Integration of polymerized ionic liquid with graphene for enhanced CO2 adsorption†
P.
Tamilarasan
and
S.
Ramaprabhu
*
Alternative Energy and Nanotechnology Laboratory (AENL), Nano Functional Materials Technology Centre (NFMTC), Department of Physics, Indian Institute of Technology Madras (IITM), Chennai, India. E-mail: ramp@iitm.ac.in
First published on 13th October 2014
Abstract
In this study, we have integrated an ionic liquid (IL) or polymerized ionic liquid (PIL) with graphene to demonstrate enhanced carbon dioxide adsorption properties. Graphene was non-covalently functionalized by IL or PIL, and the carbon dioxide adsorption and desorption properties were determined at low-pressures (<100 kPa). Upon functionalization, IL uniformly covers the graphene surface, while PIL forms highly distributed porous nanoparticles. The PIL functionalized graphene shows 22% higher adsorption capacity than graphene, while IL functionalization improves it only by 2%. This highlights the advantage of polymerizing the ionic liquid. Interestingly, the adsorption capacities of integrated system are higher than those of individual constituents (either graphene or IL or PIL). It is found that PIL functionalization offers more favorability of adsorption with a high adsorption energy. Isosteric heats of adsorption are calculated to be in the range of 18–28 kJ mol−1, suggesting an ease of adsorbent regeneration. These results encourage the integration of PIL with other high surface area nanostructures for further improvement in the adsorption capacity.
1. Introduction
Global warming is mainly caused by CO2, as it is a highly abundant greenhouse gas, released by several anthropogenic factors. Adsorption is one of the promising methods in post emission CO2 management. Solid state adsorbents have comprehensive advantages over aqueous amine based adsorbents due to their thermal stability and easy adsorbent regeneration.1 Several adsorbents have been developed for CO2 capture, including zeolites,2 metal oxides,3 hydrotalcites,4 organic–inorganic hybrids5 and metal–organic frameworks,6 carbon nanomaterials (CNMs).7 Among them, CNMs are unique due to their high surface area, porosity, low density, chemical stability, fast adsorption–desorption kinetics and ease of functionalization, which render them as suitable materials for gas adsorption related applications. The structure of adsorbed CO2 along with its thermodynamic parameters has been determined, where it is reported that CO2 molecules find grooves as suitable sites for accommodation.8Graphene, the two dimensional lattice of carbon atoms with one atom thickness, has gained immense importance in adsorption related applications due to its unique properties among carbon nanomaterials. The role of graphene oxide in the carbon dioxide adsorption properties of chitosan based aerogel has been reported, which reveals that the capacity is doubled upon 20% addition of graphene oxide due to the improved specific surface area.9 Moreover, it is reported that the hydrogen assisted exfoliation-co-reduction of graphite oxide results in highly wrinkled (groove-like structure) graphene, which are high affinity sites for anchoring the CO2 molecules.8 Ghosh et al. have reported the CO2 adsorption on graphene viz. up to 35 wt% at ∼100 kPa and 195 K sample temperature.10 Theoretical investigations of CO2 adsorption properties of graphene show that the adsorption energy of physisorption is in the range of 8.8–13.8 kJ mol−1, while that of chemisorption lies in the range of 297.9–301.7 kJ mol−1, depending on the orientation of the CO2 molecule.11 The adsorption properties depend on the lateral surface properties (specific surface area and porosity) and surface anchoring sites. Task-specific functionalization with suitable moiety is expected to tune the adsorption properties of graphene.12
On the other hand, ionic liquids (ILs) are identified as good CO2 capture medium due to their high solubility and rapid CO2 uptake.13 The advantages of IL include its low vapour pressure, high thermal stability, wide liquid range and good chemical stability.14
It is found that the solubility of CO2 in ionic liquids can be enhanced through supporting ILs with solid substrate.15,16 The atomistic simulations studies on CO2 sorption properties of IL incorporated CNTs show that CO2 solubility is good when it is confined due to a favorable negative transferring energy, but is poor in the absence of confinement due to a very large, unfavorable positive transferring energy.17
Thus, integration of ionic liquids with CNMs has potential advantages in a wide range of applications, including CO2 adsorption. Our previous study demonstrates the enhancement in CO2 adsorption capacity upon IL functionalization, which shows a doubled performance in comparison with pure graphene at high pressures. Here, IL functionalization results in a uniform distribution of anchoring sites on graphene to hold the CO2 molecules. Moreover, the isosteric heat of adsorption was found to be in the physisorption range.18 It is reported that the polymerized ionic liquids (PIL) have even higher CO2 sorption capacity, along with faster sorption–desorption rates, than monomers.19,20 These polymers are porous in nature, which enable a high sorption capacity. Hence, PIL functionalization of graphene is expected to enhance the CO2 adsorption capacity better than the IL monomers.
In this study, we have experimentally determined the low pressure (<100 kPa) CO2 adsorption properties of PIL functionalized graphene and compared the results with those of graphene and IL monomer functionalized graphene. To the best of our knowledge, this is the first study on the low pressure CO2 adsorption properties of PIL or IL functionalized graphene.
2. Experimental section
2.1 Material synthesis
2.2 Characterization techniques
An X'Pert Pro PANalytical powder X-ray diffractometer was employed for structural analysis. The surface morphology of carbon nanomaterials was determined by transmission electron microscopy (TEM) using Technai G-20. A Perkin-Elmer FTIR spectrometer was used for vibrational characteristics of the synthesized materials. Surface area and porosity of CNMs were analysed by Micromeritics ASAP 2020 V3.00H surface area analyzer. Carbon dioxide adsorption properties of CNMs were determined at various temperatures by the same analyzer equipped with a water bath.2.3 Analysis procedure
Low pressure carbon dioxide adsorption–desorption studies were carried out by a pressure reduction technique (see ESI 2† for more information), and the number of moles of adsorbed gas was calculated using van der Waals equation, | (1) |
At pressures far lower than saturation pressures (around 100 kPa), it can be presumed that each adsorption site holds only one adsorbate molecule and can be modelled by two well-known models, known as Langmuir and Freundlich adsorption isotherm models. In the Langmuir model, adsorption sites are homogeneous with equal energy, while they are heterogeneous with well distributed adsorption energies in the Freundlich model.
The Langmuir isotherm is represented as,
 | (2) |
On the other hand, the Freundlich equation is,
 | (3) |
The isosteric heat of adsorption was calculated from the slope of adsorption isosteres (regression lines of ln (pressure) vs. 1/T at constant adsorbed amount, known as the van't Hoff plot), using the Clausius Clapeyron equation,
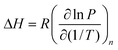 | (4) |
3. Results and discussions
3.1 Morphological analysis
The TEM image (Fig. 1(a)) of HEG shows the wrinkled nature of few layered graphene sheets, where the transparency confirms the few-layered nature of HEG. The rapid removal of functional groups from the hexagonal lattice results in exfoliation, which introduces a large disorder in the graphite structure and produces highly wrinkled sheets.22,23 The wrinkles are produced by the structural defects, such as heptagons and pentagons, which have significant advantages in CO2 adsorption.28 Here, structural defects and residual functional groups can act as anchoring sites by interacting with CO2 molecules.29 The highly wrinkled structure offers large amount of grooves, which may facilitate a high CO2 adsorption capacity.8 | ||
| Fig. 1 TEM images of (a) HEG, (b) HEG–IL (inset: magnified portion of HEG–IL) and (c) HEG–PIL (inset: magnified portion of HEG–PIL). | ||
The change in surface morphology of HEG after IL functionalization is clearly seen in Fig. 1(b), while the inset in Fig. 1(b) shows the presence of ionic liquid on the surface and inside the channel, formed by the wrinkled morphology. It is difficult to record the IL on HEG surface, since it is distributed uniformly over the surface. Molecular vibrational spectrum can provide persuasive evidence to confirm the presence of IL moieties on the HEG surface. Fig. 1(c) shows the presence of PIL on the surface of HEG. Since HEG was introduced in the free radical polymerization process, radicals are accumulated on the surface and grown as polymeric particles in order to have a low free energy. The inset in Fig. 1(c) reveals 50 nm of particle size with uniform distribution. Here, the structural defects (dangling bonds, heptagons and pentagons) and residual functional groups may act as anchoring sites, which interact with the ions of IL or PIL.29
3.2 Surface area and porosity analysis of HEG
The specific surface area (SSA) of HEG is determined from the N2 adsorption–desorption isotherm (Fig. 2), which shows a fast adsorbate intake at the low-pressure end, suggesting a high surface area and micropore volume. The isotherm follows type-IV characteristics of Brunauer–Emmett–Teller (BET) isotherms.30 Hence, the BET theory was used to determine SSA, while the Barrett, Joyner and Halenda (BJH) method31 was employed for porosity determination (inset in Fig. 2). The SSA of HEG has been found to be 344 m2 g−1, which is approximately 1/7 times of the theoretical surface area of single layer graphene (2630 m2 g−1). In other words, the few layered graphene has approximately 7 layers per stack, which is in good agreement with the literature.22,23 The large adsorbate retention in the desorption isotherm indicates a wide distribution of pores and large spatial surface area. The rapid removal of functional groups results in a highly wrinkled structure. The pore size distribution shows maxima at two distinct regions, viz. (i) at near microporous (<3 nm) due to the interlayer galleries and pinholes of graphene sheets and around 10–100 nm due to wrinkles and folded edges. The total pore volume and average pore width of HEG was calculated by BJH equation and was found to be 1.94 cm3 g−1and 25 nm, respectively. | ||
| Fig. 2 Nitrogen adsorption–desorption analysis of HEG. Inset: pore volume distribution as a function of pore width. | ||
However, IL or PIL functionalized HEG is expected to have a very low surface area and porosity, because IL moieties fill the pores and cover the surface of HEG upon functionalization. It is reported that ILs have good selectivity towards CO2 in a mixture of gases.20 Hence, the pores are inaccessible to nitrogen atoms, which cannot probe the surface area and porosity of HEG.
3.3 Molecular vibrational spectrum analysis
FTIR spectra of HEG, HEG–IL and HEG–PIL are shown in Fig. 3. The band corresponding to the stretching vibrations of hydroxyl group at ∼3450 cm−1 is due to the presence of moisture and surface hydroxyl functionalities, which commonly occurs in all the three materials. Anti-symmetric and symmetric stretching vibrations of![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 are located at 2925 and 2855 cm−1, which clearly suggest the presence of a large amount of residual
CH2 are located at 2925 and 2855 cm−1, which clearly suggest the presence of a large amount of residual ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 functional groups even after reduction in a hydrogen environment. The stretching vibration of aromatic rings, from the hexagonal honeycomb lattice, has been found at ∼1630 cm−1 in all the samples. The signals at fingerprint region (500–2000 cm−1) can be assigned to various stretching and bending modes of residual functional groups on HEG (such as
CH2 functional groups even after reduction in a hydrogen environment. The stretching vibration of aromatic rings, from the hexagonal honeycomb lattice, has been found at ∼1630 cm−1 in all the samples. The signals at fingerprint region (500–2000 cm−1) can be assigned to various stretching and bending modes of residual functional groups on HEG (such as ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, –OH, –COOH, and >C
CH2, –OH, –COOH, and >C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O). These residual functional groups are anticipated to have a strong influence on the CO2 adsorption properties of HEG.28,32
O). These residual functional groups are anticipated to have a strong influence on the CO2 adsorption properties of HEG.28,32
The presence of IL or PIL moieties on the surface of HEG has been confirmed by FTIR spectroscopy (Fig. 3). The band corresponding to imidazolium ring in-plane bending vibrations (1560 cm−1) is present for both HEG–IL and HEG–PIL samples. Furthermore, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching (1743 cm−1) has been strengthened upon IL or PIL functionalization. This, along with the peak at 1560 cm−1, substantially screens the aromatic ring stretching vibrations of graphene (∼1630 cm−1). The peak at 1110 cm−1 can be attributed to the C–C stretching vibrations, which occurs in all the studied materials. Upon IL or PIL functionalization, a bunch of signals arises between 1100 and 1250 cm−1, which can be assigned to the C–N stretching vibrations from the imidazolium ring. Various modes of 1,3-disubstituted ring vibrations are also found at the low frequency region (around 750 cm−1).33,34
N stretching (1743 cm−1) has been strengthened upon IL or PIL functionalization. This, along with the peak at 1560 cm−1, substantially screens the aromatic ring stretching vibrations of graphene (∼1630 cm−1). The peak at 1110 cm−1 can be attributed to the C–C stretching vibrations, which occurs in all the studied materials. Upon IL or PIL functionalization, a bunch of signals arises between 1100 and 1250 cm−1, which can be assigned to the C–N stretching vibrations from the imidazolium ring. Various modes of 1,3-disubstituted ring vibrations are also found at the low frequency region (around 750 cm−1).33,34
Further, the presence of IL or PIL moieties can be confirmed by Raman spectrum analysis (Fig. 4). Raman spectrum of HEG shows the D-band (1352 cm−1) and G-band (1590 cm−1) with ID/IG value close to unity. The G-band corresponds to the tangential modes (E2g) of vibrations, and the D-band corresponds to the presence of defects in HEG. The Raman spectrum of HEG–IL shows the characteristic C–H stretching vibrational modes around 2850 cm−1 from the butyl chain of [BMIM] cation. The merged Raman band observed at 1470 cm−1 may be assigned to imidazolium ring in-plane anti-symmetric stretching. In HEG–PIL, a strong signal has been found at 909 cm−1 corresponding to the CH2 out-of-plane wag from the vinyl group of PIL. These observations, along with FTIR analysis, confirm the presence of IL or PIL moieties.
4. Carbon dioxide adsorption analysis
4.1 Adsorption isotherm studies
Carbon dioxide adsorption–desorption properties of adsorbents (HEG, HEG–IL and HEG–PIL) were determined at low pressures (<100 kPa) at multiple temperatures. The equilibrium adsorbed amount has been calculated from equilibrium pressure using eqn (1).The adsorption isotherms of HEG (Fig. 5(a)) show 695 μmol g−1 adsorption capacity at ∼100 kPa and 283 K sample temperature, while that at 298 K is 512 μmol g−1. This is almost 30% higher than that of the reported values for graphene at nearly similar conditions (395 μmol g−1 STP).35 The highly wrinkled morphology, high surface area (344 m2 g−1) and pore volume (1.94 cm3 g−1) are the main factors for the high adsorption capacity. It is reported that the structural defects trap excess electrons around the defect site, which produces localized LUMO in the carbon lattice and may improve the interaction between CO2 and the graphene surface.36 The high porosity of graphene is advantageous for CO2 (molecular size 0.33 nm) adsorption. The wrinkled structure results in a large amount of slit like sites, which are known to be high potential adsorption sites for the CO2 molecules. Moreover, the residual functional groups also can contribute to the adsorption.
 | ||
| Fig. 5 CO2 adsorption–desorption isotherms of (a) HEG, (b) HEG–IL and (c) HEG–PIL at different sample temperatures. | ||
The adsorption capacity is slightly improved upon IL functionalization (Fig. 5(b)). This must be attributed to the better interaction of surface IL moieties with the CO2 molecules. It has been extensively reported that CO2 has good solubility in ionic liquids due to the interaction with the functional groups present in the anions and/or cations.37,38 However, the improvement is significantly small (∼2%) for consideration in the present study. It is widely documented in the literature that IL ions produce short range ordering on the graphitic surfaces, which reduces the CO2 storage capacity. Particularly, hydrophobic ionic liquids exhibit this phenomenon with a wide variety of substrates.39 Although the IL ([BMIM][BF4]) used in this study is hydrophilic in nature, it is expected to make an ordered structure in a moisture free (vacuum) atmosphere.
It is suggested by Tang et al. that simply developing the ionic liquids into polymeric forms can significantly increase the CO2 sorption capacity.40 The adsorption–desorption isotherms of PIL functionalized HEG are presented in (Fig. 5(c)). At 100 kPa equilibrium pressure, HEG–PIL adsorbs 794, 718, 678 and 618 μmol g−1 at 283, 288, 293 and 298 K, respectively. These are 14–22% higher values than those of pure HEG and 12–18% than those of HEG–IL. Here, PIL produces porous nanoparticles on the surface of graphene unlike its monomer. This effectively increases the amount of adsorption sites in the adsorbent.
It is interesting to notice that PIL with a similar molecular structure shows only 2 mol% (∼70 μmol g−1) of adsorption at nearly identical conditions.40,41 However, these functional moieties show higher adsorption in their supported nano-cluster forms. This may be imputed to the synergic effect, where the support improves the accessible anchoring sites in PIL.
Adsorption of CO2 on pure and IL or PIL functionalized graphene is modeled by both Langmuir and Freundlich models using eqn (2) and (3), and the parameters are given in Table 1. In general, the adsorption in the present case is more close to the Freundlich model, suggesting a distribution of the surface adsorption sites. However, the Langmuir model also fits better to a certain extent, viz. R2 is ∼0.99 except HEG at 283 K. This may be due to the narrow adsorption energy distribution. The surface is not functionalized with strongly interacting moieties, such as amines. Hence, the difference between the adsorption energy of lateral sites and IL or PIL moieties is not very high. Supporting this discussion, the fitting parameter of Langmuir equation shows that the maximum adsorption capacity reduces with PIL functionalization, which is contrary to the fact. But, the Langmuir coefficient KL clearly shows that the initial adsorption rate is significantly improved due to PIL functionalization. In addition, KL and Qmax values decrease with an increase in temperature, indicating that there is no competing temperature assisted chemical reaction between CO2 and the functional moieties.42
| Material | T (K) | Q eq | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|---|
| Q max | K L | R 2 | n | K F | R 2 | |||
| a Units: Qeq@100 kPa (μmol g−1), Qmax (μmol g−1), KL (kPa−1), n (A.U.) and KF (μmol g−1 kPa−(1/n)). | ||||||||
| HEG | 283 | 695 | 1390 | 0.0095 | 0.941 | 1.47 | 30.93 | 0.999 |
| 288 | 609 | 1241 | 0.0094 | 0.992 | 1.47 | 27.64 | 0.999 | |
| 293 | 558 | 1171 | 0.0089 | 0.993 | 1.44 | 23.64 | 0.999 | |
| 298 | 512 | 1098 | 0.0082 | 0.995 | 1.42 | 20.05 | 0.999 | |
| HEG–IL | 283 | 710 | 1230 | 0.0126 | 0.989 | 1.61 | 40.68 | 0.999 |
| 288 | 640 | 1097 | 0.0133 | 0.989 | 1.62 | 37.97 | 0.999 | |
| 293 | 591 | 1036 | 0.0126 | 0.991 | 1.59 | 33.42 | 0.999 | |
| 298 | 521 | 945 | 0.0119 | 0.992 | 1.55 | 27.71 | 0.999 | |
| HEG–PIL | 283 | 794 | 1052 | 0.0259 | 0.983 | 2.03 | 82.52 | 0.999 |
| 288 | 718 | 954 | 0.0264 | 0.984 | 2.01 | 74.30 | 0.999 | |
| 293 | 678 | 930 | 0.0238 | 0.987 | 1.94 | 64.18 | 0.999 | |
| 298 | 618 | 859 | 0.0231 | 0.987 | 1.90 | 56.62 | 0.999 | |
Adsorption occurs when the interaction between the molecular orbital of adsorbate and adsorbent surface reaches the lowest energy configuration (i.e., the most stable state). Furuya et al. have reported that the electron density of both adsorbates and adsorbents is one of the major factors determining the adsorption properties.43 Interestingly, the Langmuir constant n is significantly improved by PIL functionalization, which shows the favorability of adsorption on the HEG–PIL surface. This can be attributed to the presence of lone-pair electrons, which are present in the imidazolium rings with a large positive partial charge, in the nitrogen atoms that physically interact with the CO2 molecules.44 In addition, the fluorine atoms in the tetrafluoroborate anion also influences significantly.40
Freundlich coefficients n and KF, by means of KF/n, give information about the adsorption energy, i.e., total molecular orbital energy of the adsorbate and adsorbent system. HEG–PIL shows a considerably higher KF/n value than those of HEG and HEG–IL, suggesting a higher interaction energy between CO2 and the PIL moiety.
The desorption isotherms show hysteresis for all samples, which can be assigned to the high energy adsorption sites. This hysteresis has been quantified by the quantity “isothermal adsorbate retention (IAR)”, which is defined as the amount of CO2 retained when isothermally desorbed at the same pressure. Mathematically, it is represented as
| IAR = Qdes − Qads | (5) |
Fig. 6 shows the IAR of HEG, HEG–IL and HEG–PIL at multiple temperatures. The isothermal adsorbate retentions of 45 and 36 μmol g−1 were obtained for HEG at 50 kPa desorption pressure and 283 and 298 K, respectively. However, these values were increased to 63.1 and 53.1 μmol g−1 upon functionalization with IL monomers, respectively, due to the high energy interaction with IL moieties. The IAR values at 50 kPa desorption pressure and 283 and 298 K sample temperature were increased to 77.6 and 63.8 μmol g−1 with the HEG–PIL adsorbent, respectively. Here, the porous nature of PIL offers a large amount of accessible high energy sites for adsorption. The IAR values of all adsorbents fall at a very low pressure (<10 kPa) with a very low residue (typically <1%), suggesting good recoverability of the adsorbent. The onset pressure of the IAR drop has been calculated from the intersection of extrapolated straight line fit of IAR-raise and IAR-fall regions. The approximate values of onset pressure of IAR at 283 K sample temperature are 5, 11 and 10 kPa for HEG, HEG–IL and HEG–PIL, respectively. This strengthens the claim that the IL or PIL functionalization increases the number of surface adsorption sites with a considerably wider adsorption energy distribution. Furthermore, the behavior of IAR suggests that IL or PIL sites hold CO2 reversibly through physisorption.
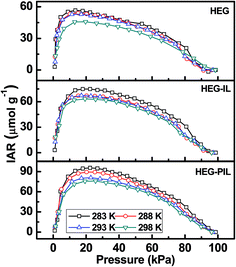 | ||
| Fig. 6 Isothermal adsorbate retention of HEG, HEG–IL and HEG–PIL as a function of equilibrium pressure. | ||
4.2 Thermodynamic parameters of adsorption
The adsorption isosteres (regression lines of ln (pressure) vs.1/T at a constant adsorbed amount of 100–488 μmol g−1) for all adsorbents have been presented in Fig. 7 (van't Hoff plot). All isosteres follow Arrhenius relation with a negative slope, suggesting the temperature-dependent exothermic adsorption (physisorption), which decreases with an increasing temperature. | ||
| Fig. 7 CO2 adsorption isosteres (van't Hoff plot) of HEG, HEG–IL and HEG–PIL with different adsorbed amounts. | ||
The strength of adsorbate–adsorbent interaction is represented by the difference between the activation energies for adsorption and desorption, known as isosteric heat of adsorption (|ΔH|), which is calculated from the slope of adsorption isosteres at multiple adsorbed amounts, using eqn (4).
The value of heat of adsorption of CO2 on HEG was found to be 18.8 ± 0.9 kJ mol−1 at 488 μmol g−1 of the adsorbed amount. This is in good agreement with the existing literature.11 This value is increased to 21 ± 1 and 23.5 ± 0.8 kJ mol−1 upon IL and PIL functionalization, respectively. As suggested by the Freundlich isotherm analysis, the total molecular orbital energy of the adsorbate and adsorbent system has been increased by the relatively energetic interaction between CO2 and nitrogen (particularly N3) sites on the imidazolium cation and fluorine atoms in the tetrafluoroborate anion.44 Although |ΔH| values are increased upon IL or PIL functionalization, they still remain in the physisorption range (<40 kJ mol−1), suggesting the ease of desorption.
Fig. 8 shows the variation in the heat of adsorption upon surface coverage, which is determined from the corresponding adsorption isosteres. The behavior of |ΔH| of HEG–IL with surface coverage is similar to that of HEG throughout the studied range. This behavior along with the R2 value of the Langmuir isotherm fit suggests the uniform surface coverage with IL moieties. Briefly, IL makes a thin layer on the graphene surface upon functionalization. The layer may arrange into short range solid-like layers in a moisture free atmosphere.39 This may be the reason for a very low improvement (∼2%) in the adsorption capacity even after IL functionalization, where the IL screens the lattice adsorption sites of graphene (structural defects and functional groups). Here, CO2 molecules are mainly anchored by the IL moieties on the surface.
 | ||
| Fig. 8 Comparison of isosteric heats of adsorption of HEG, HEG–IL and HEG–PIL as a function of the adsorbed amount. | ||
However, PIL moieties make a distribution of porous polymer nanoparticles on the surface, which allows CO2 molecules to access the lattice adsorption sites. This leads to a large amount of accessible adsorption sites with distributed adsorption energy. The variation in the heat of adsorption upon surface coverage also behaves differently, compared to those of HEG and HEG–IL. Initial surface coverage did not show a considerable change in |ΔH|, as observed for HEG and HEG–IL, due to the available unsaturated high energy sites.
The entropy change resulted by the adsorption of CO2 (−ΔS) were calculated from the van't Hoff plot, where the intercepts with ln P-axis give ΔS/R value at different adsorbed amounts. Fig. 9 shows the variation in entropy change upon surface coverage, which is quite consistent over the range of the adsorbed amount for all the three adsorbents. Generally, the change in entropy of a system does not change with surface coverage for non-uniformly distributed adsorption sites. The surfaces of all the three adsorbents are heterogeneous, as discussed in the isotherm studies. The heterogeneity is introduced by structural defects (wrinkles and edges), residual functional groups and IL or PIL moieties. The average ΔS values are found to be −175, −108.5 and −128.6 J mol−1 K−1 for HEG, HEG–IL and HEG–PIL, respectively.
 | ||
| Fig. 9 Comparison of entropy change in the adsorption of CO2 on HEG, HEG–IL and HEG–PIL as a function of the adsorbed amount. | ||
5. Conclusions
The carbon dioxide adsorption–desorption analysis of IL and PIL functionalized graphene has been carried out at low pressures (<100 kPa). The adsorption capacities of HEG, HEG–IL and HEG–PIL are 695, 710 and 794 μmol g−1 at 283 K sample temperature. The Langmuir coefficient suggests that PIL functionalization offers more favorability of adsorption with high adsorption energy. The isothermal adsorbate retention is increased upon IL or PIL functionalization; however, the onset pressure of IAR fall is reduced slightly. The isosteric heat of adsorption is in the physisorption range, even after IL or PIL functionalization, suggesting no competing chemical interaction. It is found that the surface of graphene was uniformly covered with IL, which screens the lattice adsorption sites. However, PIL resides as highly distributed porous nanoparticles on the surface, which enables a high adsorption capacity than that of the IL functionalized graphene. The change in the entropy of adsorption is independent of surface coverage, suggesting the reversible adsorption of CO2 on the non-uniformly distributed adsorption sites. This preferential uptake makes HEG–PIL suitable for the adsorption of atmospheric CO2 and the separation from gaseous mixtures. Further improvement can be achieved by the careful selection of ionic liquid monomers with amine-rich anions and cations.Acknowledgements
Authors acknowledge SAIF-IITM for FTIR analysis. One of the authors (Tamilarasan) acknowledges Indian Institute of Technology Madras (IITM) for the financial support (senior research fellowship).References
- S. D. Kenarsari, D. Yang, G. Jiang, S. Zhang, J. Wang, A. G. Russell, Q. Wei and M. Fan, RSC Adv., 2013, 3, 22739–22773 RSC.
- T.-H. Bae, M. R. Hudson, J. A. Mason, W. L. Queen, J. J. Dutton, K. Sumida, K. J. Micklash, S. S. Kaye, C. M. Brown and J. R. Long, Energy Environ. Sci., 2013, 6, 128–138 CAS.
- F.-Q. Liu, W.-H. Li, B.-C. Liu and R.-X. Li, J. Mater. Chem. A, 2013, 1, 8037–8044 CAS.
- Y. Gao, Z. Zhang, J. Wu, X. Yi, A. Zheng, A. Umar, D. O'Hare and Q. Wang, J. Mater. Chem. A, 2013, 1, 12782–12790 CAS.
- P. Tamilarasan and S. Ramaprabhu, Int. J. Greenhouse Gas Control, 2012, 10, 486–493 CrossRef CAS.
- Z. Zhang, Z.-Z. Yao, S. Xiang and B. Chen, Energy Environ. Sci., 2014, 7, 2868–2899 CAS.
- J. Wang, I. Senkovska, M. Oschatz, M. R. Lohe, L. Borchardt, A. Heerwig, Q. Liu and S. Kaskel, J. Mater. Chem. A, 2013, 1, 10951–10961 CAS.
- M. Bienfait, P. Zeppenfeld, N. Dupont-Pavlovsky, M. Muris, M. R. Johnson, T. Wilson, M. DePies and O. E. Vilches, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 035410 CrossRef.
- A. A. Alhwaige, T. Agag, H. Ishida and S. Qutubuddin, RSC Adv., 2013, 3, 16011–16020 RSC.
- A. Ghosh, K. S. Subrahmanyam, K. S. Krishna, S. Datta, A. Govindaraj, S. K. Pati and C. N. R. Rao, J. Phys. Chem. C, 2008, 112, 15704–15707 CAS.
- K.-J. Lee and S.-J. Kim, Bull. Korean Chem. Soc., 2013, 34, 3022–3026 CrossRef CAS.
- A. K. Mishra and S. Ramaprabhu, J. Mater. Chem., 2012, 22, 3708–3712 RSC.
- X. Zhang, X. Zhang, H. Dong, Z. Zhao, S. Zhang and Y. Huang, Energy Environ. Sci., 2012, 5, 6668–6681 CAS.
- M. Freemantle, An Introduction to Ionic Liquids, The Royal Society of Chemistry, Cambridge, UK, 2010 Search PubMed.
- X. Wang, N. G. Akhmedov, Y. Duan, D. Luebke and B. Li, J. Mater. Chem. A, 2013, 1, 2978–2982 CAS.
- K. M. Gupta, Y. Chen, Z. Hu and J. Jiang, Phys. Chem. Chem. Phys., 2012, 14, 5785–5794 RSC.
- W. Shi and D. C. Sorescu, J. Phys. Chem. B, 2010, 114, 15029–15041 CrossRef CAS PubMed.
- P. Tamilarasan, T. S. Remya and S. Ramaprabhu, Graphene, 2013, 1, 3–10 CrossRef.
- S. Supasitmongkol and P. Styring, Energy Environ. Sci., 2010, 3, 1961–1972 CAS.
- B. J. Adzima, S. R. Venna, S. S. Klara, H. He, M. Zhong, D. R. Luebke, M. S. Mauter, K. Matyjaszewski and H. B. Nulwala, J. Mater. Chem. A, 2014, 2, 7967–7972 CAS.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- A. Kaniyoor, T. T. Baby and S. Ramaprabhu, J. Mater. Chem., 2010, 20, 8467–8469 RSC.
- A. Kaniyoor, T. T. Baby, T. Arockiadoss, N. Rajalakshmi and S. Ramaprabhu, J. Phys. Chem. C, 2011, 115, 17660–17669 CAS.
- B. Wu, D. Hu, Y. Kuang, B. Liu, X. Zhang and J. Chen, Angew. Chem., Int. Ed., 2009, 48, 4751–4754 CrossRef CAS PubMed.
- I. Langmuir, J. Am. Chem. Soc., 1916, 38, 2221–2295 CrossRef CAS.
- H. Freundlich, Kapillarchemie: eine Darstellung der Chemie der Kolloide und verwandter Gebiete, Akademische verlagsgesellschaft mbh, 1922 Search PubMed.
- W. Gao, D. Butler and D. L. Tomasko, Langmuir, 2004, 20, 8083–8089 CrossRef CAS PubMed.
- D. Dutta, B. C. Wood, S. Y. Bhide, K. G. Ayappa and S. Narasimhan, J. Phys. Chem. C, 2014, 118, 7741–7750 CAS.
- C. Xu, X. Wang, J. Zhu, X. Yang and L. Lu, J. Mater. Chem., 2008, 18, 5625–5629 RSC.
- S. Brunauer, P. H. Emmett and E. Teller, J. Am. Chem. Soc., 1938, 60, 309–319 CrossRef CAS.
- E. P. Barrett, L. G. Joyner and P. P. Halenda, J. Am. Chem. Soc., 1951, 73, 373–380 CrossRef CAS.
- P. Cabrera-Sanfelix, J. Phys. Chem. A, 2008, 113, 493–498 CrossRef PubMed.
- N. B. Colthup, J. Opt. Soc. Am., 1950, 40, 397–400 CrossRef CAS.
- Y. Jeon, J. Sung, C. Seo, H. Lim, H. Cheong, M. Kang, B. Moon, Y. Ouchi and D. Kim, J. Phys. Chem. B, 2008, 112, 4735–4740 CrossRef CAS PubMed.
- M. E. Casco, A. Morelos-Gómez, S. M. Vega-Díaz, R. Cruz-Silva, F. Tristán-López, H. Muramatsu, T. Hayashi, M. Martínez-Escandell, M. Terrones, M. Endo, F. Rodríguez-Reinoso and J. Silvestre-Albero, Journal of CO2 Utilization, 2014, 5, 60–65 CrossRef CAS.
- H. Tachikawa and H. Kawabata, J. Phys. Chem. C, 2009, 113, 7603–7609 CAS.
- J.-J. Chen, W.-W. Li, X.-L. Li and H.-Q. Yu, Phys. Chem. Chem. Phys., 2012, 14, 4589–4596 RSC.
- B. E. Gurkan, J. C. de la Fuente, E. M. Mindrup, L. E. Ficke, B. F. Goodrich, E. A. Price, W. F. Schneider and J. F. Brennecke, J. Am. Chem. Soc., 2010, 132, 2116–2117 CrossRef CAS PubMed.
- S. Bovio, A. Podestà, C. Lenardi and P. Milani, J. Phys. Chem. B, 2009, 113, 6600–6603 CrossRef CAS PubMed.
- J. Tang, H. Tang, W. Sun, H. Plancher, M. Radosz and Y. Shen, Chem. Commun., 2005, 3325–3327 CAS.
- J. Tang, H. Tang, W. Sun, M. Radosz and Y. Shen, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 5477–5489 CrossRef CAS.
- Y. Otake, N. Kalili, T. H. Chang and E. Furuya, Sep. Purif. Technol., 2004, 39, 67–72 CrossRef CAS.
- E. G. Furuya, H. T. Chang, Y. Miura and K. E. Noll, Sep. Purif. Technol., 1997, 11, 69–78 CrossRef CAS.
- C. Cadena, J. L. Anthony, J. K. Shah, T. I. Morrow, J. F. Brennecke and E. J. Maginn, J. Am. Chem. Soc., 2004, 126, 5300–5308 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ta04808c |
| This journal is © The Royal Society of Chemistry 2015 |


