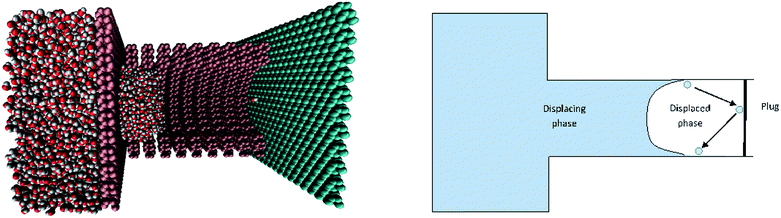
Effect of entrapped phase on the filling characteristics of closed-end nanopores
Chirodeep
Bakli
and
Suman
Chakraborty
*
Department of Mechanical Engineering, Indian Institute of Technology Kharagpur, Kharagpur 721302, India. E-mail: suman@mech.iitkgp.ernet.in
First published on 28th October 2014
Abstract
We investigated the filling dynamics in closed-end capillaries of sub-micron length scale, in which the displacing phase advances at the expense of the entrapped phase. Contrary to common intuition, we reveal that the existence of a displaced phase in a closed-end nano-scale system does not necessarily retard the meniscus advancement over all temporal regimes, unlike what is observed in cases of macro-scale capillaries, but can also sometimes augment the local filling rates. We determined that the combined effect of surface wettability and the displaced phase molecules resulted from the pinning–depinning of the meniscus, and hence, from the local dynamics of capillary filling. We also employed a simple force balance-based model to capture the essential interfacial phenomena governing this behavior, and benchmarked the same with our molecular dynamics simulations. Our results suggest a possible mechanism for modifying the effective wettabilities of nano-scale capillaries without any modification of the surface architecture or chemical treatment of the surface.
Introduction
Capillary flows are ubiquitous in several natural phenomena and also find numerous applications in a varied spectrum of industrial processes. With diminishing length scales, the increasing surface area-to-volume ratio tends to make the surface forces dominate over bulk forces and hence, aid capillarity. Thus, capillarity often becomes an effective mechanism for propelling fluids without the use of any external pressure gradients in conduits of micrometer and nanometer length scales.1,2 The implications of capillary filling over small scales, therefore, are thus wide ranging, encompassing applications spanning from nanofluidic devices,3 porous nanomaterials,4 geophysics,5 and enhanced oil recovery,6 to biomembranes.7Traditionally, in many of the above applications, the Lucas Washburn (LW) equation8,9 captures the essential features of capillary-filling dynamics, using a balance of viscous and surface tension forces. However, miniaturization induces certain extraneous factors to the classical LW description. Consequently, the phenomenon of capillarity and the associated motion of the three-phase contact line, over miniaturized scales, are considered to be profoundly affected by aspects such as substrate wettability,10–14 roughness,14–20 dynamic contact angle (DCA),21–24 and the presence of ionic impurities.25–28 Despite these investigations, the contact line motion through the course of capillary imbibition over miniaturized scales remains one of the least understood topics in the field of interface science. The contact line, which is formed at the junction of fluid(s) and the confining solid boundary, may primarily evolve with the movement of fluid with respect to a stationary solid, and such examples occur in oil extraction6 and film spreading.29 However, interfaces may also evolve as a solid moves in a stationary fluid pool such as in the case of a recording disk drive.30 As opposed to bulk fluid motion, the contact line dynamics with the no-slip boundary condition poses a singularity31 accompanied by a multi-valued behavior in the velocity. In macro-scale flows, this singularity is generally resolved by considering the fluid film moving over a thin film that instantaneously prewets the surface.32 However, this assumption becomes questionable over reduced length scales. This may be attributed to the fact that below the micron regime, molecular diffusion plays a significant role. Thus, the presumption of any film between the bulk fluid and solid surface becomes empirical. Moreover, barring the cases of completely wettable substrates, rapid propagation of a prewetting film over partially wetting materials seems to be improbable.
The effects of the displacing liquid medium as well as that of the solid substrate in contact line motion in narrow capillaries have been extensively discussed. However, insufficient insight has been available on the influence of an entrapped displaced phase in a closed-end nano-capillary. Numerous research investigations have recently been focused to elucidate the effects of an entrapped phase on capillary dynamics.33–41 One important conclusion drawn from these studies is that the displaced phase would trivially retard the motion of the displacing fluid in a closed-end capillary, owing to increasing density of the entrapped molecules. However, possible extrapolation of the underlying considerations over capillaries of nanometer scale has yet to be comprehensively addressed.
Here, we attempt to understand the implications of the displaced phase towards the filling of a closed-end capillary of nanometer length scale. We show that the presence of the trapped phase does not necessarily slow down the meniscus movement in nano-scale capillaries. Rather, the meniscus speed may turn out to be a non-monotonous function of the extent of the displaced column being trapped. In effect, the meniscus marches by a complex process that combines molecular diffusion and evaporation–condensation at the contact line.42 The pertinent interfacial phenomenon is grossly manifested as the alternate pinning–depinning of the meniscus. We demonstrate that the gas compression between the meniscus and the closed end may non-trivially affect this pinning–depinning of the contact line over nanoscopic scales (for a schematic depiction, see Fig. 1), and may either accelerate or retard the displacing fluid front depending on the concentration of the gas phase and the wettability of the substrate. Through molecular dynamics (MD) simulations, we obtain the conditions under which the displaced phase can impart pseudo-lyophilicity to the substrate, despite otherwise indications of intrinsic wettability. These results may help not only to better comprehend the contact line dynamics in nanochannels in the presence of a trapped gas, but also may facilitate in providing the means for manipulating the ‘effective’ wettability of a substrate without chemically or physically modifying it.
MD simulations
We considered a circular capillary pore attached to a large reservoir containing water molecules. The walls of the reservoir and the capillary are constituted of molecules in an FCC lattice in the <100> plane. The capillary pore forms a continuous crystal structure with the reservoir. The pore is obtained by removing the atoms within a radius of 2 nm. The reservoir contains 48204 water molecules in a dimension of 12 × 12 × 10 nm. In a typical simulation for capillary filling, the setup is confined within a simulation box of comparatively larger dimensions, and the fluid molecules moving into the gas phase have less probability to interact with the imbibing fluid once these molecules leave the bulk. In order to simulate the identical physical conditions of a closed pore, we appended an impermeable solid plug at the other end of the capillary pore that was composed of the same material as that of the capillary walls, and hence having the same intrinsic wettability. This arrangement traps the gas phase molecules in the system, and these gradually get compressed between the imbibing liquid and the capillary plug. This setup attempts to mimic the conditions that exist in a natural closed-end capillary that is widely encountered in petroleum and shale-oil extraction. The wall atoms are allowed to vibrate about their equilibrium positions instead of being rigid. This corresponds to a more physically realistic situation and removes the artifacts that may be incurred due to fixed walls.43 The wall atoms are tethered to springs of sufficiently high spring constants so as to prevent vibrations over a threshold level.44 The walls are thermostatted using a Nose–Hoover thermostat at 300 K and the fluid molecules are allowed to exchange heat via collisions with the flexible wall and hence maintain the temperature.Water molecules are modeled using the SPC/E model.45 We initially arranged the water molecules in a regular lattice by defining the three coordinates of each particle in a Cartesian coordinate system and then minimized the energy of the system. The process of energy minimization ensures that the liquid is in a random molten configuration to begin the actual simulations. The initial velocities were calculated using a Maxwellian distribution at 300 K. At first, the system is equilibrated with a hypothetical impenetrable wall at the pore entrance, and the equilibrated system is then allowed to evolve to build the vapor pressure in the pore with this hypothetical wall being removed, and finally, the imbibition simulation is conducted. The time integration of the equation was performed using a leapfrog algorithm with the potentials of the individual particle described. The van der Waals interactions were accounted for by considering hetero-nuclear Lennard-Jones (LJ) interactions applied using standard mixing rules.46 The wall molecules were chosen to be LJ particles. For the wall LJ parameters, we chose, σww = 0.35 nm, and εww was varied from 0.1–6 kJ mol−1 to obtain variation of substrate wettability from hydrophobic to completely wettable capillary pores. The electrostatic interactions were accounted for using a particle-particle particle-mesh (PPPM) algorithm. For the simulations, we chose a van der Waals cut-off of 5σ. We selected various capillary pore lengths as will be discussed later, and the simulation time depends on the length of the capillary to be filled. Typical simulations run for 2 ns (2 × 106 time steps) and take approximately 50 hours ns−1 of simulation on i7 processors. We used a leapfrog algorithm to numerically integrate the equations of motions, and the size of the time step chosen was 0.001 ps. The data were averaged over 50 simulation runs to obtain statistically reliable and robust results.
The meniscus position was determined by tracking the imbibing fluid density along the capillary axis. The location where the density drops to half of the bulk density was considered to be the location of the meniscus.24 We obtained the shape of the meniscus by binning the capillary pore, and hence, measuring the contact angle. We divided the capillary into cylindrical shells with a size that is small enough to obtain the appropriate curvature shape without causing abrupt density fluctuations in the resulting profile. The curvature gives a qualitative idea regarding the contact angle of the liquid imbibing through the capillary. The contact angle, being a macroscopic quantity, does not have a true physical counterpart for a flowthrough nanochannel. However, in this work, one of our aims is to provide a blueprint for experimental systems and for oil extraction and other similar applications in the industrial paradigm where every parameter can be physically realized. Accordingly, we attempted to quantify the channel wettability (an intrinsic physical property that is embodied into the surface by virtue of its atomic arrangement) in terms of the contact angle, which is a macroscopic property that can be easily measured in experiments. In order to provide this unified representation, we measured the contact angles of sessile droplets on surfaces similar to the one used in the nanochannel by varying the Lennard-Jones potential parameter. Following this, we replicated the same interaction parameters for the particles constituting the walls of the nanochannel, and mapped the contact angle values obtained in the sessile drop simulation. This, in turn, defines the channel wettability in terms of the contact angle.
MD results
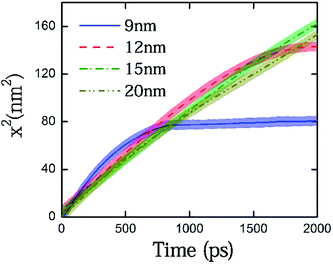
We first considered several cases of closed-end capillaries, with solid plugs being located at x = 9, 12, 15 and 20 nm and the distances being measured from the junction of the reservoir and capillary pore. All capillaries are considered to be perfectly wettable. A perfectly wettable capillary would have a thin film composed of the imbibing phase that moves ahead of the meniscus, and the bulk fluid moves on a prewetted surface. Thus, for wettable surfaces, the invading water front encounters an atomically smooth surface that intrinsically exhibits minimum contact angle hysteresis.15,47 As we discuss later in the text, if a perfectly wettable pore demonstrates flow alteration caused by the plug, the partially wettable pores would be more likely to demonstrate similar behavior that is possibly amplified. We plotted the square of meniscus height versus time to represent the primary meniscus dynamics, as depicted in Fig. 2; the meniscus height was measured from the junction of the reservoir and the capillary pore. The initial filling rates for all the closed capillaries remained similar. As the meniscus hits the plug, the level plateaus at the plug height with small fluctuations. The meniscus hitting the plug, however, is only captured for the two shortest capillaries within the time frame chosen to plot the trends. A closer observation of the plots yields an interesting trend – in each of the capillaries, before the menisci hit the plug, there occurs a temporal regime where the filling rate is larger than for the larger capillaries. For the plug placed at l = 9 nm, the zone lies somewhere between 500–700 ps, where it can be easily observed that the blue (bold) line is above the other curves. In the case of l = 12 nm, this ‘take-off’ zone lies somewhere between 800–1000 ps. For l = 15 nm and l = 17 nm, the corresponding zone exists beyond 1500 ps. For the largest capillary, a similar zone exists which has been excluded from the displayed plot, in order to obtain a magnified view of the phenomenon occurring. Analyzing from a continuum-based framework and referring to previously reported macro-scale capillary filling studies,33,40 one would conclude that shorter capillaries should have shown a filling rate that is always lower than a larger capillary, or at most, equal. A shorter pore ensures faster gas compression between the meniscus and the plug, and hence is likely to exhibit a more pronounced effect of flow retardation owing to the viscosity of the displace phase. However, our preliminary findings seem to contradict this intuitive notion.Here, we are more interested in the filling rates than the meniscus position. Therefore, we tried to directly compare the instantaneous filling rates, i.e., the local meniscus velocities for capillaries of various lengths. If we presume that the plug height would induce velocity variation in the capillaries, then in that case, plotting the velocities on the same time scale would not help in deciphering the trend. To circumvent this problem, we plotted the velocities of these four completely wettable capillaries having different plug heights with respect to the meniscus height in Fig. 3. This representation helps us to efficiently capture both the temporal march of the capillary front and the distance of the meniscus from the plug. The velocity profiles were smoothened to remove the statistical fluctuations so as to aid the eye towards capturing the trends in the evolving meniscus. The blue (bold) line for the shortest capillary has the maximum initial velocity, which gradually decays as the plug is approached and the trend finally reaches a plateau. Following this, one can also observe that the meniscus velocity assumes maximum values in orders of increasing plug heights. In the representative plot shown, the longest capillary has yet to reach the maxima, which occurs after 12 nm. This unexplained increase in velocity at an intermediate distance from the plug has not been previously captured, with the primary reason for this deficit being the macroscopic nature of the reported studies on the closed capillaries. The study we conducted here, as per our knowledge, is the first molecular-level investigation conducted for imbibition in closed capillary pores, and this can be attributed to the unprecedented results observed. We will make an endeavor to explain these trends in our subsequent discussions. Towards that, we will also bring out a non-trivial contribution of the intrinsic contact angle.
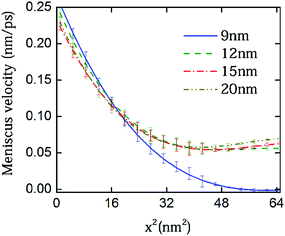 | ||
| Fig. 3 Comparison of filling rates for closed capillaries with the plug placed at different locations. The velocity of the meniscus front is plotted as a function of the square of the meniscus height. The observation of an intermediate acceleration to the meniscus made in Fig. 2 is reinforced. The profiles have been smoothened to remove statistical fluctuations. The filtered profiles clearly depict successive attaining of velocity maxima according to the capillary length. | ||
Theoretical model
We begin with a reduced order model that attempts to capture the essential physics of interest in an LW skeleton of filling dynamics, albeit considering a single fluid in an open-ended capillary, augmented with Bosanquet's analysis:8,9,37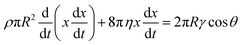 | (1) |
We next incorporate the contribution of the gas phase to the above equation, pertinent to a closed-end capillary. Towards this, we first accommodate the momentum of the gas phase and the viscous resistance offered by the gas phase by assuming a hypothetical length scale xg over which the gas in the capillary pore senses the meniscus motion:33
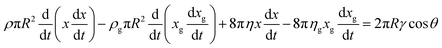 | (2) |
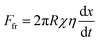 | (3) |
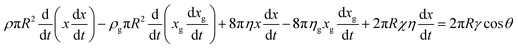 | (4) |
For a closed-end capillary, one may substitute xg with L − x to yield:
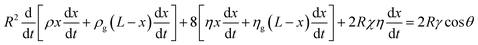 | (5) |
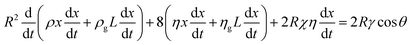 | (6) |
Integrating eqn (6) with initial conditions 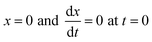 gives:
gives:
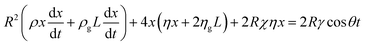 | (7) |
Furthermore, substitution of our simulation parameters in eqn (7) leads to negligible inertial effects, so that one may write:
2x(ηx + 2ηgL) + Rχηx = Rγ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θt θt | (8) |
This, upon solving for a constant value of the friction parameter, yields:
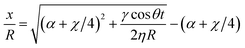 | (9) |
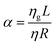 . On substituting α = 0 and χ = 0 in the above equation, we return to the original LW form.
. On substituting α = 0 and χ = 0 in the above equation, we return to the original LW form.
The above equation, considering χ to be a single valued function of the static contact angle, would indicate trivial meniscus retardation in closed-end capillaries, as compared to open-ended ones. However, the same may not be a complete representative of the prevailing physical scenario. This may be attributed to the fact that the friction force in a closed capillary system is not only a function of intrinsic wettability as in an open capillary pore, but also is likely to be a function of the plug distance (i.e., the total length of the channel) that dictates the action of molecules being reflected at the plug, thereby altering the behavior of the three-phase contact line. Therefore, we move on to describe the form of χ in the absence and presence of the plug, and the implication of the same towards altering the capillary filling dynamics.
Towards this, one may first note that the mass transfer occurring at the fluid front, and the pressure changes due to it, has a characteristic time scale longer than the time scale of establishing local mechanical equilibrium between the liquid and the vapor phases at the interface.51 This, when upscaled, transcribes to the phenomenon of pinning–depinning. In other words, many cycles of liquid–gas rearrangement precede an avalanche of the fluid in the capillary pore. This rearrangement generates the disjoining pressure which counterbalances the capillary force, and the meniscus remains pinned until the meniscus perceives an increased pressure on the liquid side due to accumulation of the molecules corresponding to the high-density phase at the interface. Drawing a simple dynamical analogy, we can say that the disjoining pressure or the pressure deficit at the meniscus acts akin to a friction force at the timescale of hydrodynamic pressure changes. The molecular phenomenon of fluid–vapor rearrangement, being of a shorter timescale, cannot be directly plugged in eqn (8); however, disjoining pressure in the form of a friction force can accommodate the effect of the gas layer adjoined to the interface on the meniscus dynamics, which, in turn, depends on the local interfacial film thickness.
At the molecular level, the notion of such a film boils down to a spike in the molecular number density near the wall, and the molecules do not have an identity of the phase in which they exist. Here, the definition of the local film thickness h combines molecules from both the displaced phase and the imbibing phase, and can be roughly estimated to be scaling with the distance of the molecular density peak from the wall. Interestingly, we observe that for closed capillary systems, the location of the density peaks remains unaltered with changes in intrinsic substrate wettability. Thus, we conclude that while for an open capillary system, the meniscus friction is a strong function of wettability, for closed capillary systems, the meniscus friction becomes a strong function of the thickness of the leading film or the concentration of the species just ahead of the meniscus rather than the wettability of the surface. Thus, with a plug in front of the capillary (i.e., a closed-end capillary), the value of h becomes invariant with the intrinsic contact angle of the substrate. Also, we observe that the location of the density peak for closed pores is quite similar to that of a completely wettable open pore, irrespective of the intrinsic wettability of the closed pore. As the film thickness strongly dominates the contact line friction, we can conclude that the contact line friction becomes independent of the wettability of the substrate for a closed capillary. With this knowledge, the dynamics of any closed capillary can be derived from eqn (8) as:
2x(ηx + 2ηgL) + Rχ0ηx = Rγ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θt θt | (10) |
Comparison with MD simulations
In order to delve deeper into the physics of meniscus formation and propulsion through nano-capillary pores in the presence of a trapped gas phase, we simulated capillary filling through open and closed capillaries of different wettabilities and compared the MD simulation predictions with the corresponding theoretical predictions. To impart visual ease to the comparison, we plotted the square of the local filling length with time, both in reduced units (see Fig. 4). For the closed capillary simulations, the plug is placed at a distance of 15 nm from the capillary pore entrance. The dynamics of the meniscus are shown for the first 2 ns during which the meniscus level remains at a considerable distance from the plug in order to avoid the dynamics of direct flow choking by the plug. We deliberately made this choice to ensure that the meniscus, even for the completely wettable case, remains at a distance greater than the van der Waals cut-off from the plug.46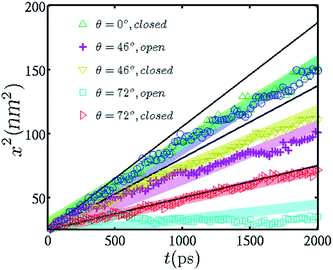 | ||
| Fig. 4 Comparison of meniscus filling for open and closed capillaries of different intrinsic wettabilities. While the filling rate remains almost the same for completely wetting capillary walls, it increases for partially wetting walls and registers a larger increase for walls with lower wettability values. The shaded lines depict the results of the LW equation extended with the contact line friction term. For open pores, the meniscus friction coefficient was determined by simulating droplet motion. In particular, we executed droplet-based simulations on surfaces similar to the capillary walls considered here and artificially tuned the ambient humidity of the droplet.54,55 For closed pores, the same was obtained as an extension to completely wettable open pores (see the text for details). | ||
Several interesting features are revealed from Fig. 4. First, for completely wettable capillary pores, the difference in meniscus dynamics for closed and open capillaries is negligible. Although the velocities might be different during the filling process, the integral distance traversed by the meniscus does not show any discernable difference between the open and closed cases. In previous studies that utilized completely wettable capillaries, only the retardation of capillary flow due to the enhanced viscous force of the entrapped gas layer was observed.33 However, non-intuitive results may be obtained as we reduce the wettability of the capillary walls and compare the data obtained for closed and open channels. The filling rate of capillaries of lower wettability shows an appreciable increase for closed capillaries as compared to open ones. This observation is completely counter-intuitive and is in direct opposition to the macro-scale results for closed capillary systems.33–38 The closed capillaries with intermediate wettability values (a representative result shows a capillary with walls having a contact angle of 46°) show a slight increase in the filling rate; however, capillaries that have walls with less intrinsic wettability (contact angle approaching 90°) depict a tremendous instantaneous increase in the filling rates. We illustrate this behavior for an intrinsic contact angle of 72° in Fig. 4.
A closer observation of Fig. 4 reveals yet another interesting phenomenon. The filling rates for both open and closed capillaries with a particular wettability remain the same at the initial stages of filling. The rates of filling for closed capillaries start increasing after a certain time has elapsed. Again, the time at which this filling rate ‘take-off’ occurs is increased for more wettable capillaries. For example, for a contact angle of 46°, the closed capillary filling rate ‘take-off’ occurs at approximately 1100 time units, whereas for a contact angle of 72°, it occurs at approximately 700 time units. Thus, there occurs an intermediate temporal regime over which an intrinsically less wettable channel exhibits augmented filling rates as compared to an otherwise intrinsically more wettable channel. This indicates that the presence of the plug would thwart the effect of hydrophobicity. Lesser wettability of the walls in a closed capillary seems to increase the driving force propelling the liquid in the meniscus. In all cases, there is very good agreement between the MD predictions and the theoretical model predictions, within an error bar of ±5%.
Discussion
The results shown above clearly indicate that the impermeable capillary plug has a two-way effect on the meniscus motion. At a distance greater than the range of van der Waals force, the plug aids the ascent of fluid in the capillary; however, within the van der Waals force range, the plug begins to retard the progress of the meniscus. The process of meniscus retardation is well understood and occurs due to the increased resistance offered by the gas molecules that are being progressively compressed between the meniscus and the plug.33–38 However, the initial acceleration of the meniscus signals is a phenomenon that has yet to be elucidated.We note that the plug in a closed capillary interferes with the pinning–avalanche phenomenon occurring at the meniscus. The plug acts as a concentrator and reflector of the molecules in the gas phase, which helps to augment the contact angle at the meniscus and aids in reaching the advancing contact angle value faster. The effect of the plug was observed to be more pronounced in cases of lower intrinsic wettability. Because the meniscus moves forward more favorably by evaporation–condensation rather than surface diffusion for capillaries with lower intrinsic wettability, the presence of the plug dramatically increases the probability of gas molecules condensing back to the meniscus zone and leads to faster occurrences of pinning–avalanche cycles. The plug alters the gas concentration and dynamics in the confined zone above the meniscus. This, in turn, alters the pinning and depinning of the meniscus.
For a completely wettable pore, a prewetting film spreads rapidly ahead of the meniscus, leading to minimum contact angle hysteresis. Pores having lower wettability, however, are expected to have greater contact angle hysteresis and intrinsically lower filling rates of a capillary with intermittent pinning phases that are prolonged. The presence of a plug, in such a case, accelerates the pinning–depinning cycle on account of the increased number of molecules moving into the gas phase due to lower pore wettability. Thus, with a plug, there is a two-way increase in the filling rates of pores of lower wettability. Firstly, compared to the open pore, the closed pore enhances the depinning of the meniscus, and secondly, the increased number of gas molecules due to lower wettability again serves as a precursor for movement of the meniscus via diffusion. We observe the same in Fig. 4, where the filling rate for a capillary with a contact angle of 72° for pure water shows a colossal increase as compared to the open capillary case upon introduction of the plug. The take-off in the filling rates of the closed capillaries occurs when the particles in the gas phase start interacting with the plug. A lower intrinsic wettability of the capillary walls is accompanied by an increase in the escape of molecules into the gas phase, and hence, the take-off would occur at an earlier stage. The combined effect of hydrophobicity and the presence of a plug increases the gas concentration near the meniscus, resulting in a faster capillary rise.
For higher values of the static contact angle, the film ahead of the imbibing fluid is not rich in molecules; however, the interfacial gas phase has more molecules owing to substrate hydrophobicity. Substrates with lower static contact angle values have an extended film that compensates for the poor concentration of trapped gas molecules. This implies that a closed capillary would have a constant meniscus friction coefficient irrespective of the intrinsic wettability. With this hypothesis, one may equate the value of the meniscus friction coefficient of a completely wettable closed pore to that of a completely wettable open pore (assuming completely wettable pores lead to instantaneous film formation ahead of the meniscus as soon as the meniscus is formed at the interface) and substitute the same value in eqn (9) for pores with different contact angles. The above conceptual paradigm has been verified by solid agreement between our theoretical predictions and MD predictions.
Conclusions
Using a comparison between the interfacial phenomena characterizing meniscus dynamics in open and closed capillaries of nanoscopic length scales under similar conditions, we demonstrated through MD simulations that the existence of a closed end does not trivially oppose the fluid filling in the capillary, but may also accelerate the rate of filling over an intermediate temporal regime. We attribute the same to the physics of meniscus formation and a pinning–depinning mechanism over nanometer-length scales. This, in turn, offers the possibility of accelerating the capillary filling rate in nanopores by realizing the interplay of dynamical interactions between the displacing and the displaced phase towards altering the interfacial friction. This opens several prospects in natural systems, for example, in oil recovery systems in which closed-end nanocapillaries are omnipresent. Interestingly, the presence of a closed end enhances the instantaneous throughput for a partially wettable system more than a completely wettable system, and this property can be used for many industrial purposes.Notes and references
- J. Boreyko and C.-H. Chen, Phys. Rev. Lett., 2009, 103, 184501 CrossRef.
- G. R. Willmott, C. Neto and S. C. Hendy, Soft Matter, 2011, 7, 2357 RSC.
- P. Abgrall and N. T. Nguyen, Anal. Chem., 2008, 80, 2326–2341 CrossRef CAS PubMed.
- S. Darwich, K. Mougin and H. Haidara, Soft Matter, 2012, 8, 1155 RSC.
- D. Lohse, Phys. Today, 2003, 56, 36–41 CrossRef CAS PubMed.
- H.-Q. Sun, L. Zhang, Z.-Q. Li, L. Zhang, L. Luo and S. Zhao, Soft Matter, 2011, 7, 7601 RSC.
- X. Li, X. Ma, D. Fan and C. Zhu, Soft Matter, 2012, 8, 3781 RSC.
- R. Lucas, Kolloid-Z., 1918, 23, 15–22 CrossRef CAS.
- E. W. Washburn, Phys. Rev., 1921, 17, 391–283 CrossRef.
- X. Xu and T. Qian, Phys. Rev. E, 2012, 85, 1–17 Search PubMed; P. K. Mondal, D. DasGupta and S. Chakraborty, Phys. Rev. E, 2014, 90, 013003 CrossRef.
- S. Granick, Y. Zhu and H. Lee, Nat. Mater., 2003, 2, 221–227 CrossRef CAS PubMed.
- J. Janecek and R. R. Netz, Langmuir, 2007, 23, 8417–8429 CrossRef CAS PubMed.
- M. Nosonovsky, Nature, 2011, 477, 412–413 CrossRef CAS PubMed.
- C. Bakli and S. Chakraborty, Appl. Phys. Lett., 2012, 101, 153112 CrossRef PubMed.
- D. Quéré, Annu. Rev. Mater. Res., 2008, 38, 71–99 CrossRef.
- A. Niavarani and N. V. Priezjev, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 81, 1–10 CrossRef.
- T. M. Galea and P. Attard, Langmuir, 2004, 20, 3477–3482 CrossRef CAS.
- B. Xu, Y. Li, T. Park and X. Chen, J. Chem. Phys., 2011, 135, 144703 CrossRef PubMed.
- D. Ebert and B. Bhushan, J. Colloid Interface Sci., 2012, 368, 584–591 CrossRef CAS PubMed.
- A. Vadakkepatt, Y. Dong, S. Lichter and A. Martini, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2011, 84, 1–11 CrossRef.
- N. Fries and M. Dreyer, J. Colloid Interface Sci., 2009, 338, 514–518 CrossRef CAS PubMed.
- M. Hilpert, J. Colloid Interface Sci., 2009, 337, 131–137 CrossRef CAS PubMed.
- G. Martic, F. Gentner, D. Seveno, D. Coulon, J. De Coninck and T. D. Blake, Langmuir, 2002, 18, 7971–7976 CrossRef CAS.
- G. Martic, T. D. Blake, J. De Coninck and J. D. Coninck, Langmuir, 2005, 21, 11201–11205 CrossRef CAS PubMed.
- S. Chakraborty, D. Chatterjee and C. Bakli, Phys. Rev. Lett., 2013, 110, 184503 CrossRef; C. Bakli and S. Chakraborty, J. Chem. Phys., 2013, 138, 054504 CrossRef PubMed.
- C. Bakli and S. Chakraborty, Electrophoresis, 2014 DOI:10.1002/elps.201400317.
- C. Bouzigues, P. Tabeling and L. Bocquet, Phys. Rev. Lett., 2008, 101, 114503 CrossRef CAS.
- L. Joly, C. Ybert, E. Trizac and L. Bocquet, J. Chem. Phys., 2006, 125, 204716 CrossRef PubMed; L. Joly, C. Ybert, E. Trizac and L. Bocquet, Phys. Rev. Lett., 2004, 93, 257805 CrossRef.
- C. Chen, L. Zhuang, X. Li, J. Dong and J. Lu, Langmuir, 2012, 28, 1330–1336 CrossRef CAS PubMed.
- Y. Chen, J. Weng, J. R. Lukes, A. Majumdar and C.-L. Tien, Appl. Phys. Lett., 2001, 79, 1267 CrossRef CAS PubMed.
- V. E. B. Dussan and S. H. Davis, J. Fluid Mech., 2006, 65, 71 CrossRef.
- V. Ludviksson and E. N. Lightfoot, AIChE J., 1968, 14, 674–677 CrossRef CAS.
- M. Hultmark, J. M. Aristoff and H. A. Stone, J. Fluid Mech., 2011, 678, 600–606 CrossRef.
- F. Chauvet, S. Geoffroy, A. Hamoumi, M. Prat and P. Joseph, Soft Matter, 2012, 8, 10738 RSC.
- A. V. Pesse, G. R. Warrier and V. K. Dhir, Int. J. Heat Mass Transfer, 2005, 48, 5150–5165 CrossRef CAS PubMed.
- B. V. Zhmud, F. Tiberg and K. Hallstensson, J. Colloid Interface Sci., 2000, 228, 263–269 CrossRef CAS PubMed.
- C. H. Bosanquet, Philos. Mag. Ser. 6, 1923, 45, 525–531 CrossRef CAS.
- R. Fazio and S. Iacono, in Proc. World Congress on Engineering, London, UK, 2009, Vol. II Search PubMed.
- D. Schneider, R. Valiullin and P. A. Monson, Langmuir, 2014, 30, 1290–1294 CrossRef CAS PubMed.
- H. Lim, A. Tripathi and J. Lee, Langmuir, 2014, 9390–9396 CrossRef CAS PubMed.
- V. N. Phan, N.-T. Nguyen, C. Yang, P. Joseph, L. Djeghlaf, D. Bourrier and A.-M. Gue, Langmuir, 2010, 26, 13251–13255 CrossRef CAS PubMed.
- F. B.-W. P.-G. de Gennes, Capillarity and Wetting Phenomena: Drops, Bubbles, Pearls, Waves, Springer, New York, 2003 Search PubMed.
- A. Martini, H. Hsu, N. A. Patankar and S. Lichter, Phys. Rev. Lett., 2008, 206001, 1–4 Search PubMed.
- P. Thompson and M. Robbins, Phys. Rev. Lett., 1989, 63, 766–769 CrossRef CAS.
- H. J. C. Berendsen, J. R. Grigera and T. P. Straatsma, J. Phys. Chem., 1987, 91, 6269–6271 CrossRef CAS.
- M. Allen and D. Tildesley, Computer Simulation of Fluids, Clarendon Press, 1987 Search PubMed.
- N. R. Tas, J. Haneveld, H. V. Jansen, M. Elwenspoek and A. van den Berg, Appl. Phys. Lett., 2004, 85, 3274 CrossRef CAS PubMed.
- L. Joly, J. Chem. Phys., 2011, 135, 214705 CrossRef PubMed.
- a. Hamraoui, J. Colloid Interface Sci., 2000, 226, 199–204 CrossRef CAS.
- T. Andrukh, D. Monaenkova, B. Rubin, W.-K. Lee and K. G. Kornev, Soft Matter, 2014, 10, 609 RSC.
- K. Kornev, A. Neimark and A. Rozhkov, Adv. Colloid Interface Sci., 1999, 82, 127–187 CrossRef CAS.
- K. Kornev and G. Shugai, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1998, 58, 7606–7619 CrossRef CAS.
- A. G. Egorov, K. G. Kornev and A. V. Neimark, Phys. Fluids, 2003, 15, 3134 CrossRef CAS PubMed.
- M. J. de Ruijter, T. D. Blake and J. De Coninck, Langmuir, 1999, 15, 7836–7847 CrossRef CAS.
- A. Balankin, H. Zapata López, E. Pineda León, D. Morales Matamoros, L. Morales Ruiz, D. Silva López and M. Rodríguez, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 87, 014102 CrossRef.
| This journal is © The Royal Society of Chemistry 2015 |