3-Nitro-1-(2H-tetrazol-5-yl)-1H-1,2,4-triazol-5-amine (HANTT) and its energetic salts: highly thermally stable energetic materials with low sensitivity†
Chengming
Bian
a,
Man
Zhang
a,
Chuan
Li
a and
Zhiming
Zhou
*ab
aSchool of Chemical Engineering and the Environment, Beijing Institute of Technology, Beijing, 100081, P.R. China. E-mail: zzm@bit.edu.cn
bState Key Laboratory of Explosion Science and Technology, Beijing Institute of Technology, Beijing, 100081, P.R. China
First published on 8th September 2014
Abstract
A new family of nitrogen-rich energetic salts based on 3-nitro-1-(2H-tetrazol-5-yl)-1H-1,2,4-triazol-5-amine (HANTT) were synthesized and characterized by 1H and 13C nuclear magnetic resonance, infrared spectroscopy and elemental analysis. The crystal structures of neutral HANTT (2), its guanidinium salt (3), and 1,5-diamino-tetrazolium salt (9) were determined by single-crystal X-ray diffraction. All energetic salts exhibit excellent thermal stabilities with decomposition temperatures ranging within 264–321 °C and are insensitive to impact, friction and electrostatic discharge. The densities of salts 3–10 ranged from 1.65 g cm−3 to 1.81 g cm−3. Theoretical performance calculations (Gaussian 03 and EXPLO5) provided detonation pressures and velocities for the energetic salts within the ranges of 22.6–32.6 GPa and 7742–8779 m s−1, respectively, making them competitive energetic materials.
Introduction
Recently, nitrogen-rich heterocyclic energetic compounds have been the focus of several research groups worldwide,1 as they proved to be promising high-energy-density materials (HEDMs) with high thermal and mechanical stabilities in suitable designs. For example, dihydroxylammonium 3,3′-dibitroamino-4,4′-azoxyfurazanate2 which contains the azoxy [–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(O)–] moiety has high density (1.9 g cm−3), acceptable impact sensitivity (IS = 19 J, 120 N), excellent detonation pressure (P = 42.2 GPa) and velocity (vD = 9511 m s−1); dihydroxylammonium 3,3′-dinitro-5,5′bis-1,2,4-triazole-1,1′-diol3 bearing N-oxide moieties is thermally stable (Td = 217 °C), exceedingly powerful (P = 39 GPa, vD = 9087 m s−1) and safe to handle (IS > 40 J).
N(O)–] moiety has high density (1.9 g cm−3), acceptable impact sensitivity (IS = 19 J, 120 N), excellent detonation pressure (P = 42.2 GPa) and velocity (vD = 9511 m s−1); dihydroxylammonium 3,3′-dinitro-5,5′bis-1,2,4-triazole-1,1′-diol3 bearing N-oxide moieties is thermally stable (Td = 217 °C), exceedingly powerful (P = 39 GPa, vD = 9087 m s−1) and safe to handle (IS > 40 J).
Among these nitrogen-rich heterocycles, 1,2,4-triazole shows a perfect balance between thermal stability and high positive heat of formation, and has drawn much attention as a backbone molecule for HEDMs. Moreover, 5-amino-3-nitro-1H-1,2,4-triazole (ANTA) is a typical thermally stable (Td = 238 °C)4 and insensitive explosive based on 1,2,4-triazole.5 Researchers have reported several derivatives of ANTA in the past few years, which were synthesized either by modifying the original amino group to generate new energetic compounds, such as 3-nitro-5-nitramino-1H-1,2,4-triazole6 and 5-azido-3-nitro-1H-1,2,4-triazole;7 using a diazo bridge, such as 5,5′-dinitro-3,3′-azo-1,2,4-triazole (DNAT);8 or with a direct C–N/N–N connection of energetic groups/compounds to triazole, such as 1,5-diamino-3-nitro-1,2,4-triazole (BANT),9 4,6-bis-(3-amino-5-nitro-1H-1,2,4-triazole-1-yl)-5-nitropyrimidine (DANTNP),10,11 and 1-nitroguanyl-3-nitro-5-amino-1,2,4-triazole (ANTA-NQ).12 However, these derivatives more or less have some drawbacks. Decomposition temperatures of 3-nitro-5-nitramino-1H-1,2,4-triazole, 5-azido-3-nitro-1H-1,2,4-triazole and ANTA-NQ are 135 °C, 174 °C and 200 °C, respectively, which are much lower than that of ANTA; whereas DNAT and DANTNP are less energetic than ANTA due to the moderate charge density of DNAT powder (d = 1.67 g cm−3)8 and the poor oxygen balance (−52.74%) and the lower energy of exothermic decomposition (reduced by approximately 324 J g−1) of DANTNP.10
The tetrazole ring is an excellent building block of HEDMs owing to its high-nitrogen content, ready availability and high positive heat of formation, especially the good thermal stability derived from its aromaticity. The C–N combination of tetrazole (ΔHof = 273 kJ mol−1)13 with ANTA (ΔHof = 88 kJ mol−1)14 will greatly improve heat of formation, which is associated with the increased detonation performance.15 The introduction of tetrazole would also lead to improved stability and density due to intermolecular hydrogen bonds, represented as plane, pseudo-fused heterocycles from the close proximity between the hydrogen atom in the amino group and the nitrogen atom in the tetrazole ring, which is especially obvious when tetrazole deprotonated to obtain a tetrazolate anion.
Based on the considerations above, we report on the synthesis, characterization and calculation of the detonation properties of 3-nitro-1-(2H-tetrazol-5-yl)-1H-1,2,4-triazol-5-amine (HANTT) and its energetic salts.
Results and discussion
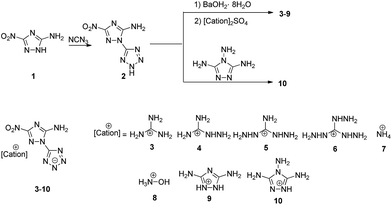
The synthetic pathway to HANTT (2) and its energetic salts is shown in Scheme 1. An initial attempt to synthesize HANTT (2) by selective diazotization of 3,5-diamino-1-(1H-tetrazol-5-yl)-1H-1,2,4-triazole with sodium nitrite in dilute sulfuric acid followed by denitrification and nitration proved unsuccessful and gave rise to 3,5-dinitro-1H-1,2,4-triazole as the main product. Nevertheless, the desired product 2 was finally prepared from ANTA with the reaction of cyanogen azide according to the method reported by Joo and Shreeve.16Energetic salts 3–9 were readily synthesized through the metathesis reactions of Ba(ANTT)2, which was formed in situ with one equivalent of the corresponding sulphate salt. HANTT (2) reacted directly with 3,4,5-triamino-1,2,4-triazole to form salt 10. Colorless crystals of 2, 3 and 9 suitable for single-crystal X-ray analysis were obtained by slowly evaporating the solvent at room temperature and their crystallographic and structural refinement data are listed in Table 2.
All of the energetic salts are soluble in hot water or methanol, stable in air and could be stored for extended periods of time. Their structures were confirmed by IR spectroscopy, 1H, 13C NMR spectroscopy and elemental analysis. The data are listed in the Experimental section.
The signal at δ = 7.71 ppm in 1H NMR spectra of the energetic salts is assigned to the amino group in the ANTT anion, while the rest signals are assigned to the cations. In this study, the 13C NMR shifts related to cations are consistent with previously recorded shifts for such cations.17,18 The 13C NMR resonances observed for the triazole carbon bonded to the nitro group and the other bonded to the amino group in all salts are approximately at δ = 157.9 and 155.7 ppm, respectively. These resonances are downfielded compared with those of precursor 2 (δ = 156.8 and 154.6 ppm). The signal at δ = 160.7 is attributed to the tetrazole carbon, which is upfielded from that of 2 (δ = 161.8 ppm).
HANTT and its salts had been heated at 60 °C for 2 h before their thermal stabilities were measured by differential scanning calorimetry (Table 1) at a heating rate of 10 °C min−1. Salt formation is usually an efficient method of improving thermal stability. Decomposition temperatures of new energetic salts ranging from 264 °C (8) to 321 °C (3) are higher than that of HANTT at 246 °C. Thus, the energetic salts of HANTT exhibit excellent thermal stabilities which are superior to that of 1,3,5-trinitro-1,3,5-triazinane (RDX) (Td = 230 °C). It is noteworthy that the increase of amino substituent in analogous guanidine cations gives rise to a decrease in thermal stability, as salts 4, 5 and 6 melts at 246, 239 and 231 °C, respectively.
| Compd | T m | T d | d | ΔfHLd | ΔfHsale | ΔExUosalf | P cj | v D | ISi | FSj | ESDk | OBl | Nm % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Melting point [°C]. b Thermal degradation [°C]. c Measured density (gas pycnometer) [g cm−3]. d Calculated molar lattice energy [kJ mol−1]. e Calculated molar enthalpy of formation of salts [kJ mol−1]. f Energy of formation [kJ kg−1]. g Detonation pressure [GPa]. h Detonation velocity [m s−1]. i Impact sensitivity. j Friction sensitivity (BAM friction apparatus) [N]. k Electrostatic discharge device [J]. l Oxygen balance (OB) is an index of the deficiency or excess of oxygen in a compound required to convert all C into CO2 and all H into H2O, for the compound with the molecular formula of CaHbNcOd (without crystal water), OB(%) = 1600[(d−2a−b/2)/Mw] [%]. m Nitrogen content. n No sharp decomposition point. o From ref. 20. | |||||||||||||
| 2 | — | 246 | 1.77 | — | 487.2 | 2559.9 | 28.8 | 8316 | >40 | >360 | 7.04 | −44.6 | 64.0 |
| 3 | — | 321 | 1.65 | 477.5 | 392.2 | 1637.2 | 22.6 | 7742 | >40 | >360 | >44 | −62.4 | 65.6 |
| 4 | 246 | 269 | 1.72 | 475.2 | 486.0 | 1901.8 | 25.9 | 8149 | >40 | >360 | >44 | −61.9 | 67.1 |
| 5 | 239 | —n | 1.69 | 467.3 | 595.5 | 2193.3 | 26.4 | 8249 | >40 | >360 | >44 | −61.5 | 68.5 |
| 6 | 231 | —n | 1.69 | 461.0 | 707.9 | 2465.2 | 27.5 | 8390 | >40 | >360 | >44 | −61.1 | 69.7 |
| 7 | — | 281 | 1.79 | 511.1 | 409.1 | 2014.5 | 29.6 | 8507 | >40 | >360 | >44 | −52.3 | 65.4 |
| 8 | 217 | 264 | 1.81 | 502.5 | 460.8 | 2104.6 | 32.6 | 8779 | >40 | >360 | >44 | −41.7 | 60.8 |
| 9 | — | 277 | 1.68 | 462.3 | 595.5 | 2110.7 | 23.6 | 7840 | >40 | >360 | >44 | −64.8 | 66.2 |
| 10 | — | 301 | 1.74 | 460.2 | 711.2 | 2388.6 | 26.7 | 8217 | >40 | >360 | >44 | −64.2 | 67.5 |
| TATB | 350 | ∼360 | 1.93 | — | −154.2 | −511 | 31.2 | 8114 | 50 | >360 | — | −55.8 | 32.5 |
| RDX | — | 230 | 1.82 | — | 92.6 | 617.3 | 34.8o | 8748o | 7 | 120 | 0.2 | −21.6 | 37.8 |
Density is one of the most important properties contributing to the performance of an explosive. As shown in Table 1, HANTT possesses a density of 1.77 g cm−3, while the densities of new salts range from 1.65 g cm−3 (3) to 1.81 g cm−3 (8; RDX = 1.82 g cm−3), as measured using a gas pycnometer.
Oxygen balance (OB) is an expression which indicates the degree to which an explosive can be oxidized.21,22 All salts have negative OB which range from −64.8% (9) to −41.7% (8) and display high nitrogen contents ranging from 60.8% (8) to 69.7% (6).
Impact sensitivity (IS) was determined using the BAM fall hammer BFH-10 apparatus test with approximately 20 mg samples (5.0 kg drop hammer).23 The IS values of HANTT and its salts are higher than 40 J, and their FS values are higher than 360 N, placing them in the insensitive class. This result may be attributed to intramolecular hydrogen bonds and extensive hydrogen-bonding interactions between the cation and the anion in salt formation. It is interesting that all salts are insensitive to electrostatic discharge with the ESD values above 44 J while the neutral compound 2 is relatively insensitive (ESD = 7.04 J).
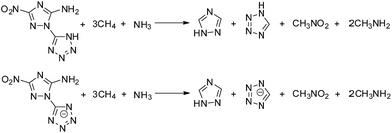
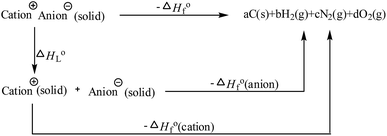
Heat of formation is fundamentally important in evaluating compounds for their possible value as explosives. More positive values lead to potentially better detonation properties. The ΔHf of HANTT and its anion salt are 487.2 kJ mol−1 and 293.8 kJ mol−1, respectively, as calculated using Gaussian 03 (Revision E.01) suite of programs based on isodesmic reactions (Scheme 2). The cation values are provided in the literature.19 The positive ΔHf values of salts 3–10 are much higher than that of ANTA (ΔHof = 88 kJ mol−1), and range from 392.2 kJ mol−1 (3) to 711.2 (10) kJ mol−1 as computed using the Born–Haber energy cycle (Fig. 1).
The detonation pressures (P) and velocities (vD) of the new energetic salts were determined using the EXPLO5 program (Version 5.05) according to the measured density and the calculated ΔHf.24 The calculated detonation pressures range between 22.6 GPa and 32.6 GPa, and the calculated detonation velocities fall between 7742 m s−1 to 8779 m s−1. Salt 7 (vD = 8507 m s−1, P = 29.6 GPa) has an excellent detonation performance due to its high density and moderate oxygen balance. Salt 8 possesses the highest density and the best oxygen balance, thus confirming its excellent detonation performance (vD = 8779 m s−1, P = 32.6 GPa) which is comparable to RDX (vD = 8748 m s−1, P = 34.8 GPa).
X-ray crystallography
Suitable colourless crystals of HANTT (2) were obtained for single-crystal X-ray analysis by slowly evaporating ethyl alcohol/water at room temperature. Compound 2 crystallizes in the monoclinic space group P21/c with a calculated density of 1.710 g cm−3. Fig. 2 shows that the unit consists of one HANTT molecule and one H2O molecule. Table 2 lists the crystallographic and structural refinement data.| 2 | 3 | 9 | |
|---|---|---|---|
| a w = 1/[σ2(Fo2) + (0.0671P)2 + 0.106P], where P = (Fo2 + 2Fc2)/3. b w = 1/[σ2(Fo2) + (0.0391P)2], where P = (Fo2 + 2Fc2)/3. c w = 1/[σ2(Fo2) + (0.0786P)2 + 1.260 P], where P = (Fo2 + 2Fc2)/3. | |||
| Formula | C3H5N9O3 | C4H9N12O2.50 | C5.46H9.92N14O2.50 |
| M w | 215.16 | 265.23 | 311.73 |
| Crystal system | Monoclinic | Triclinic | Monoclinic |
| Space group | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
PC2/c |
| a [Å] | 10.830(4) | 7.0181(17) | 17.869(6) |
| b [Å] | 5.0641(18) | 8.833(2) | 7.864(2) |
| c [Å] | 15.865(6) | 18.795(6) | 18.347(6) |
| V [Å3] | 835.9(5) | 1073.5(5) | 2499.2(14) |
| Z | 4 | 4 | 8 |
| T [K] | 153(2) | 163(2) | 163(2) |
| λ [Å] | 0.71073 | 0.71073 | 0.71073 |
| ρ calcd [mg m−3] | 1.710 | 1.641 | 1.657 |
| μ [mm−1] | 0.15 | 0.14 | 0.14 |
| F(000) | 440 | 548 | 1286 |
| Crystal size [mm−3] | 0.59 × 0.13 × 0.05 | 0.50 × 0.25 × 0.13 | 0.52 × 0.37 × 0.07 |
| θ range[°] | 2.7–29.1 | 2.2–31.5 | 2.4–31.0 |
| Index ranges | −14 ≤ h≤ 14 | −9 ≤ h ≤ 10 | −24 ≤ h ≤ 25 |
| −6 ≤ k ≤ 6 | −12 ≤ k ≤ 12 | −11 ≤ k ≤ 11 | |
| −21 ≤ l ≤ 21 | −27 ≤ l ≤ 27 | −26 ≤ l ≤ 25 | |
| Reflns collected | 8820 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 124 124 |
14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 198 198 |
| Independent reflns (Rint) | 2245[0.026] | 7031 | 3929 |
| Data/retraints/parameters | 2245/0/156 | 7031/0/406 | 3929/1/245 |
| GOF on F2 | 1.003 | 1.002 | 1.002 |
| R[F2 > 2σ(F2)] | 0.041 | 0.039 | 0.050 |
| wR(F2) | 0.112a | 0.089b | 0.137c |
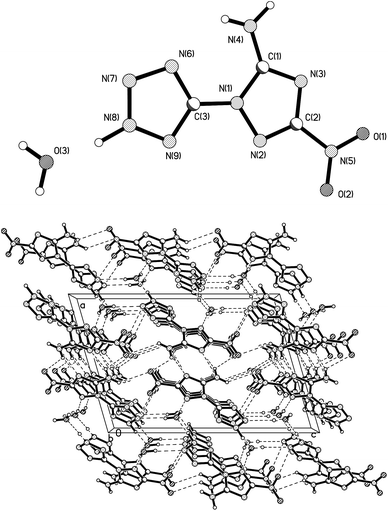
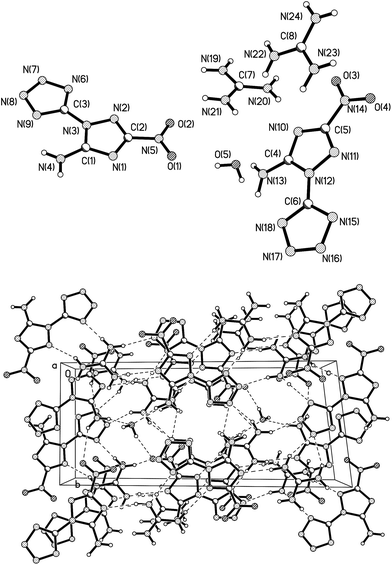
All atoms of 2 are roughly coplanar, and the largest torsion angle is 169.55(12)°. This angle is located among N2–N1–C3–N6. The length of the C3–N1 bond which combines the triazole to tetrazole is 1.3939(15) Å. The dihedral angle between triazole (C1–N1–N2–C2–N3) and tetrazole (C3–N6–N7–N8–N9) is 9.46°. The 1.3013(16) Å C2–N2 bond length is the shortest while the 1.3746(16) Å C1–N1 bond length is the longest in the two heterocyclic rings. This may be caused by the electron-donating amino group and the strong electron-withdrawing inductive effects of the nitro group. As expected, an intramolecular hydrogen bond is represented in the form of a six-membered ring by the close proximity of N4–H4B⋯N6 (2.298 Å). The amino (H4A–N4–H4B) and nitro (O1–N5–O2) groups are approximately coplanar with the triazole ring. The dihedral angles between the triazole and the amino and nitro groups are 1.79° and 2.35°, respectively, which are smaller than those in 3-nitro-1H-1,2,4-triazol-5-amine,25 at 21.83° and 10.32°, respectively. This result suggests that the introduction of the tetrazole ring lead to a strong conjugation of the charge throughout the whole HANTT molecule. The packing structure of 2 was built up and linked to a 2D double layer by various hydrogen bonds, as shown along the b axis in Fig. 2b.
A crystal of 3 suitable for X-ray diffraction analysis was obtained by slow evaporation of water at room temperature. Compound 3 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with a calculated density of 1.641 g cm−3. Its crystallographic data are summarized in Table 2.
with a calculated density of 1.641 g cm−3. Its crystallographic data are summarized in Table 2.
Fig. 3a shows the presence of one H2O molecule and two asymmetric units. Each unit contains one ANTT anion and one guanidinium cation, in which proton transfers from the tetrazole of HANTT to guanidine is confirmed. All atoms of the ANTT anion are roughly coplanar with the largest torsion angle of 171.96(10)° between N11–N12–C6–N18. Intramolecular hydrogen bonds between N13 of the amino group and N18 of the tetrazole [N13–H13A⋯N18 (2.212 Å)], N4–H4B and N9[N4–H4B⋯N9 (2.246 Å)] are the strongest in the three confirmed crystals of 2, 3 and 9, which greatly improve the thermal stability of 3 at the highest decomposition temperature (Td = 321 °C). The dihedral angles between triazole and tetrazole are 2.98° and 7.40°, respectively, which are smaller than that of HANTT. From a structural point of view, the whole ANTT anion looks like a plane pseudo-fused aza-cyclic compound by the connection of intramolecular hydrogen bonds. In an asymmetric unit, the cation and triazole ring in ANTT tend to be coplanar with a dihedral angle between them of 4.28°. The N–N bond lengths in the tetrazolate ring of the ANTT anion vary from 1.3094(14) Å to 1.3551(13) Å and thus lie between those of N–N single bonds (1.454 Å) and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds (1.245 Å).26 This result indicates delocalization of the negative charge throughout the aromatic rings, as observed in other tetrazolate salts.27,28 Viewed along the a axis, the packing structure of 3 shows a variety of hydrogen bonds in the salt structure.
N double bonds (1.245 Å).26 This result indicates delocalization of the negative charge throughout the aromatic rings, as observed in other tetrazolate salts.27,28 Viewed along the a axis, the packing structure of 3 shows a variety of hydrogen bonds in the salt structure.
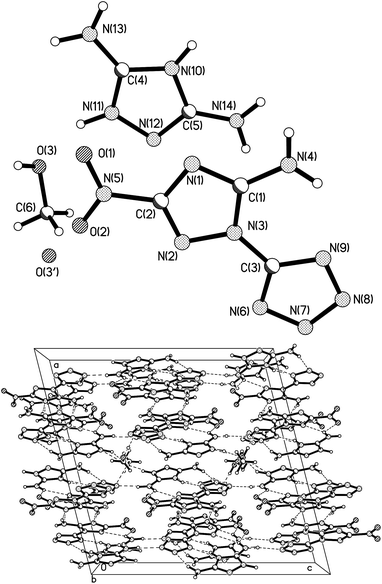
Suitable colourless crystals of 9 were obtained for single-crystal X-ray analysis by slowly evaporating methanol/water at room temperature. Compound 9 crystallizes in the monoclinic space group PC2/c with a calculated density of 1.657 g cm−3.
As shown in Fig. 4a, the salt is formed through transfer of proton from the N7/N8 position in the ANTT anion to the resulting cation. Disorder of CH3OH and H2O molecules was observed in the crystal. The ratio of solvent molecules and salts is 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, while the former consist of 0.461
1, while the former consist of 0.461![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.039 CH3OH and H2O ratio. The intramolecular hydrogen bond is shorter than that of the neutral compound 2, as represented by the close proximity of N4–H4A⋯N9 (2.259 Å), which lead to high thermal stability (Td = 277 °C). The dihedral angle between triazole and tetrazole in the ANTT anion is 1.40° and all atoms of the ANTT anion are nearly in one plane with the largest torsion angle of 175.59° (O2–N5–C2–N9). The 3,5-diamino-1,2,4-triazolium cation and the ANTT anion tend to be coplanar with a dihedral angle of 4.23° between the two triazole rings. Similar to the situation in 3, the N–N bond lengths in the tetrazolate ring of the ANTT anion vary from 1.3095(16) to 1.3612(17) Å and thus lie between those of N–N single bonds (1.454 Å) and N
0.039 CH3OH and H2O ratio. The intramolecular hydrogen bond is shorter than that of the neutral compound 2, as represented by the close proximity of N4–H4A⋯N9 (2.259 Å), which lead to high thermal stability (Td = 277 °C). The dihedral angle between triazole and tetrazole in the ANTT anion is 1.40° and all atoms of the ANTT anion are nearly in one plane with the largest torsion angle of 175.59° (O2–N5–C2–N9). The 3,5-diamino-1,2,4-triazolium cation and the ANTT anion tend to be coplanar with a dihedral angle of 4.23° between the two triazole rings. Similar to the situation in 3, the N–N bond lengths in the tetrazolate ring of the ANTT anion vary from 1.3095(16) to 1.3612(17) Å and thus lie between those of N–N single bonds (1.454 Å) and N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds (1.245 Å). This result indicates the conjugation of the negative charge throughout the aromatic rings. As shown in Fig. 4b, the discrete ANTT anions and 3,5-diamino-1,2,4-triazolium cations are linked into a 3D network by extensive hydrogen bonds.
N double bonds (1.245 Å). This result indicates the conjugation of the negative charge throughout the aromatic rings. As shown in Fig. 4b, the discrete ANTT anions and 3,5-diamino-1,2,4-triazolium cations are linked into a 3D network by extensive hydrogen bonds.
Conclusions
In this study, HANTT (2) and its salts 3–10 were synthesized and fully characterized. The structures of neutral compound 2, salts 3 and 9 have been analyzed by single-crystal X-ray diffraction analysis. The densities of all nitrogen-rich salts lie in the range between 1.65 g cm−3 (3) and 1.81 g cm−3 (8). The intramolecular hydrogen bond represented in the form of plane pseudo-fused heterocycles by the close proximity between the hydrogen atom in the amino group and the nitrogen atom in the tetrazole ring greatly improves thermal stability and IS, which is especially obvious when tetrazole deprotonated to obtain a tetrazolate anion as we expected. It is notable that all energetic salts exhibit excellent thermal stabilities with decomposition temperatures ranging within 264–321 °C and are insensitive to impact, friction and electrostatic discharge. Their detonation velocities and detonation pressures were calculated to be 7742 m s−1 to 8779 m s−1 and 22.6 GPa to 32.6 GPa, respectively. Salt 7 (vD = 8507 m s−1, P = 29.6 GPa) shows an excellent detonation performance due to its high density and moderate oxygen balance. Salt 8 possesses the highest density and the best oxygen balance, thus confirming its excellent detonation performance (vD = 8779 m s−1, P = 32.6 GPa). Finally, in view of thermal and mechanical stabilities, salt 8 is superior to RDX.Experimental section
Caution! Although we experienced no difficulties in handling these energetic materials, small scale and best safety practices (leather gloves and face shield) are strongly encouraged!General methods
1H, 13C NMR spectra were recorded using a 500 MHz nuclear magnetic resonance spectrometer operating at 400 and 100 MHz, respectively. Chemical shifts are reported relative to TMS for 1H and 13C NMR spectra. The solvent was [D6] dimethyl sulfoxide ([D6]DMSO) unless otherwise specified. The melting and decomposition points were recorded using differential scanning calorimetry (DSC) at a scan rate of 10 °C min−1 in a dynamic nitrogen atmosphere (flow rate = 50 mL min−1). Infrared spectra were recorded using a Bruker Alpha with a ATR-Ge device. Densities were measured at 25 °C using a Micromeritics Accupyc II 1340 gas pycnometer. Elemental analyses were obtained on an Elementar Vario MICRO CUBE (Germany) elemental analyzer.X-ray crystallography
Crystals of 2, 3 and 9 were removed from the flask and covered with a layer of hydrocarbon oil. A suitable crystal was then selected, attached to a glass fiber, and placed in the low-temperature nitrogen stream. Data for 2 were collected at 153(2) K while for 3 and 9 were collected at 163(2) K, using a Rigaku Saturn724 CCD (AFC10/Saturn724+ for 7) diffractometer equipped with a graphite-monochromatized MoKα radiation (λ = 0.71073 Å) using omega scans. Data collection and reduction were performed and the unit cell was initially refined by using Crystalclear-SM Expert 2.0 r2 software.29 The reflection data were also corrected for Lp factors. The structure was solved by direct methods and refined by the least squares method on F2 using the SHELXTL-97 system of programs.30 Structures were solved in the space group P21/c for 2, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) for 3, PC2/c for 9, by analysis of systematic absences. In this all-light-atom structure the value of the Flack parameter did not allow the direction of polar axis to be determined and Friedel reflections were then merged for the final refinement. Details of the data collection and refinement are given in Table 2.
for 3, PC2/c for 9, by analysis of systematic absences. In this all-light-atom structure the value of the Flack parameter did not allow the direction of polar axis to be determined and Friedel reflections were then merged for the final refinement. Details of the data collection and refinement are given in Table 2.
3-Nitro-1-(2H-tetrazol-5-yl)-1H-1,2,4-triazol-5-amine (HANTT, 2)
At 0 °C, 10 mmol cyanogen bromide was dissolved in 50 mL anhydrous acetonitrile to which was added 40 mmol sodium azide. The reaction mixture was stirred at 0–5 °C for 4 h. The inorganic salt was filtered off. The cyanogen azide solution is added to a solution containing 5 mmol ANTA which has been neutralized in 20 mL water at 0 °C. After stirring over 4 h at ambient temperature, the solvent was removed in air. The product was purified by washing with water and acetonitrile. Yield 78%, yellow solid, m.p. 246 °C. 1H NMR ([D6]DMSO, 400 MHz, 25 °C, TMS): δ = 7.96 (s, 1H), 6.31 (brs, 2H) ppm. 13C NMR ([D6]DMSO, 100 MHz, 25 °C): δ = 161.8, 156.8, 154.6 ppm. IR (neat): 3568, 3417, 3167, 1639, 1582, 1554, 1518, 1404, 1307, 1218, 1155, 1020, 990, 846, 766, 722, 612, 478 cm−1. MS (ESI): 196 (M − H)−. Anal. calcd for C3H3N9O2: C 18.28, H 1.53, N 63.95%; found C 18.28, H 1.54, N 63.93%.General procedures for the preparation of energetic salts 3–9
Ba(OH)2·8H2O (158 mg, 0.5 mmol) was added to a solution of HANTT (197 mg, 1.0 mmol) in H2O (10 mL) and the resulting mixture was stirred at 60 °C until all of the solids had dissolved, and the sulfate salt (0.5 mmol) was added and the mixture was stirred at 60 °C for an additional 2 h. After removal of BaSO4, the solvent was evaporated in vacuo and the residue was recrystallized from H2O to give the target product.References
- (a) P. Yin, J. Zhang, C. He, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2014, 2, 3200–3208 RSC; (b) D. Chand, D. A. Parrish and J. M. Shreeve, J. Mater. Chem. A, 2013, 1, 15383–15389 RSC; (c) V. Thottempudi, P. Yin, J. Zhang, D. A. Parrish and J. M. Shreeve, Chem.–Eur. J., 2014, 20, 542–548 CrossRef CAS PubMed; (d) Y. Tang, H. Yang, J. Shen, B. Wu, X. Ju, C. Lu and G. Cheng, Eur. J. Inorg. Chem., 2014, 7, 1231–1238 CrossRef; (e) Q. Zhang, C. He, P. Yin and J. M. Shreeve, Chem. – Asian J., 2014, 9, 212–217 CrossRef CAS PubMed; (f) D. Srinivas, V. D. Ghule and K. Muralidharan, RSC Adv., 2014, 4, 7041–7051 RSC; (g) D. Fischer, T. M. Klapötke, M. Reymann and J. Stierstorfer, Chem.–Eur. J., 2014, 20, 6401–6411 CrossRef CAS PubMed; (h) N. Fischer, K. Hüll, T. M. Klapötke and J. Stierstorfer, J. Heterocycl. Chem., 2014, 51, 85–95 CrossRef CAS; (i) P. Yin, D. A. Parrish and J. M. Shreeve, Chem. – Asian J., 2014, 20, 6707–6712 CAS; (j) D. Izsak, T. M. Klapötke and S. Reuter, Eur. J. Inorg. Chem., 2013, 5641–5651 CrossRef CAS; (k) A. A. Dippold, D. Izsak and T. M. Klapötke, Chem.–Eur. J., 2013, 19, 12042–12051 CrossRef CAS PubMed.
- J. Zhang and J. M. Shreeve, J. Am. Chem. Soc., 2014, 136, 4437–4445 CrossRef CAS PubMed.
- A. A. Dippold and T. M. Klapötke, J. Am. Chem. Soc., 2013, 135, 9931–9938 CrossRef CAS PubMed.
- A. K. Sikder, M. Geetha, D. B. Sarwade and J. P. Agrawal, J. Hazard. Mater., 2001, 82, 1–12 CrossRef CAS.
- K. Y. Lee, C. B. Storm, M. A. Hiskey and M. D. Coburn, J. Energ. Mater., 1991, 9(5), 415–428 CrossRef CAS.
- A. A. Dippold, T. M. Klapötke, F. A. Martin and S. Wiedbrauk, Eur. J. Inorg. Chem., 2012, 2429–2443 CrossRef CAS.
- D. Izsak, T. M. Klapötke, R. Scharf and J. Stierstorfer, Z. Anorg. Allg. Chem., 2013, 639(10), 1746–1755 CrossRef CAS.
- D. L. Naud, M. A. Hiskey and H. H. Harry, J. Energ. Mater., 2003, 21, 57–62 CrossRef CAS.
- S. Jia, B. Wang, C. Hao, H. Zhang, Q. Zhou and X. Wang, Chin. J. Explos. Propellants, 2012, 35, 23–26 CAS.
- A. K. Sikder, M. Geetha, D. B. Sarwade and J. P. Agrawal, J. Hazard. Mater., 2001, 82, 1–12 CrossRef CAS.
- S. Jia, X. Wang, B. Wang, H. Zhang and C. Xiong, Chin. J. Energ. Mater., 2008, 16(3), 251–253 CAS.
- D. E. Chavez and D. A. Parrish, Propellants, Explos., Pyrotech., 2012, 37, 536–539 CrossRef CAS.
- W. S. Mcewan and M. W. Rigg, J. Am. Chem. Soc., 1951, 73, 4725–4727 CrossRef.
- L. E. Fried, M. R. Manaa, P. F. Pagoria and R. L. Simpson, Annu. Rev. Mater. Res., 2001, 31, 291–321 CrossRef CAS.
- G. Drake, T. Hawkins, A. Brand, L. Hall, M. McKay, A. Vij and I. Ismail, Propellants, Explos., Pyrotech., 2003, 28, 174–180 CrossRef CAS.
- (a) Y. Joo and J. M. Shreeve, Eur. J. Org. Chem., 2009, 3573–3578 CrossRef CAS; (b) Y. Joo, B. Twamley, S. Garg and J. M. Shreeve, Angew. Chem., Int. Ed., 2008, 47, 6236–6239 CrossRef CAS PubMed; (c) Y. Joo and J. M. Shreeve, Org. Lett., 2008, 10, 4665–4667 CrossRef CAS PubMed; (d) Y. Joo and J. M. Shreeve, Angew. Chem., Int. Ed., 2010, 49, 7320–7323 CrossRef CAS PubMed; (e) Y. Joo and J. M. Shreeve, Angew. Chem., Int. Ed., 2009, 48, 564–567 CrossRef CAS PubMed; (f) G. Tao, B. Twamley and J. M. Shreeve, J. Mater. Chem., 2009, 19, 5850–5854 RSC.
- L. Liang, K. Wang, C. Bian, L. Ling and Z. Zhou, Chem.–Eur. J., 2013, 19, 14902–14910 CrossRef CAS PubMed.
- H. Huang, Z. Zhou, L. Liang, J. Song, K. Wang, D. Cao, W. Sun, C. Bian and M. Xue, Chem. – Asian J., 2012, 7, 707–714 CrossRef CAS PubMed.
- K. Wang, D. A. Parrish and J. M. Shreeve, Chem.–Eur. J., 2011, 17, 14485–14492 CrossRef CAS PubMed.
- J. Song, Z. Zhou, X. Dong, H. Huang, D. Cao, L. Liang, K. Wang, J. Zhang, F. Chen and Y. Wu, J. Mater. Chem., 2012, 22, 3201–3209 RSC.
- J. P. Agrawal and R. D. Hodgson, Organic Chemistry of Explosives, Wiley, New York, 2007 Search PubMed.
- J. P. Agrawal, High Energy Materials: Propellants, Explosives and Pyrotechnics, Wiley–VCH, Weinheim, 2010 Search PubMed.
- (a) http://www.bam.de ; (b) A portion of 20 mg of ANTT salts 3–10 was subjected to a fall hammer test using 5 kg weight. A range in impact sensitivities from UN recommendations: Insensitive >40 J; less sensitive ≥35 J; sensitive ≥4 J; very sensitive ≤3 J.
- M. Suceska, EXPLO5.05 program, Croatia, Zagreb, 2011 Search PubMed.
- E. Garcia and K. Lee, Acta Cryst., 1992, 48, 1682–1683 Search PubMed.
- F. H. Allen, R. Taylor, D. G. Watson, O. Kennard, A. G. Orpen and R. Taylor, Int. Tables Crystallogr., 2006, vol. C, 790–811 Search PubMed.
- L. Liang, H. Huang, K. Wang, C. Bian, J. Song, L. Ling, F. Zhao and Z. Zhou, J. Mater. Chem., 2012, 22, 21954–21964 RSC.
- H. Huang, Z. Zhou, L. Liang, J. Song, K. Wang, D. Cao, C. Bian, W. Sun and M. Xue, Z. Anorg. Allg. Chem., 2012, 638(2), 392–400 CrossRef.
- CrystalClear, SM Expert 2.0 r2, An Integrated Program for the Collection and Processing of Area Detector Data, Rigaku Corporation, 2009 Search PubMed.
- G. M. Sheldrick, SHELXTL–97, Structure Determination Software Suite, Bruker AXS, Madison WI, 2008 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Ab initio computational data. CCDC 1017630 (2), 1017631 (3) and 1017632 (9). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ta04107k |
| This journal is © The Royal Society of Chemistry 2015 |