Pt/C catalyst from E-TEK (1.05 mA cm−2).42 The comparison in current densities indicated that the Au–Pt5 NDs showed a higher specific activity than that of the commercial Pt/C catalyst (E-TEK) or the commercial Pt black.To demonstrate the durability of the catalytic performances, the long-term oxidation of methanol by commercial Pt black, spherical hyperbranched Pt particles and Au–Pt5 NDs was studied. On the basis of their chronoamperometric (CA) curves, the electrochemical stability of the Au–Pt5 NDs is superior to that of the spherical hyperbranched Pt nanoparticles or commercial Pt black (Fig. 9C). The oxidation current of the spherical hyperbranched Pt nanoparticles is a little better than commercial Pt black and is not comparable to that of the Au–Pt5 NDs. The oxidation current of the Au–Pt5 NDs is higher than that of the spherical hyperbranched Pt particles or commercial Pt black in the entire time range and the current decay is also slower than that of the spherical hyperbranched Pt particles or commercial Pt black. These results suggest that the Au–Pt5 NDs have a long-term high electrocatalytic activity for methanol oxidation in acid media.
In comparison with commercial Pt black, the higher catalytic performance of Au–Pt5 NDs on methanol oxidation may be attributed to their core–shell structure and multi-branched features. The presence of the Au core in each Au–Pt ND not only facilitates the growth of Pt branches but also plays a key role in the enhanced activity of the Au–Pt NDs relative to pure hyperbranched Pt particles due to the d-center theory.43 On the basis of the electronegativity difference between Au and Pt (2.54 and 2.28, respectively), the potential electron-withdrawing effect from Au to the neighboring Pt may induce an increase of 5d vacancies in Pt, thus significantly improving the adsorption of methanol on the surface of Pt sites and thus favoring the methanol oxidation.44 X-ray photoelectron spectroscopy (XPS) was further used to investigate the valence states of Pt in spherical hyperbranched Pt particles and Au–Pt5 NDs (Fig. S8, ESI†). As expected, one can see that Pt 4f7/2 and 4f5/2 binding energies of the Au–Pt5 NDs are 71.1 and 74.45 eV, respectively. The Pt 4f signal consisted of two pairs of Pt peaks. The most intense peaks (71.05 and 74.4 eV) were attributed to Pt (0). Compared with the Pt 4f7/2 and 4f5/2 binding energies of hyperbranched Pt particles (70.9 and 74.2 eV), a slight shift to higher values (about 0.15 eV) was observed in Au–Pt5 NDs, suggesting that electrons indeed were transferred from Pt shells to Au cores. This result is attributed to the fact that the thick Pt shells on the small Au cores weaken the electron-withdrawing effect. In addition, the abundant atomic steps, edges, and corner atoms in the Pt nanobranches of the Au–Pt5 NDs also act as highly active sites for methanol oxidation.25,34,45 More importantly, these active sites can tolerate undesirable agglomeration due to the special dendritic structure. Thus, the superior electrocatalytic activity of the bimetallic Au–Pt catalysts for methanol oxidation was achieved through structure design with an appropriate Pt-to-Au molar ratio.
To understand the structure and morphology effect of Au–Pt NDs on the CO electrooxidation efficiency, a series of CO-stripping voltammetry tests was performed (Fig. S9, ESI†). The strong stripping peak of CO on the electrode modified by Au–Pt5 NDs is centered at 0.52 V, which is rather close to that of other Au–Pt NDs. However, it is 10 mV, and 160 mV more negative than those on the electrode modified by hyperbranched Pt particles and Pt black, respectively. This suggests that CO can be more easily oxidized on Au–Pt5 NDs, indicating that the bifunctional mechanism may play a more important role than the electronic effect arising from the increase of 5d vacancies, and the improved electrocatalytic activities of Au–Pt5 NDs could be explained by the bifunctional mechanism.46
The potential cycling stabilities of GCEs modified by Au–Pt5 NDs and commercial Pt black are also compared by using CV cycling (Fig. S10, ESI†). It can be seen that the oxidation peak current density of GCEs modified by Au–Pt5 NDs is reduced to about 20% while that of commercial Pt black is reduced to about 42% after 500 cycles of electrochemical oxidation of ethanol. The decrease in the current density of Au–Pt5 NDs is attributed to the fact that the atomic steps, kinks, and corner atoms on the surfaces of the Pt branches disappear gradually and sinter together to form smooth surfaces during the cycling stability test (Fig. S11, ESI†). However, the remaining current density (0.09 A mgPd−1) of Au–Pt5 NDs is still comparable to the original current density of commercial Pt black (0.12 A mgPd−1). Thus, the stability of our Au–Pt NDs still needs to be improved in order to achieve their application in fuel cells.
4 Conclusions
In summary, a simple and efficient strategy has been developed for synthesis of well-dispersed CS Au–Pt5 NDs via overgrowth of platinum on gold nanoparticles in situ formed in water at room temperature. After optimization of the reaction conditions such as the incubation time of citrate–HAuCl4 solution, the mixing time of citrate–HAuCl4–K2PtCl4 solution before AA addition, Pt-to-Au and AA-to-Pt molar ratios, Au–Pt5 NDs with their superior catalytic performance can be obtained at high yield. They have a uniform CS dendritic shape, and narrowly distributed size; the average size is about 14 nm and the average dimensions of the Pt nanobranches are about 2.6 nm × 4.2 nm. This unique structural feature renders the resulting Au–Pt5 NDs with significantly increased electrochemically active surface areas. In comparison with commercial Pt black (0.12 A mgPd−1), the Au–Pt5 NDs have superior catalytic activity towards methanol oxidation (0.45 A mgPd−1) due to the electronic interaction between the Au cores and Pt branches in bimetallic Au–Pt5 NDs and high ratios of atomic steps, kinks, and corner atoms on the surfaces of the Pt branches.Acknowledgements
This work is financially supported by the Natural Science Foundation of China (51172126, 21473105, 51002086, 51227002 and 51272129), and the 973 program (2010CB630702). H. X. is grateful to the Program for New Century Excellent Talents in University (NCET-10-0553), and Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry for the financial support. D. W. thanks the Australian Research Council for the financial support (DP 120102959).Notes and references
- B. Y. Xia, H. B. Wu, X. Wang and X. W. Lou, Angew. Chem., Int. Ed., 2013, 52, 12337–12340 CrossRef CAS PubMed.
- X. L. Sun, D. G. Li, Y. Ding, W. L. Zhu, S. J. Guo, Z. L. Wang and S. H. Sun, J. Am. Chem. Soc., 2014, 136, 5745–5749 CrossRef CAS PubMed.
- B. Y. Xia, H. B. Wu, Y. Yan, X. W. Lou and X. Wang, J. Am. Chem. Soc., 2013, 135, 9480–9485 CrossRef CAS PubMed.
- C. H. Cui and S. H. Yu, Acc. Chem. Res., 2013, 46, 1427–1437 CrossRef CAS PubMed.
- L. Kuai, S. Wang and B. Geng, Chem. Commun., 2011, 47, 6093–6095 RSC.
- Y. Xu and B. Zhang, Chem. Soc. Rev., 2014, 43, 2439–2450 RSC.
- B. Y. Xia, H. B. Wu, X. Wang and X. W. Lou, J. Am. Chem. Soc., 2012, 134, 13934–13937 CrossRef CAS PubMed.
- S. Guo, S. Zhang and S. Sun, Angew. Chem., Int. Ed., 2013, 52, 8526–8544 CrossRef CAS PubMed.
- H. Ataee-Esfahani, L. Wang and Y. Yamauchi, Chem. Commun., 2010, 46, 3684–3686 RSC.
- B. Lim, M. Jiang, P. H. Camargo, E. C. Cho, J. Tao, X. Lu, Y. Zhu and Y. Xia, Science, 2009, 324, 1302–1305 CrossRef CAS PubMed.
- Y. G. Guo, J. S. Hu, H. M. Zhang, H. P. Liang, L. J. Wan and C. L. Bai, Adv. Mater., 2005, 17, 746–750 CrossRef CAS.
- N. S. Porter, H. Wu, Z. Quan and J. Fang, Acc. Chem. Res., 2013, 46, 1867–1877 CrossRef CAS PubMed.
- D. S. Wang, P. Zhao and Y. D. Li, Sci. Rep., 2011, 1, 37 Search PubMed.
- Q. Yuan, Z. Y. Zhou, J. Zhuang and X. Wang, Chem. Mater., 2010, 22, 2395–2402 CrossRef CAS.
- L. Wang, M. Imura and Y. Yamauchi, ACS Appl. Mater. Interfaces, 2012, 4, 2865–2869 CAS.
- L. Wang and Y. Yamauchi, J. Am. Chem. Soc., 2009, 131, 9152–9153 CrossRef CAS PubMed.
- B. Y. Xia, Y. Yan, X. Wang and X. W. Lou, Mater. Horiz., 2014, 1, 379 RSC.
- L. Wang and Y. Yamauchi, Chem. Mater., 2009, 21, 3562–3569 CrossRef CAS.
- L. Wang, Y. Nemoto and Y. Yamauchi, J. Am. Chem. Soc., 2011, 133, 9674–9677 CrossRef CAS PubMed.
- L. Wang and Y. Yamauchi, J. Am. Chem. Soc., 2013, 135, 16762–16765 CrossRef CAS PubMed.
- H. Ataee-Esfahani, M. Imura and Y. Yamauchi, Angew. Chem., Int. Ed., 2013, 52, 13611–13615 CrossRef CAS PubMed.
- L. Wang and Y. Yamauchi, Chem. Mater., 2011, 23, 2457–2465 CrossRef CAS.
- J. Zhang, K. Sasaki, E. Sutter and R. R. Adzic, Science, 2007, 315, 220–222 CrossRef CAS PubMed.
- S. J. Guo, J. Li, S. J. Dong and E. K. Wang, J. Phys. Chem. C, 2010, 114, 15337–15342 CAS.
- Y. Feng, H. Liu and J. Yang, J. Mater. Chem. A, 2014, 2, 6130–6137 CAS.
- S. Y. Wang, N. Kristian, S. P. Jiang and X. Wang, Nanotechnology, 2009, 20, 025605 CrossRef PubMed.
- S. Wang, L. Kuai, Y. Huang, X. Yu, Y. Liu, W. Li, L. Chen and B. Geng, Chem.–Eur. J., 2013, 19, 240–248 CrossRef CAS PubMed.
- L. Wang, C. P. Hu, Y. Nemoto, Y. Tateyama and Y. Yamauchi, Cryst. Growth Des., 2010, 10, 3454–3460 CAS.
- W. Sheng, H. A. Gasteiger and Y. Shao-Horn, J. Electrochem. Soc., 2010, 157, B1529–B1536 CrossRef CAS PubMed.
- K. J. J. Mayrhofer, D. Strmcnik, B. B. Blizanac, V. Stamenkovic, M. Arenz and N. M. Markovic, Electrochim. Acta, 2008, 53, 3181–3188 CrossRef CAS PubMed.
- S. W. Lee, S. Chen, W. Sheng, N. Yabuuchi, Y. T. Kim, T. Mitani, E. Vescovo and Y. Shao-Horn, J. Am. Chem. Soc., 2009, 131, 15669–15677 CrossRef CAS PubMed.
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732–735 CrossRef CAS PubMed.
- Z. Y. Zhou, Z. Z. Huang, D. J. Chen, Q. Wang, N. Tian and S. G. Sun, Angew. Chem., Int. Ed., 2010, 49, 411–414 CrossRef CAS PubMed.
- H. B. Xia, Y. Ran, H. S. Li, X. T. Tao and D. Y. Wang, J. Mater. Chem. A, 2013, 1, 4678–4684 CAS.
- Y. J. Song, Y. Yang, C. J. Medforth, E. Pereira, A. K. Singh, H. Xu, Y. Jiang, C. J. Brinker, F. van Swol and J. A. Shelnutt, J. Am. Chem. Soc., 2004, 126, 635–645 CrossRef CAS PubMed.
- H. B. Xia, S. Bai, J. Hartmann and D. Y. Wang, Langmuir, 2010, 26, 3585–3589 CrossRef CAS PubMed.
- W. C. Ding, Y. Liu, Y. J. Li, Q. R. Shi, H. S. Li, H. B. Xia, D. Y. Wang and X. T. Tao, RSC Adv., 2014, 4, 22651–22659 RSC.
- P. N. Zhang, C. X. Xi, C. Feng, H. B. Xia, D. Y. Wang and X. T. Tao, CrystEngComm, 2014, 16, 5268–5274 RSC.
- J. Turkevich, P. C. Stevenson and J. A. Hillier, Discuss. Faraday Soc., 1951, 11, 55–75 RSC.
- B. K. Pong, H. I. Elim, J. X. Chong, W. Ji, B. L. Trout and J. Y. Lee, J. Phys. Chem. C, 2007, 111, 6281–6287 CAS.
- X. Ji, X. Song, J. Li, Y. Bai, W. Yang and X. Peng, J. Am. Chem. Soc., 2007, 129, 13939–13948 CrossRef CAS PubMed.
- A. B. A. A. Nassr, I. Sinev, M.-M. Pohl, W. Grünert and M. Bron, ACS Catal., 2014, 4, 2449–2462 CrossRef CAS.
- B. Hammer and J. K. Norskov, Adv. Catal., 2000, 45, 71–129 CAS.
- X. G. Ding, Y. Zou, F. Ye, J. Yang and J. Jiang, J. Mater. Chem. A, 2013, 1, 11880–11886 CAS.
- M. A. Mahmoud, C. E. Tabor, M. A. El-Sayed, Y. Ding and Z. L. Wang, J. Am. Chem. Soc., 2008, 130, 4590–4591 CrossRef CAS PubMed.
- A. Chen and P. Holt-Hindle, Chem. Rev., 2010, 110, 3767–3804 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Selected area electron diffraction (SAED) pattern, HRTEM, TEM and HAADF-STEM-EDS mapping images of the CS Au–Pt5 NDs, TEM image of nanowire-like Au nanoparticles extracted from the citrate–HAuCl4–K2PtCl6 solution with 11 min incubation, TEM images of other Au–Pt5 NDs obtained with 9 min and 18 min incubation, TEM image of the hyperbranched Pt particles, Table S1: summary of the redox potential values of the half-reaction of AuCl4−/Au0 and PtCl62−/PtCl42−, Table S2: summary of ECSAs, current densities normalized by mass and current densities normalized by ECSA of the GCE modified by Au–Pt products, and Table S3: summary of the lengths and widths of the branches on Au–Pt5 NDs prepared under different AA-to-Pt molar ratios. See DOI: 10.1039/c4ta04940c |
| This journal is © The Royal Society of Chemistry 2015 |