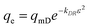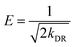Polyvinylamine modified polyester fibers – innovative textiles for the removal of chromate from contaminated groundwater†
Received
14th August 2014
, Accepted 5th November 2014
First published on 5th November 2014
Abstract
The soil and groundwater of many old industrial areas are polluted by different environmental hazards. Because of their high toxicity and carcinogenic potential, chromate contaminations are especially problematic and a complete cleanup of such areas is necessary to avoid fatal environmental and sanitary impacts. Conventionally, decontamination is carried out by the removal of the soil and a long-term filtration of groundwater with various chromate-adsorbing materials over a period of many years. Efficient, reusable and cheap adsorbing materials, however, are still missing. Here, we present a new, cheap and reusable chromate-adsorbing substrate based on polyvinylamine-coated polyester fibers. The surface modification of the fibrous material is realized by common methods in textile finishing yielding a durable, high-performing and reusable adsorbent for water-dissolved chromate. The functionalized nonwoven fabric has a high binding capacity for chromate and the chromate concentration of highly polluted waters (with concentrations around 50 mg L−1) can easily be decreased below the limit of 50 μg L−1 recommended by the WHO. Moreover, the material is reusable after regeneration under aqueous alkaline conditions. The adsorption properties at different pH values were determined with different adsorption models. In addition, adsorption kinetics were evaluated using artificial and real life chromate-contaminated water samples.
1. Introduction
Since the 19th century, chromate has been a widely used metal in tannery, electroplating and dyestuff production. With the development of stainless and heat-resistant steel in the early 20th century, the importance of this metal has further grown rapidly. In past times when the legal environmental regulations/provisions did not conform to modern standards, these production processes caused the pollution of soil and groundwater with different toxins and often a high level of chromate in soil (0.65 and 25.9 g m−3).1–3 For example, in an old chromium plating ground in Düsseldorf (Germany) 11 g kg−1 of chromate in soil were found, resulting in 30 mg L−1 in groundwater.4 Chromate has a high environmental impact because of its good water solubility and high mobility in soil, which results in groundwater contamination. In humans and animals, chromate causes acute poisoning and cancer even at low concentrations.5 Moreover, teratogen and mutagenic effects were found in several studies, although the data are not complete. For this reason and in accordance with the WHO recommendations,6 in most Western countries the legal limit of total chromium (Cr(III) and Cr(VI)) in drinking water amounts to 50 μg L−1.
Once contaminated, the affected areas have to be cleaned up by costly and time-consuming procedures. Depending on the degree of chromate contamination, this environmental remediation typically comprises the relocation of polluted soil to specialized landfills and a long-term adsorptive filtration of the groundwater that spans 10 to 30 years. For the efficient removal of chromate, however, cheaper and more effective adsorbents than the commonly used ion exchange and active charcoal filters are still missing. As possible alternatives, many studies have demonstrated the use of various minerals and natural substances for this purpose, such as clarified sludge (steel industry derived sludge), rice husk ash, activated alumina, fuller's earth, neem bark, chitosan, and different clays, e.g. bentonite or montmorillonite.7–14 Nevertheless, their reusability was not investigated in these studies.
Here, we present an alternative fiber-based chromate-adsorbing filter system. Woven and nonwoven textiles that consist of various fiber-forming polymers are commonly applied as filter materials. Especially polyester fibers (PET) are broadly used, because they have the advantage of good workability and low price. The filtration properties are easily controllable by the fiber diameter, the mesh size and the absolute material thickness.
On the other hand, polyvinylamine (PVAm) and other polyelectrolytes are already used as flocculants15–17 in different areas, e.g., participation/removal of heavy metals and floating particles from sewage water. Other applications are the surface modification of paper18–20 and textiles.21 Here, we use immobilized PVAm to form pH-labile complexes with metal oxoanions like chromate, by permanent immobilization of the polyelectrolyte on a polyester nonwoven. These metal-adsorbing properties of polyelectrolytes have also been exploited by other groups for a variety of different applications.7,11,12,22–27 However, until now, the binding of metal oxoanions by PVAm and the selectivity for this process have not been reported. Gao et al.28 have shown that PVAm-grafted microspheres are capable of adsorbing chromate, without reporting other properties of the system, whereas the use of chitosan, a polyamine biopolymer, for this purpose was reported by different groups.7,9,13 Recently Ferrero et al.29 showed the application of chitosan-modified cotton fabric for the adsorption of chromate and copper. Accordingly, the application of PVAm modified PET to generate a smart textile filter material with a chemisorbing surface is easily conceivable. The practicality of the basic concept, i.e. metal binding by textile fibers modified with PVAm, has already been demonstrated by Meilikhov et al.30 in the context of metal–organic frameworks.
In previous studies21 we have developed an easy thermal process for the functionalization of polyester fibers with PVAm, which can be performed using common textile machinery on an industrial scale. The process is demonstrated schematically in Fig. 1A and B.
 |
| | Fig. 1 Immobilization of PVAm to PET and the proposed chemisorption mechanism. (A) PVAm in solution on the PET surface. (B) Bound PVAm and amide bonds after drying and heating the PVAm solution on the fabric. (C) Formation of the ammonium groups at low pH values, and (D) Coulomb interaction of positive charged ammonium groups with chromate anions; chemisorption of chromate on PVAm modified PET fibers. | |
In this study, we report the preparation and chromate adsorption behavior of PVAm modified PET textile filter materials (Fig. 1). Investigations were carried out using model chromate solutions as well as a real-life sample of highly polluted groundwater (56 mg L−1) from a contaminated industrial area, where galvanic chrome plating was carried out over a period of several decades.
2. Experimental
2.1. PET surface modification with PVAm and characterization
PET nonwoven filter material with a surface weight of 109 g m−2, a thickness of 1.07 mm and an average fiber diameter of 15 μm and a specific surface of about 0.19 m2 g−1 (calculated by the fiber diameter) was donated by TAG Carpets & Composites (Krefeld, Germany) and was extracted using a Soxhlet apparatus with ethanol–water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 8 h) and petroleum ether (8 h) to remove production related finishes. PVAm (Lupamin® 9095) was donated by BASF (Ludwigshafen, Germany). The solution contains 10–15 wt% PVAm with an average molecular weight of 340
1 v/v, 8 h) and petroleum ether (8 h) to remove production related finishes. PVAm (Lupamin® 9095) was donated by BASF (Ludwigshafen, Germany). The solution contains 10–15 wt% PVAm with an average molecular weight of 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a degree of hydrolysis >90%. All other chemicals were of analytical grade.
000 g mol−1 and a degree of hydrolysis >90%. All other chemicals were of analytical grade.
The PET material was wetted with PVAm solution of a pH value of 3, 7 and 11 (adjusted with HCl and NaOH), pre-dried for 30 min at 80 °C and afterwards thermally fixed for 15 min at 130 °C. To remove unbound PVAm, the material was extracted with water–ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) for 24 h. The amount of immobilized PVAm loading was determined gravimetrically (weight/weight% (w/w)) and the amount of nitrogen from PVAm by Kjeldahl analysis in μmol g−1. The limit of detection for nitrogen is 10 μmol g−1. The results of a triple measurement are shown in Table 1. The fabrics are weighed after one day in a standardized climate chamber (20 ± 2 °C and 65 ± 4% humidity). SEM images are given in the ESI.†
1 v/v) for 24 h. The amount of immobilized PVAm loading was determined gravimetrically (weight/weight% (w/w)) and the amount of nitrogen from PVAm by Kjeldahl analysis in μmol g−1. The limit of detection for nitrogen is 10 μmol g−1. The results of a triple measurement are shown in Table 1. The fabrics are weighed after one day in a standardized climate chamber (20 ± 2 °C and 65 ± 4% humidity). SEM images are given in the ESI.†
Table 1 Results of the PVAm loading on a PET fabric at a specific pH value
| pH |
PVAm loading [% (w/w)] |
Nitrogen [μmol g−1] |
| 3 |
0.6 ± 0.1 |
52.2 ± 14.3 |
| 7 |
6.2 ± 0.6 |
417.5 ± 59.3 |
| 11 |
4.0 ± 0.5 |
116.4 ± 9.5 |
2.2. Measurement of chromate concentration
The measurements of the chromate concentration were carried out using two different methods. First, the concentration was measured using a Varian 720-ES ICP/OES (DE-Darmstadt) calibrated with a Merck multi-element standard solution VIII and a detection limit of 40 μg L−1. For digestion of the fabrics a microwave by CEM MarsXpress (DE-Kamp-Lintfort) was used. To 200 mg of the fabric 8 mL of HNO3 (65%) was added and heated up to 180 °C, afterwards the solution was diluted to 25 mL and measured by ICP/OES. Second, the time dependent measurements of chromate (kinetic and breakthrough curves) were performed with a UV/Vis Varian Cary 5E-Spectrometer (DE-Darmstadt) with a calibration from 0.5–100 mg L−1 at a wavelength of 370 nm.
2.3. Determination of the pH-dependent chromate adsorption properties
To determine the pH influence of the chromate adsorption, the different loaded PVAm modified PET nonwoven (PVAm@PET) was tested using chromate solutions (1 g L−1) of pH 3–13. Samples with a size of about 280 mg (5 × 5 cm) were put into 10 mL of the appropriate solution for 5 h. After removal from the chromate solution, the sample was washed with distilled water and dried, and the loading was determined by ICP/OES after microwave digestion. The loading q [mg g−1] was calculated according to eqn (1), with cI = concentration of chromate [mg L−1] in the solution after microwave digestion, V = volume [L] of the digestion solution and M = mass of the adsorbent [g].| |  | (1) |
2.4. Determination of the adsorption isotherms
The adsorption isotherms were determined for 4% PVAm@PET nonwoven fabrics in the concentration range of 1 mg L−1 to 5 g L−1 at a pH of 1, 3, 5 and 7. The modified fabric sample (280 mg, 5 × 5 cm) was stored for 1 h in 5 mL of a K2CrO4 solution before the chromate concentration was analyzed by ICP/OES.
2.5. Determination of the adsorption kinetics
250–3500 mg of PVAm@PET with 4.0% loading was immersed into a 23.2 mg L−1 chromate solution and the real-life sample with 56.6 mg L−1 chromate. A detailed analysis is given in ESI Table S1.† The change of the concentration over time was determined photometrically.
2.6. Determination of the desorption properties
About 280 mg of a fabric loaded with 0.8 mg g−1 chromate is added into 25 mL water in the pH range of 1–14. The chromate concentration of the solution was measured by ICP/OES after 2 h.
2.7. Breakthrough curves
Adsorption experiments were performed in a glass column packed with 50 g of the adsorbent (modified PET nonwoven with 4% PVAm loading) cut into pieces of about 1.5 × 1.5 cm. The column was filled with water, and then the real-life chromate containing groundwater sample (56.6 mg L−1, detailed analysis in ESI Table S1†) was dropped onto the fabric, so that it was permanently covered by the solution. The groundwater was pumped over the column with a flow rate of 10 mL min−1 through a UV-Vis flow cell and the concentration over time was measured at 370 nm. The column was regenerated by 100 mL NaOH (0.1 mol L−1) with a flow rate of 3.5 mL min−1, followed by washing with 50–250 mL of water with a flow rate of 10 mL min−1. On the addition of NaOH onto the column the adsorption at 370 nm raises until all chromate is eluted. In all steps the adsorbent was constantly covered by the solution. (Scheme Fig. 7B, a picture of the column setup and the column at different process stages is given in ESI Fig. S5 and the analysis of run one and two is given in Table S1.†)
3. Results and discussion
Polyvinylamine is a widely used industrial chemical product, which is often applied as a flocculant in waste water treatment.15–17 The aim of our approach was to demonstrate that PVAm is also capable of adsorption of chromate. In particular, our goal was the development of a reusable, textile-based filtration material and its feasibility for the purification of real-life waste waters.
3.1. Textile modification
As a substrate, we have chosen a PET-based nonwoven fabric, because of its higher surface to mass ratio compared to a woven fabric. The immobilization of PVAm onto the nonwoven was examined at different pH values. As shown in Table 1, the amount of PVAm bound on the fabric varies between 0.6 and 6.2% depending on the pH-value. The real amount of PVAm can be measured by the Kjeldahl method. Overall Kjeldahl measurements show a similar trend to the loading by weight. The non-linear behavior of nitrogen by PVAm to loading by weight depends on the change in the hydrophilicity of the fabric, and therefore, a higher water retention value of the fabrics.
The difference in the loading with PVAm can be explained by a pH-dependent change of the conformation of the polymer in aqueous solution. In acidic solution, the Bjerrum length of polyvinylamine is maximized because of the electrostatic repulsion of the protonated ammonium groups. With an increasing pH value, the solution conformation changes to a random coil structure. The different structures of the polymer are fixed as a result of the immobilization step and determine the loading and porosity of the network and, therefore, the film thickness of the fabric. Under SEM no change of the fiber thickness and morphology is observable (ESI Fig. S1a and b†). Also in the immobilized state the PVAm conformation changes with the pH and can influence the capacity of the material.
As a result of the PET modification with PVAm, the fiber surface resulting PVAm@PET fabrics are of basic character, i.e. the amino groups are easily protonated to form polyvinyl ammonium salts on the surface (Fig. 1C). This polycation can then adsorb the negatively charged chromate ion by means of an anion exchange reaction (Fig. 1C and D). Accordingly, addition of a chromate solution to PVAm@PET fabrics results in discoloration (Fig. 2).
 |
| | Fig. 2 Chromate solutions (50 mg L—1 and pH 5) before (left) and after the addition (right) of PVAm@PET. The discoloration of the solution is easily recognizable. | |
3.2. Probing the pH and loading influence
An initial point of interest of this study was how the loading of PVAm on the nonwoven fabric and the pH of the sorbent solution influence the adsorption power. The amount of chromate adsorbed on the fabric should depend mainly on two factors: the total amount of amino groups on the surface, which is controlled by the PVAm loading onto the PET fiber, and the pH value of the solution, which has a more complex influence on the adsorption behavior. With decreasing pH, the number of ammonium ions increases, whereas the amount of chromate10,31,32 decreases because of the formation of H2CrO4 and HCr2O7−. Accordingly, an optimal pH value for chromate adsorption should be observable. This was confirmed qualitatively by the visual inspection of dried fabric samples that were treated with chromate solutions at different pH values (Fig. 3), which showed the most intense color after adsorption at pH 5. In Fig. 4, the influence of chromate adsorption on PVAm@PET nonwoven fabric with different PVAm loadings is shown for different pH values of the sorbent solution. The untreated PET material shows no adsorption of chromate, independent of the pH value. The modified PET has the highest loading capacity between pH 5 and 7, supporting the qualitative observations presented in Fig. 3. In addition, the observation that under strongly basic conditions only minor amounts of chromate are bound suggests that a regeneration of the filtration material at pH values of 11–14 should be possible. The decreased adsorption properties at lower pH show that the shift in the chromate/dichromate equilibrium10,31,32 to H2CrO4 and HCr2O7− can reduce the adsorption power at low pH values.
 |
| | Fig. 3 4% PVAm@PET fabrics after the adsorption of chromate at different pH values. | |
 |
| | Fig. 4 Adsorption of chromate from a 1 g L−1 solution at different pH values with different loadings of PVAm@PET. | |
The PVAm loading has a significant influence on the adsorption performance. Interestingly, the highest PVAm loading does not show the highest adsorption ratio, which is observed for the sample with 4.0% PVAm loading. In accordance with SEM observations (ESI Fig. S1a and b†) of treated fiber morphology being unchanged, the reason for the adsorption performance influence could be that the 6.2% PVAm layer on the nonwoven is too thick so that the diffusion into deeper layers is inhibited. For that reason we have chosen to evaluate the adsorption isotherms and kinetics with the 4.0% loaded nonwoven filtration material.
3.3. Adsorption isotherms
To understand and determine the optimal pH area for PVAm@PET based adsorption of chromate from groundwater samples – where it is desired, if no pre-treatment of the groundwater before the adsorption step is needed – the influence of the pH and the chromate concentration on the loading was determined. These measurements were done covering a pH range of 1–7 at chromate concentrations of 1–5000 mg L−1. The obtained adsorption isotherms (Fig. 5A) for pH 3–7 are similar at low concentrations, for qm a pH influence is observable, at pH 3 and 5 more chromate is adsorbed in comparison to pH 7. A special case is the adsorption isotherm at pH 1, because a stepwise adsorption is found. For C0 from 0–250 mg L−1, the slope of the adsorption is lower compared to higher pH values (Fig. 5A). At C0 = 500 mg L−1 a jump occurs with a strong increase of the sorption power. These data show that at low pH values a concentration dependent change occurs in the adsorption mechanism. The role of the pH could be – as mentioned before – the shift in the chromate/dichromate equilibrium10,31,32 at higher chromate concentrations and an acid induced condensation of dichromate to higher surface bound polychromates which could explain the stronger adsorption for higher C0 values. Further investigation regarding the details of these processes is in progress.
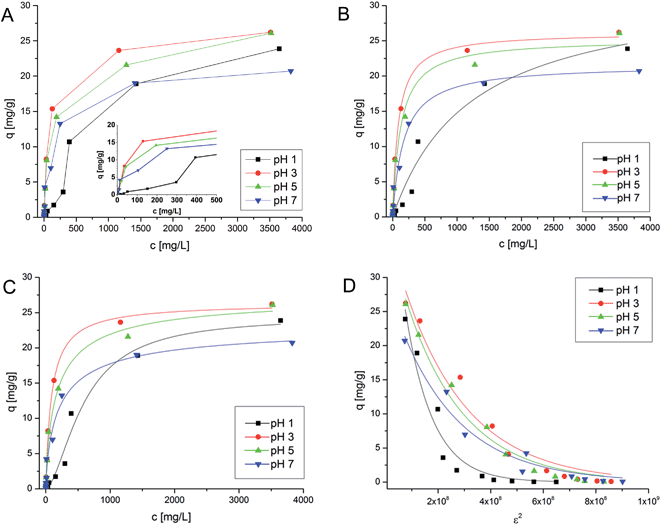 |
| | Fig. 5 Adsorption isotherms for the chromate adsorption with 4% PVAm@PET at different pH-values. (A) Unfitted isotherms, (B) isotherms with Langmuir fits, (C) isotherms with Hill fits, and (D) isotherms with DR fits. | |
Adsorption isotherms are useful for the understanding of the adsorption mechanism. The observed isotherms were analyzed according to a Langmuir, Dubinin–Radushkevich (DR) and Hill behavior, respectively, which are common models for polyelectrolyte metal adsorption.5,7–9,11,24,33–36 The isotherm fitting was processed using Microcal Origin 9.0 with a least squares approach. The adsorption isotherm describes the concentration of the metal on the surface and in solution at the equilibrium. The adsorption isotherms were measured at pH values of 1, 3, 5 and 7. At pH 1, the stepwise change/behavior cannot be described with the above mentioned models, therefore we waive the fitting results. The quality of the fit was judged by the correlation coefficient (R2). On this basis the chosen isotherm models (Langmuir, Hill and DR) seem to be suitable for a description of the adsorption process. The best fit, however, is reached with the Hill model. With all models it is possible to determine the maximum capacity of the adsorbent, which is higher at pH 3–5 than at pH 7. In Fig. 5 the adsorption isotherms and the possible fits are given. Table 2 shows the isotherm constants.
Table 2 Fitting parameters of the different isotherms
|
|
Langmuir |
|
q
mD [mg g−1] |
K
L [L mg−1] |
ΔG [kJ mol−1] |
R
2
|
| pH 3 |
26.18 |
1.14 × 10−2 |
17.8 |
0.997 |
| pH 5 |
25.26 |
8.12 × 10−3 |
17.0 |
0.988 |
| pH 7 |
21.62 |
5.83 × 10−3 |
16.2 |
0.970 |
|
|
DR |
|
q
m [mg g−1] |
k
DR [mol2 J−2] |
E [kJ mol−1] |
R
2
|
| pH 3 |
38.38 |
4.1 × 10−9 |
11.0 |
0.964 |
| pH 5 |
37.94 |
4.5 × 10−9 |
10.5 |
0.981 |
| pH 7 |
29.21 |
4.3 × 10−9 |
10.8 |
0.963 |
|
|
Hill |
|
q
mH [mg g−1] |
K
H [L mg−1] |
n
H
|
R
2
|
| pH 3 |
26.39 |
77.5 |
0.97 |
0.997 |
| pH 5 |
27.66 |
58.2 |
0.78 |
0.992 |
| pH 7 |
23.45 |
58.0 |
0.75 |
0.981 |
3.3.1. Langmuir isotherm.
The Langmuir isotherm7–9,11 is derived from the law of mass action, and can describe the surface saturation of an adsorbent. The premises of a Langmuir-type behavior are non-interacting binding sites. The Langmuir adsorption7–9,11 is described by eqn (2), with qe [mg g−1] = equilibrium adsorption capacity, Ce = equilibrium concentration in solution, and qmL [mg g−1] = maximum Langmuir adsorption capacity. KL is the Langmuir constant related to the energy of adsorption [L mg−1]. From the Langmuir constant KL, additional information can be obtained. ΔG, which can be calculated from KL according to eqn (4), has a negative value independent of the pH. This shows that the adsorption process is thermodynamically favored. In addition, the separation factor RL (eqn (3)) can be derived. According to Weber et al.,37 this factor is a useful tool for judging the technical applicability of an adsorbent. RL describes the adsorption efficiency in relation to a certain starting concentration C0. For 0 < RL < 1, the adsorption is favored, whereas for RL > 1, adsorption is disfavored. In the case of chromate adsorption, a RL < 1 is found for the full concentration range of the used chromate solution. This shows that adsorption is favored in all cases, and emphasizes the technical usefulness of the system. A plot of RL against C0 is shown in the ESI.† Furthermore, the free energy of adsorption (ΔG) is calculable from KL (eqn (4)).7–9,11| |  | (2) |
| | 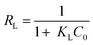 | (3) |
| | ΔG = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL KL | (4) |
3.3.2. Hill isotherm.
With the Hill11 modification of the Langmuir isotherm (eqn (5)), the binding sites lose their independency during the adsorption process, because their interaction with the sorbent is taken into account.11,12,38–43 It can be used to determine how different binding sites of a macromolecule interact with each other and if a cooperative or a disturbing binding behavior exists. This behavior is described by the Hill exponent nH with nH > 1 for a cooperative binding, nH = 1 if no interaction of the binding sites occurs (Langmuir behavior) and nH < 1 if the binding sites are disturbing each other. KH is the Hill equilibrium dissociation constant and qmH is the saturation loading.| |  | (5) |
In the case of polyvinylamine, e.g., a decrease of chromate binding should be observable after a certain level of adsorption places are blocked, because the binding sites are close-by. Via the Hill exponent nH it is possible to recognize if this kind of interaction occurs and if occupied binding sites inhibit or promote further binding events. Using this model, an explanation for the pH influence can be derived, because with increasing pH nH becomes smaller, i.e. the interaction at the bonding sites becomes stronger. At pH 3, nH is nearly 1, meaning that there is quasi or no interaction between the binding sites. At higher pH values nH becomes smaller, so an inhibition by the bound chromate occurs, because at higher pH the positive charge of the surface is lower as it is more shielded by the negatively charged chromate. This also explains the decrease of the qm values at higher pHs.
3.3.3. Dubinin–Radushkevich isotherm.
Another possibility for the description of the adsorption isotherm is the Dubinin–Radushkevich (DR)7,11,12,24,44,45 approach (eqn (6)), which connects the monolayer capacity qmD [mg g−1] with the Polanyi potential (ε) (eqn (7)), the gas constant (R), the absolute temperature (T) and ce,m = equilibrium concentration [mol L−1] and the DR constant (kDR [mol2 J−2]) which is related to the sorption energy. The sorption energy E [kJ mol−1] can be derived from kDR by eqn (8). Values of E in the range of 1.00–8.00 kJ mol−1 show physisorption, whereas E > 9.00 kJ mol−1 is characteristic for an ion exchange process. For the adsorption from pH 3–7 sorption energies of 10.5–11.0 kJ mol−1 are calculated. This is characteristic for an ion exchange process, like we have proposed in Fig. 1D.| | 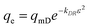 | (6) |
| |  | (7) |
| | 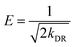 | (8) |
3.4. Adsorption kinetics
For a possible application as an adsorbent, not only the pH influence and the adsorption isotherm are of relevance but also the kinetics of the adsorption reaction is important, especially if a continuous flow application is desired. The adsorption of chromate on different sorbents has previously been investigated by different groups. Bhattacharya et al.8 and Aydın et al.7 each described independently that the kinetics of chromate adsorption on polyelectrolytes is best described by pseudo 2nd order kinetics. Accordingly, the time-dependent adsorptions determined in this study were linearized to fit the 2nd order kinetics46 (eqn (9)), with qt [mg g−1] = chromate loading at a certain time t [min], k [g mg−1 min−1] = adsorption rate constant and qe [mg g−1] = capacity in equilibrium. The initial adsorption rate h for t → 0 is given in eqn (10).| |  | (9) |
We analyzed the kinetics of the adsorption process using a pure chromate solution (23.2 mg L−1) and a groundwater sample (56.6 mg L−1 chromate), to see how strong the adsorption is influenced/disturbed by other compounds. In Fig. 6 the kinetic curves for different amounts of the adsorber material and their linearized plots are shown. Table 3 shows the fitting and the resulting kinetic parameters. We found a fast kinetics, within a minute, the half-life concentration is reached; after 5 minutes most of the absorbable chromate is bound and the equilibrium is almost reached. This system is faster than other reported materials.9,12,44,47–49 With more adsorbent material, the reaction is accelerated; k becomes larger with higher amounts of adsorbent. The adsorption kinetics of the artificial and real solutions are fast and comparable, and no change in the reaction speed is observed; this shows that the adsorption process is not disturbed by other ions, so a technical process should be possible.
 |
| | Fig. 6 Kinetic plots of the chromate adsorption. (A and C) Depletion of chromate from the solution. (B and D) Linearized 2nd order kinetics. (A and B) Chromate adsorption from a labor solution. (C and D) Chromate adsorption from contaminated groundwater. | |
Table 3 Kinetic parameters derived by linearization for the different waters (lab = pure chromate solution and ground = groundwater sample). For qe experimental (exp.) data and calculated (cal.) fitting results are given
| Water |
Adsorbent amount [mg] |
Intercept |
Slope |
R
2
|
q
e [mg g−1] |
k [g mg−1 min−1] |
h [mg g−1 min−1] |
| Cal. |
Exp. |
| Lab |
240 |
0.086 |
0.119 |
0.999 |
8.41 |
8.31 |
0.16 |
11.6 |
| 468 |
0.057 |
0.223 |
0.999 |
4.49 |
4.46 |
0.87 |
17.4 |
| Ground |
492 |
0.053 |
0.222 |
0.999 |
4.51 |
4.49 |
0.93 |
19.0 |
| 982 |
0.05 |
0.313 |
1 |
3.20 |
3.19 |
1.96 |
20.0 |
| 3442 |
0.143 |
0.635 |
1 |
1.58 |
1.57 |
2.82 |
7.0 |
3.5. Regeneration and reusability
For a real application, a repeated use of the adsorption material is desirable instead of an incineration process, because the remediation process should be as economical as possible. To prove this concept we built a simple column adsorption system (Scheme Fig. 7B) that is comparable to the state of the art processes. With this apparatus we can measure the breakthrough properties repeatedly. At first the desorption conditions of the materials were evaluated by a batch process with a chromate pre-loaded textile at different pH values. In Fig. 7A the pH dependent desorption profile is shown. In accordance with the mechanistic data, a weak desorption under acidic conditions, a low desorption in the range of pH 5–7 and a high desorption for alkaline solutions are found. The highest desorption is observed at pH 13 (0.1 mol L−1 NaOH). We therefore decided to apply this concentration for the elution of chromate from the column filled with PVAm@PET.
 |
| | Fig. 7 (A) Desorption profile for a PET nonwoven with 4% PVAm. (B) Scheme of the measuring apparatus for the breakthrough curves and (C) different breakthrough curves for the recycling circles of the chromate loaded PVAm@PET adsorbent. | |
3.6. Breakthrough curve measurement
For the measurement of the breakthrough curve a groundwater sample from an old electroplating ground with 56.6 mg L−1 chromate was used. To determine the reusability the groundwater was pumped over the adsorbent until the breakthrough was reached; afterwards the adsorbent was eluted with 0.1 mol L−1 NaOH solution following an equilibration step with water and then the breakthrough curve was measured again. The change of the chromate concentration was measured with an UV-Vis at 370 nm. The amount of NaOH needed for elution was measured after the 1st run by measuring the adsorption in the eluate. After 100 mL the adsorption dropped to the blind value and the column (ESI Fig. S4†) was washed with water to equilibrate the material. In the 1st run the breakthrough is at about 40 min, in the 2nd run the breakthrough is much earlier, so we raised the amount of washing water after each run stepwise from 50–250 mL. Upon raising the amount of washing water, the breakthrough shifted to later breakthrough times until in the 7th run the original breakthrough time was reached. The 8th run was done to confirm the results of the 7th; this shows that the material is fully regenerated and reusable. The breakthrough curves for the different runs with the used amount of water are shown in Fig. 7C. In addition to the UV-Vis measurement, the chromate concentration before and after breakthrough was measured by ICP/OES and it is reduced to <50 μg L−1. To find out if a PVAm leaching is observable, we measured the amount of total organic carbon during the breakthrough measurements. No change was observed. Further information about PVAm leaching is given in the ESI.†
This simple column filtration system proves that the application of PVAm@PET as an adsorbent for chromate could easily be realized and integrated into a state of the art adsorption system. For a real application e.g. classical adsorber column systems can be used, here an optimization of column packing with a textile to achieve good grain boundaries or flow speed has to be done. The construction of cartridge type filter systems would be an alternative possibility. Further investigation on the best way for a technical application is in progress and it is planned to use these results in a pilot plant at two different sites where chromate contaminations are found.
4. Conclusions
We demonstrated for the first time that a textile modified with a polyelectrolyte can be used as a recyclable adsorbent material for the removal of toxic chromate water contaminations. Both artificial chromate solutions and actual chromate contaminated groundwater samples were successfully purified, with the latter result demonstrating that a good selectivity for chromate under realistic conditions is achieved. The use of modified PET nonwoven combines the advantages of textiles like high chemical resistance, good mechanical properties, high durability, easy and flexible processing and a broad accessibility with excellent adsorbent properties. A further advantage of our modified fabric is pH-independency for typical pH values and chromate concentrations that are realistic for groundwater. In addition, the good recycling properties of the material – which has not yet been investigated for comparable systems – show the potential of this type of intelligent textile filtration material for practical applications. With a simple set-up, similar to the state of the art systems, we have proven that our material has a high adsorption efficiency that can be reused for at least eight adsorption/desorption cycles. Furthermore, we have carried out some pre-investigation on other toxic metal oxoanions such as arsenate or selenates where we also got promising results. These results demonstrate that we have developed a selective smart textile as an adsorbent for different kinds of environmental pollution problems.
Acknowledgements
We thank the Federal State North Rhine-Westphalia and the AAV – Verband für Flächenrecycling und Altlastensanierung NRW for the funding of this research. We thank Markus Oberthür for the valuable discussion about the paper. We thank DTNW Öffentliche Prüfstelle GmbH for the ICP measurements.
Notes and references
- C. D. Palmer and P. R. Wittbrodt, Environ. Health Perspect., 1991, 92, 25 CrossRef CAS.
- M. A. Armienta, R. Rodríguez, N. Ceniceros, F. Juárez and O. Cruz, Environ. Pollut., 1996, 91, 391–397 CrossRef CAS.
-
L. M. Calder, in Chromium in the Natural and Human Environments, ed. J. O. Nriagu and E. Nieboer, John Wiley & Sons, Inc., New York, 1988, pp. 215–229 Search PubMed.
- G. Schellartz, AAV Fachtagung 2008, http://www.aav-nrw.de/Downloads/manuskripte/boden_grundwasser2008/08_schellartz.pdf, accessed September 2014.
- J. O. Nriagu, Environ. Pollut., 1988, 50, 139–161 CrossRef CAS.
-
Health criteria and other supporting information, Guidelines for Drinking-Water Quality, ed. WHO, World Health Organization, Geneva, 2nd edn, 1996, vol. 2, pp. 1–13 Search PubMed.
- Y. A. Aydın and N. D. Aksoy, Chem. Eng. J., 2009, 151, 188–194 CrossRef PubMed.
- A. K. Bhattacharya, T. K. Naiya, S. N. Mandal and S. K. Das, Chem. Eng. J., 2008, 137, 529–541 CAS.
- F. Wang and M. Ge, Text. Res. J., 2013, 83, 628–637 CrossRef CAS PubMed.
- D. Mohan and C. U. Pittman Jr, J. Hazard. Mater., 2006, 137, 762–811 CrossRef CAS PubMed.
- K. Y. Foo and B. H. Hameed, Chem. Eng. J., 2010, 156, 2–10 CrossRef CAS PubMed.
- B. Sarkar, Y. Xi, M. Megharaj, G. S. R. Krishnamurti, D. Rajarathnam and R. Naidu, J. Hazard. Mater., 2010, 183, 87–97 CrossRef CAS PubMed.
- S. Pandey and S. B. Mishra, J. Colloid Interface Sci., 2011, 361, 509–520 CrossRef CAS PubMed.
- M. C. Brum, J. L. Capitaneo and J. F. Oliveira, Miner. Eng., 2010, 23, 270–272 CrossRef CAS PubMed.
-
G. R. Rose and M. R. S. John, in Encyclopedia of Polymer Science and Technology, John Wiley & Sons, Inc., 2002 Search PubMed.
-
A. L. Wigsten and R. A. Stratton, in ACS Symposium Series, American Chemical Society, 2013, vol. 240, pp. 429–444 Search PubMed.
- V. A. Kabanov, A. B. Zezin, V. A. Kasaikin, A. A. Yaroslavov and D. A. Topchiev, Russ. Chem. Rev., 1991, 60, 288–291 CrossRef PubMed.
- A. Marais and L. Wågberg, Cellulose, 2012, 19, 1437–1447 CrossRef CAS.
- C. Miao, M. Leduc and R. Pelton, J. Pulp Pap. Sci., 2008, 34, 69–75 CAS.
- Z. Wu, S. Chen and H. Tanaka, J. Appl. Polym. Sci., 2001, 80, 2185–2190 CrossRef CAS.
- R. Wittke, D. Knittel, R. Kaufmann and E. Schollmeyer, Z. Naturforsch., A: Phys. Sci., 2009, 64, 653 CAS.
- E. Bayer, K. Geckeler and K. Weingärtner, Makromol. Chem., 1980, 181, 585–593 CrossRef CAS.
- S. L. Davydova and N. A. Plate, Coord. Chem. Rev., 1975, 16, 195–225 CrossRef CAS.
- C. Gerente, V. K. C. Lee, P. L. Cloirec and G. McKay, Crit. Rev. Environ. Sci. Technol., 2007, 37, 41–127 CrossRef CAS.
- K. Kimura, Y. Inaki and K. Takemoto, Makromol. Chem., 2003, 175, 83–93 CrossRef.
- B. L. Rivas, G. V. Seguel and K. E. Geckeler, Angew. Makromol. Chem., 1997, 251, 97–106 CrossRef CAS.
- E. Bayer, H. Eberhardt and K. Geckeler, Angew. Makromol. Chem., 1981, 97, 217–230 CrossRef CAS.
- B. Gao, J. Lu, R. Zhuang and G. Zhang, J. Appl. Polym. Sci., 2009, 114, 3487–3494 CrossRef CAS.
- F. Ferrero, C. Tonetti and M. Periolatto, Carbohydr. Polym., 2014, 110, 367–373 CrossRef CAS PubMed.
- M. Meilikhov, K. Yusenko, E. Schollmeyer, C. Mayer, H.-J. Buschmann and R. A. Fischer, Dalton Trans., 2011, 40, 4838 RSC.
- F. Brito, J. Ascanio, S. Mateo, C. Hernández, L. Araujo, P. Gili, P. Martín-Zarza, S. Domínguez and A. Mederos, Polyhedron, 1997, 16, 3835–3846 CrossRef CAS.
-
L. D. Benefield, J. F. Judkins Jr and B. L. Weand, in Process Chemistry for Wastewater Treatment, Prentice-Hall, Englewood Cliffs, NJ, 1982, pp. 433–439 Search PubMed.
- M. Dakiky, M. Khamis, A. Manassra and M. Mer'eb, Adv. Environ. Res., 2002, 6, 533–540 CrossRef CAS.
- D. W. O'Connell, C. Birkinshaw and T. F. O'Dwyer, J. Chem. Technol. Biotechnol., 2006, 81, 1820–1828 CrossRef.
- P. S. Kumar and R. Gayathri, J. Eng. Sci. Technol. Rev., 2009, 4, 381–399 Search PubMed.
- T. A. Davis, B. Volesky and A. Mucci, Water Res., 2003, 37, 4311–4330 CrossRef CAS.
- T. W. Weber and R. K. Chakravorti, AIChE J., 1974, 20, 228–238 CrossRef CAS.
- S. Goutelle, M. Maurin, F. Rougier, X. Barbaut, L. Bourguignon, M. Ducher and P. Maire, Fundam. Clin. Pharmacol., 2008, 22, 633–648 CrossRef CAS PubMed.
- D. Ringot, B. Lerzy, K. Chaplain, J.-P. Bonhoure, E. Auclair and Y. Larondelle, Bioresour. Technol., 2007, 98, 1812–1821 CrossRef CAS PubMed.
- T. J. V. Prazeres, A. M. Santos, J. M. G. Martinho, A. Elaïssari and C. Pichot, Langmuir, 2004, 20, 6834–6840 CrossRef CAS PubMed.
- V. M. Dronnet, C. Renard, M. Axelos and J.-F. Thibault, Carbohydr. Polym., 1996, 30, 253–263 CrossRef CAS.
- A. Hashidzume, S.-I. Watanabe and Y. Morishima, Langmuir, 2007, 23, 2191–2197 CrossRef CAS PubMed.
- F. Delben, P. Gabrielli, R. Muzzarelli and S. Stefancich, Carbohydr. Polym., 1994, 24, 25–30 CrossRef CAS.
- G. Alberti, V. Amendola, M. Pesavento and R. Biesuz, Coord. Chem. Rev., 2012, 256, 28–45 CrossRef CAS PubMed.
- Y. Liu and Y.-J. Liu, Sep. Purif. Technol., 2008, 61, 229–242 CrossRef CAS PubMed.
- Y.-S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- S. S. Gupta and K. G. Bhattacharyya, J. Colloid Interface Sci., 2006, 295, 21–32 CrossRef PubMed.
- K. Inoue, T. Yamaguchi, M. Iwasaki, K. Ohto and K. Yoshizuka, Sep. Sci. Technol., 1995, 30, 2477–2489 CrossRef CAS.
- S. E. Bailey, T. J. Olin, R. M. Bricka and D. D. Adrian, Water Res., 1999, 33, 2469–2479 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM images of modified and unmodified PET non-woven. A picture of the filtration column. RL plots for all pHs and concentrations. Analytical details and groundwater analysis. See DOI: 10.1039/c4ta04212c |
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, 8 h) and petroleum ether (8 h) to remove production related finishes. PVAm (Lupamin® 9095) was donated by BASF (Ludwigshafen, Germany). The solution contains 10–15 wt% PVAm with an average molecular weight of 340
1 v/v, 8 h) and petroleum ether (8 h) to remove production related finishes. PVAm (Lupamin® 9095) was donated by BASF (Ludwigshafen, Germany). The solution contains 10–15 wt% PVAm with an average molecular weight of 340![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 and a degree of hydrolysis >90%. All other chemicals were of analytical grade.
000 g mol−1 and a degree of hydrolysis >90%. All other chemicals were of analytical grade.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) for 24 h. The amount of immobilized PVAm loading was determined gravimetrically (weight/weight% (w/w)) and the amount of nitrogen from PVAm by Kjeldahl analysis in μmol g−1. The limit of detection for nitrogen is 10 μmol g−1. The results of a triple measurement are shown in Table 1. The fabrics are weighed after one day in a standardized climate chamber (20 ± 2 °C and 65 ± 4% humidity). SEM images are given in the ESI.†
1 v/v) for 24 h. The amount of immobilized PVAm loading was determined gravimetrically (weight/weight% (w/w)) and the amount of nitrogen from PVAm by Kjeldahl analysis in μmol g−1. The limit of detection for nitrogen is 10 μmol g−1. The results of a triple measurement are shown in Table 1. The fabrics are weighed after one day in a standardized climate chamber (20 ± 2 °C and 65 ± 4% humidity). SEM images are given in the ESI.†



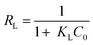
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KL
KL