A facile approach to fabricate a UV/heat dual-responsive triple shape memory polymer†
Y.
Wu
a,
J.
Hu
*ab,
C.
Zhang
a,
J.
Han
a,
Y.
Wang
a and
B.
Kumar
a
aInstitute of Textiles & Clothing, The Hong Kong Polytechnic University, Hung Hom, Kowloon, Hong Kong. E-mail: jin-lian.hu@polyu.edu.hk
bThe Hong Kong Polytechnic University Shenzhen Base, 518057, Shenzhen, China
First published on 24th October 2014
Abstract
In the present work, a facile approach was employed to fabricate a UV/heat dual-responsive triple shape memory polymer (SMP) by simply mixing Zn(Mebip)2(NTf2)2, a metallosupramolecular unit formed by coordinating 2,6-bis(N-methyl-benzimidazolyl)-pyridine (Mebip) ligands to zinc di[bis(trifluoromethylsulfonyl)-imide] (Zn(NTf2)2), into one part of epoxy resin. Dissimilar to previously reported UV sensitive SMPs with sophisticated molecular structure and relatively poor mechanical properties, the advantage of this approach is that the UV-sensitivity was simply achieved by employing Zn(Mebip)2(NTf2)2, a UV-heat transfer compound, into the polymer matrix without significant sacrifice of thermal and mechanical properties. Also thanks to the light–heat transfer nature of Zn(Mebip)2(NTf2)2 and segmented material structure, the resulting polymer displayed UV sensitivity to the composite part, while leaving the shape of the neat SMP part unchanged under the UV source (solely thermal sensitive). Thus the resulting composite displayed an excellent UV and heat selective localized triple shape memory effect.
Shape memory polymers (SMPs) are a class of stimuli-responsive materials which are able to revert to the pre-defined shape upon application of a stimulus.2,3 Traditional SMPs are generally realized by introducing net-points into a polymer with appropriate phase transition temperature (Ttrans), forming thermally induced one-way dual-shape memory polymers (1WDSMPs).4 The net-points determine the permanent shape, while the rest of the polymer acts as a switch to immobilize the temporary shape. A typical programming cycle for 1WDSMPs consists of four steps. First, the SMP was heated above its Ttrans. Then the SMP was deformed and fixed by an external force. Then the fixed SMP was cooled down (below the Ttrans) and the external force was released, and the network reached a thermodynamically unstable but dynamically stable state (so-called temporary shape). Finally, the SMP was heated above its transition temperature, the switch was on (the mobility of the polymer increased) and the network reached a dynamically and thermodynamically stable state (so-called permanent shape).5,6
SMPs which could also memorize 2 temporary shapes and realize shape changing among three shapes are called triple shape memory polymers (3SMPs).7–9 Typically 3SMPs were realized by introducing additional switches (either chemically or physically) into a normal 1WDSMP network, which could be a polymeric switch10–12/network13 with distinct Ttrans, self-complementary hydrogen bonding moieties,14 or even utilize the two phase change in a crystalline type switch (for example, PCL13). These unique abilities of 1WDSMPs and 3SMPs have been utilized for a number of applications in biomedical, packaging, textile and aerospace areas.3,15,16
Despite commonly utilized direct heating, other alternative triggering mechanisms like light and magnetic fields have also been developed.17 These methods enable remote triggering of shape recovery and make SMPs more feasible for in vivo applications. In a previously demonstrated light-induced shape memory system,18 light-sensitive components such as gold nanoparticles (responsive to the laser in the visible light region), graphene oxide (responsive to the infrared light region), organo-metal compounds and cinnamic groups (responsive to the UV region) were introduced into the polymer network either covalently or non-covalently to realize such purposes. Zhao et al. introduced functionalized AuNPs into biodegradable SMPs to realize the visible light triggered shape recovery.19,20 Rowan et al. reported covalently cross-linked metallosupramolecular shape memory polymers with metal salt and poly(butadiene) which was end-capped with Mebip.1,21 The key component in fixing and releasing the temporary shape is the UV induced heating effect on metal–ligand units that leads to their dissociation–association. This mechanism was previously proved by Weder et al.1 for fabricating UV-induced self-healing polymers, where new bonding could be reversibly formed among the neighbor fracture surfaces. For SMPs with cinnamic groups, their SME is realized by reversibly photofixing (r > 260 nm) and photocleaving (λ < 260 nm) cinnamic groups.22 Although polymers with cinnamic groups and OMebip are UV responsive, they are covalently connected to the polymers to realize light sensitivity. Therefore, compared to other commercial polymers like epoxy resin and polyurethane, the polymers mentioned above are difficult to fabricate and their mechanical properties are relatively poor. Compared to tuning molecular structures, blending light–heat transfer components into the original polymer matrix is better because they are easy to process and maintain (even enhance) the original mechanical performance of the matrix. However, to the best of our knowledge, so far there have been few reports on the fabrication of UV-sensitive SMPs using a composite approach.
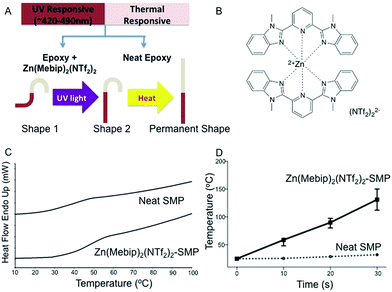
Driven by increasing sophisticated demands of materials and devices, developing SMPs with additional functions like dual-responsiveness8 is fueled by technological needs.23,24 Here, we report a triple shape memory polymer composite featuring both remote triggering of the shape recovery with an UV source and heat. Our approach for light-sensitivity is based on the principle that Zn(Mebip)2(NTf2)2, as shown in Fig. 1B, can transfer the UV source into heat.1 Mebip and the related zinc complex were synthesized according to the literature with slight modification.1,25,26 Epoxy resin was chosen as the matrix because of its facile synthesis routine, tunable transition temperature in different regions and great mechanical properties (for example, high recovery force27). The shape memory behavior of epoxy resin has been investigated elsewhere.28 The bi-responsive SMPs in the present work were realized by casting the Zn(Mebip)2(NTf2)2 embedded epoxy pre-polymer to the surface of pre-cured neat epoxy resin. Such a conceptual material design is shown in Fig. 1A. The target SMP consists of two regions: the Zn(Mebip)2(NTf2)2–SMP (right region) and neat SMP. The permanent straight shape was first deformed into shape 1, in which strains were introduced into each region. For shape 1, the recovery of the Zn(Mebip)2(NTf2)2–SMP region can be remotely actuated by the UV source without enacting the recovery of the other region. As such, after UV irradiation, it could recover to the permanent shape by heating. Since the approach relies on selective localized actuations, a broad shape memory transition or two distinct shape memory transitions, which are required for other known triple shape memory polymers, are not needed here.
Detailed raw materials for synthesis and characterization methods are available in the ESI.† The molar ratio of the liquid epoxy precursor for the SMP samples was E-51/NGDE/Jeffamine (D230) = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 because, according to our previous experience, the Tg of the resulting polymer with this recipe is in the body temperature region. Weighed nanoparticles (0.2 g bulk Zn(Mebip)2(NTf2)2 which was dissolved in CH2Cl2/MeCN) were dispersed into the mixture of E51 (1.0 g) and NGDE (0.3 g), then the solvent was removed under vacuum. The nanoparticles were further dispersed by ultrasonication for 10 min. Next, Jeffamine (0.47 g) was added into the dispersion. The obtained mixture was further mixed mechanically for 30 s before curing. Inspired by Xie's work,17 the SMP was synthesized in two steps. First, the neat SMP monomer solution, a mixture of E51 (2.5 g), NGDE (0.7 g) and Jeffamine (1.1 g), was partially cured for 60 min at 80 °C in a 7.5 cm × 2.5 cm Teflon mold. Then, the neat SMP was evenly cut into 2 pieces in the long axis and the right part was left in the mold (the left side was left blank). Finally, the Zn(Mebip)2–SMP monomer solution was cast into the related blank part of the mold and fully cured. The overall measurement of the demonstration sample is 75 mm × 2 mm × 1 mm. Two regions are both around 37 mm in length. The mass ratio of Zn(Mebip)2(NTf2)2 was 10%.
3 because, according to our previous experience, the Tg of the resulting polymer with this recipe is in the body temperature region. Weighed nanoparticles (0.2 g bulk Zn(Mebip)2(NTf2)2 which was dissolved in CH2Cl2/MeCN) were dispersed into the mixture of E51 (1.0 g) and NGDE (0.3 g), then the solvent was removed under vacuum. The nanoparticles were further dispersed by ultrasonication for 10 min. Next, Jeffamine (0.47 g) was added into the dispersion. The obtained mixture was further mixed mechanically for 30 s before curing. Inspired by Xie's work,17 the SMP was synthesized in two steps. First, the neat SMP monomer solution, a mixture of E51 (2.5 g), NGDE (0.7 g) and Jeffamine (1.1 g), was partially cured for 60 min at 80 °C in a 7.5 cm × 2.5 cm Teflon mold. Then, the neat SMP was evenly cut into 2 pieces in the long axis and the right part was left in the mold (the left side was left blank). Finally, the Zn(Mebip)2–SMP monomer solution was cast into the related blank part of the mold and fully cured. The overall measurement of the demonstration sample is 75 mm × 2 mm × 1 mm. Two regions are both around 37 mm in length. The mass ratio of Zn(Mebip)2(NTf2)2 was 10%.
Generally, the value of Ttrans is important as it determined the application area of the resulting polymer.29,30 For example, Ttrans close to (usually slightly below) 37 °C is preferred for biomedical applications,31 while high Ttrans (say, 100 °C) is required for aerospace applications.16 Either dynamic mechanical analysis (DMA) or differential scanning calorimetry (DSC) can be employed to study the Ttrans. In our work, the Ttrans of the resulting SMPs was evaluated by DSC with a scanning rate of 10 °C min−1. As the material is with two parts (neat SMP and composite SMP), their Ttrans was studied separately. As shown in Fig. 1C (the 2nd heating curve of the sample, as the first heating curve is to eliminate thermal history), the phase change of two parts is of glass transition nature and their glass transition temperatures (Tg) are close to or slightly higher than body temperature. For the neat SMP, an apparent Tg was found at 37 °C, while the Tg of Zn(Mebip)2(NTf2)2–SMP is slightly shifted to 48 °C. This phenomenon may be caused by the rigid nature of the organo-metal additives which hindered the mobility of the molecular chain. This result suggested that the Ttrans of both parts is close to body temperature. The Tg of Zn(Mebip)2(NTf2)2–SMP could be further tuned to body temperature by slightly increasing the ratio of NGDE in the matrix.
The pre-requirement of triple shape memory effects with two types of stimuli is that at least one part of the sample is selectively responsive to stimuli while the other part is less affected under the same stimuli. In our case, we anticipated that the Zn(Mebip)2(NTf2)2–SMP should be sensitive to both UV and heat while the neat SMP part is solely thermal responsive. To prove the selective activation of the sample under an UV source, the temperature–time relationship of each region (neat SMP part and composite part) was determined. The UV source was generated from a SunSpot SM 2 from Shenzhen Wisbay M&E Co., LTD. The sample surface temperature during heating was recorded with an IR camera. As shown in Fig. 1D, the temperature of the Zn(Mebip)2(NTf2)2–SMP part rapidly increased above its transition temperature after 10 seconds while the temperature of the neat SMP part remained below its transition temperature. This indicates that Zn(Mebip)2(NTf2)2 is an efficient light–heat transfer agent. The light–heat transfer mechanism has been reported elsewhere.1 Besides, the slight temperature increase from the neat SMP may be caused by the heat effect from the UV source, but such a heat effect is not high enough to trigger the shape recovery of the neat SMP part.
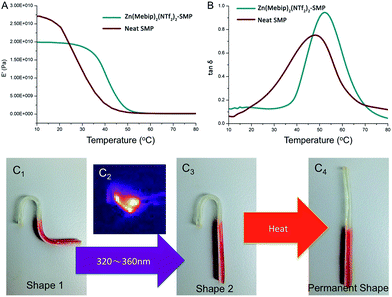
The mechanical properties of the SMPs were further studied by dynamic mechanical analysis (DMA) on a PerkinElmer DMA 8000 by the bending method and the heating rate is 2 °C min−1. As shown in Fig. 2A and B, the elastic modulus of the neat SMP and Zn(Mebip)2(NTf2)2–SMP below the Tg is around 2.0 × 104 MPa and 2.7 × 104 M, while decreasing to 1.3 × 104 MPa and 1.6 × 102 MPa above Tg separately. The Tgs determined by tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ are slightly higher than the ones determined by DSC. The shape memory effect of the sample was determined by the bending test with angle as a recovery factor.17,32 The shape fixity (Rf) and shape recovery (Rr) were evaluated by comparing the bending angle, fixed angle, and recovered angle. Samples were heated at 80 °C and bent to 180° (bending angle) and then quenched in ice water for 1 min. The fixed angle of the sample was measured afterwards. Each region of the sample was then exposed to UV and subsequently heated. The final angle was measured as the recovered angle. The shape fixity and shape recovery ratio of each part are all close to 100%. Furthermore, a demonstration was made to illustrate the UV/heat sensitive triple shape memory effect. As shown in Fig. 2, the sample was first deformed into “s” shape #1, upon exposure to the 320–360 nm UV source which was applied by using a horizontally fixed optical fiber, as shown in C2; the IR image indicates the selected activation of the Zn(Mebip)2(NTf2)2-SMP part while the neat SMP part maintains the original temperature. After 15 second UV irradiation, the SMP changed to shape #2, which was subsequently recovered to its permanent shape by immersing the SMP into hot water with 60 °C.
δ are slightly higher than the ones determined by DSC. The shape memory effect of the sample was determined by the bending test with angle as a recovery factor.17,32 The shape fixity (Rf) and shape recovery (Rr) were evaluated by comparing the bending angle, fixed angle, and recovered angle. Samples were heated at 80 °C and bent to 180° (bending angle) and then quenched in ice water for 1 min. The fixed angle of the sample was measured afterwards. Each region of the sample was then exposed to UV and subsequently heated. The final angle was measured as the recovered angle. The shape fixity and shape recovery ratio of each part are all close to 100%. Furthermore, a demonstration was made to illustrate the UV/heat sensitive triple shape memory effect. As shown in Fig. 2, the sample was first deformed into “s” shape #1, upon exposure to the 320–360 nm UV source which was applied by using a horizontally fixed optical fiber, as shown in C2; the IR image indicates the selected activation of the Zn(Mebip)2(NTf2)2-SMP part while the neat SMP part maintains the original temperature. After 15 second UV irradiation, the SMP changed to shape #2, which was subsequently recovered to its permanent shape by immersing the SMP into hot water with 60 °C.
The nanoparticulate dispersion and morphologies of the SMP composites were further characterized using scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As shown in Fig. 3A, the fracture surface of the epoxy is relatively rough at low magnification of SEM. This may be caused by imperfect compatibility of the epoxy matrix and Zn(Mebip)2(NTf2)2. Although some projections may be regarded as Zn(Mebip)2(NTf2)2 clusters, unlike carbon nanotubes, they did not display a specific microstructure. Thus their dispersion cannot be proved simply by SEM. To further study the dispersion of Zn(Mebip)2(NTf2)2 in the epoxy matrix, TEM was employed as zinc is the only heavy element available in the composite system. As shown in Fig. 3B, the dark spots, which may be considered as zinc, are dispersed relatively normally in the polymer matrix at high magnification. Energy-dispersive X-ray spectroscopy (EDX) (Fig. 3C) further supported the existence of Zn in the matrix. TEM proved that Zn(Mebip)2(NTf2)2 can be dispersed relatively normally at high magnification. The compatibility may be further improved by modifying the p-C on pyridine with an additional aliphatic branch.
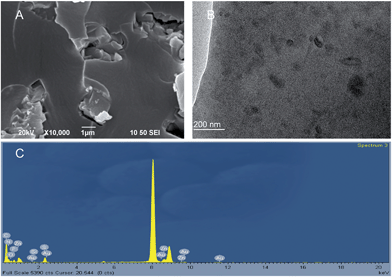 | ||
Fig. 3 SEM, TEM and EDX images of the SMP composites. (A) SEM image of Zn(Mebimpy)2(NTf2)2–SMP at ×10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 magnification. (B) TEM image of Zn(Mebimpy)2(NTf2)2–SMP and (C) EDX image of the composite. 000 magnification. (B) TEM image of Zn(Mebimpy)2(NTf2)2–SMP and (C) EDX image of the composite. | ||
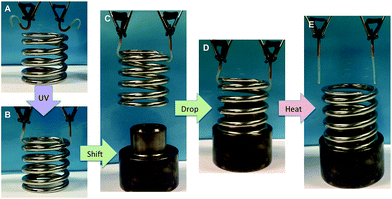
One practical application was demonstrated by employing the SMP as robot hands. As shown in Fig. 4, (A) the initial state of the robot hands was fabricated into an S shape and exposed to the UV source, resulting in the fact that (B) the metal spring was hold by the SMP. (C) Then the spring was shifted above the metal base and (D) installed on the base. (E) The robot hands were further released by heat.
In summary, this work concerns a facile approach to fabricate a UV/heat dual-responsive triple shape memory polymer. The molecular strategy of fabricating such a polymer composite was realized by mixing Zn(Mebip)2(NTf2)2 partially into epoxy resin (10 wt%). Unlike other reported UV-sensitive SMPs where a sophisticated polymer structure is required and which displayed relatively poor mechanical properties, the advantage of this approach is that UV-sensitivity can be introduced into SMPs by simply employing Zn(Mebip)2(NTf2)2 into any polymer matrix without significant sacrifice of thermal and mechanical properties. Also thanks to the light–heat transfer nature of the Zn(Mebip)2(NTf2)2 segmented material structure, the resulting polymer displayed UV sensitivity to the composite part, while leaving the shape of the neat SMP part unchanged. Thus the resulting composite displayed an excellent UV and heat selective localized triple shape memory effect.
Acknowledgements
The authors of this paper would like to acknowledge the financial support by projects of PolyU 5162/12E and RD/PR/004/09. The authors would also like to acknowledge Shenzhen Wisbay M&E Co., LTD for their generous free support for the UV source. The authors would also acknowledge Prof. Tao Xie (Zhejiang University) and Mr Yifu Wang for inspiration of the idea from their work and presentations.Notes and references
- M. Burnworth, L. M. Tang, J. R. Kumpfer, A. J. Duncan, F. L. Beyer, G. L. Fiore, S. J. Rowan and C. Weder, Nature, 2011, 472, 334-U230 CrossRef PubMed.
- J. L. Hu, Y. Zhu, H. H. Huang and J. Lu, Prog. Polym. Sci., 2012, 37, 1720–1763 CrossRef CAS PubMed.
- J. S. Leng, X. Lan, Y. J. Liu and S. Y. Du, Prog. Mater. Sci., 2011, 56, 1077–1135 CrossRef CAS PubMed.
- T. Xie, Polymer, 2011, 52, 4985–5000 CrossRef CAS PubMed.
- Y. Wu, J. L. Hu, H. H. Huang, J. Li, Y. Zhu, B. Z. Tang, J. P. Han and L. B. Li, J. Polym. Sci., Part B: Polym. Phys., 2014, 52, 104–110 CrossRef CAS.
- Y. Wu, J. Hu, J. Han, Y. Zhu, H. Huang, J. Li and B. Tang, J. Mater. Chem. A, 2014, 2, 18816–18822 CAS.
- S. J. Chen, H. M. Yuan, H. T. Zhuo, S. G. Chen, H. P. Yang, Z. C. Ge and J. H. Liu, J. Mater. Chem. C, 2014, 2, 4203–4212 RSC.
- Y. K. Bai, Y. Chen, Q. H. Wang and T. M. Wang, J. Mater. Chem. A, 2014, 2, 9169–9177 CAS.
- Y. K. Bai, X. R. Zhang, Q. H. Wang and T. M. Wang, J. Mater. Chem. A, 2014, 2, 4771–4778 CAS.
- M. Behl and A. Lendlein, J. Mater. Chem., 2010, 20, 3335–3345 RSC.
- T. Pretsch, Polym. Degrad. Stab., 2010, 95, 2515–2524 CrossRef CAS PubMed.
- J. Zotzmann, M. Behl, D. Hofmann and A. Lendlein, Adv. Mater., 2010, 22, 3424–3429 CrossRef CAS PubMed.
- X. F. Luo and P. T. Mather, Adv. Funct. Mater., 2010, 20, 2649–2656 CrossRef CAS.
- T. Ware, K. Hearon, A. Lonnecker, K. L. Wooley, D. J. Maitland and W. Voit, Macromolecules, 2012, 45, 1062–1069 CrossRef CAS PubMed.
- J. L. Hu and S. J. Chen, J. Mater. Chem., 2010, 20, 3346–3355 RSC.
- Y. J. Liu, H. Y. Du, L. W. Liu and J. S. Leng, Smart Mater. Struct., 2014, 23, 023001 CrossRef.
- Z. W. He, N. Satarkar, T. Xie, Y. T. Cheng and J. Z. Hilt, Adv. Mater., 2011, 23, 3192–3196 CrossRef CAS PubMed.
- D. Iqbal and M. H. Samiullah, Materials, 2013, 6, 116–142 CrossRef CAS PubMed.
- H. J. Zhang and Y. Zhao, ACS Appl. Mater. Interfaces, 2013, 5, 13069–13075 CAS.
- H. J. Zhang, H. S. Xia and Y. Zhao, ACS Macro Lett., 2014, 3, 940–943 CrossRef CAS.
- J. R. Kumpfer and S. J. Rowan, J. Am. Chem. Soc., 2011, 133, 12866–12874 CrossRef CAS PubMed.
- A. Lendlein, H. Y. Jiang, O. Junger and R. Langer, Nature, 2005, 434, 879–882 CrossRef CAS PubMed.
- C. Liu, H. Qin and P. T. Mather, J. Mater. Chem., 2007, 17, 1543–1558 RSC.
- W. X. Wang, H. B. Lu, Y. J. Liu and J. S. Leng, J. Mater. Chem. A, 2014, 2, 5441–5449 CAS.
- S. Coulibaly, A. Roulin, S. Balog, M. V. Biyani, E. J. Foster, S. J. Rowan, G. L. Fiore and C. Weder, Macromolecules, 2014, 47, 152–160 CrossRef CAS.
- W. W. Yang, Y. W. Zhong, S. Yoshikawa, J. Y. Shao, S. Masaoka, K. Sakai, J. N. Yao and M. Haga, Inorg. Chem., 2012, 51, 890–899 CrossRef PubMed.
- T. Xie and X. C. Xiao, Chem. Mater., 2008, 20, 2866–2868 CrossRef CAS.
- I. A. Rousseau and T. Xie, J. Mater. Chem., 2010, 20, 3431–3441 RSC.
- Q. H. Meng, J. L. Hu, Y. Zhu, J. Lu and Y. Liu, J. Appl. Polym. Sci., 2007, 106, 2515–2523 CrossRef CAS.
- Q. H. Meng and J. L. Hu, Composites, Part A, 2008, 39, 314–321 CrossRef PubMed.
- R. M. Baker, J. H. Henderson and P. T. Mather, J. Mater. Chem. B, 2013, 1, 4916–4920 RSC.
- W. Wagermaier, K. Kratz, M. Heuchel and A. Lendlein, Adv. Polym. Sci., 2010, 226, 97–145 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ta04881d |
| This journal is © The Royal Society of Chemistry 2015 |