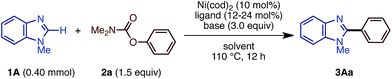Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
C–H arylation and alkenylation of imidazoles by nickel catalysis: solvent-accelerated imidazole C–H activation†
Kei
Muto
a,
Taito
Hatakeyama
b,
Junichiro
Yamaguchi
*a and
Kenichiro
Itami
*ac
aInstitute of Transformative Bio-Molecules (WPI-ITbM) and Graduate School of Science, Nagoya University, Chikusa, Nagoya 464-8602, Japan. E-mail: itami@chem.nagoya-u.ac.jp; junichiro@chem.nagoya-u.ac.jp
bCentral Research Laboratory Technology and Development Division, Kanto Chemicals Co. Inc., Saitama 340-0003, Japan
cJST, ERATO, Itami Molecular Nanocarbon Project, Nagoya University, Chikusa, Nagoya 464-8602, Japan
First published on 8th September 2015
Abstract
The first nickel-catalyzed C–H arylations and alkenylations of imidazoles with phenol and enol derivatives are described. Under the influence of Ni(OTf)2/dcype/K3PO4 (dcype: 1,2-bis(dicyclohexylphosphino)ethane) in t-amyl alcohol, imidazoles can undergo C–H arylation with phenol derivatives. The C–H arylation of imidazoles with chloroarenes as well as that of thiazoles and oxazoles with phenol derivatives can also be achieved with this catalytic system. By changing the ligand to dcypt (3,4-bis(dicyclohexylphosphino)thiophene), enol derivatives could also be employed as coupling partners achieving the C–H alkenylation of imidazoles as well as thiazoles and oxazoles. Thus, a range of C2-arylated and alkenylated azoles can be synthesized using this newly developed nickel-based catalytic system. The key to the success of the C–H coupling of imidazoles is the use of a tertiary alcohol as solvent. This also allows the use of an air-stable nickel(II) salt as the catalyst precursor.
Introduction
Imidazoles, including benzimidazoles, are recognized as important chemical motifs since they are frequently found in a range of natural products, and are exploited as core structures in pharmaceuticals, agrochemicals, and organic materials. Because of the high versatility of imidazole-containing compounds (particularly C2-arylated and alkenylated imidazoles; Fig. 1),1 functionalization and derivatization thereof are of significant importance in synthetic organic chemistry. Numerous synthetic methods to construct C2-aryl and alkenyl imidazoles have been reported thus far. Although cyclization and annulation reactions have found wide use, these methods often suffer from multi-step reaction sequences.2 Transition metal-catalyzed cross-coupling reactions of arylmetal compounds and aryl halides have also been employed, albeit requiring the pre-functionalization of metalated or halogenated imidazoles prior to the coupling reactions.3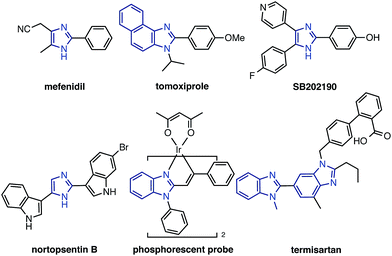 | ||
| Fig. 1 C2-arylated and alkenylated imidazoles and benzimidazoles in natural products, pharmaceuticals, and organic materials. | ||
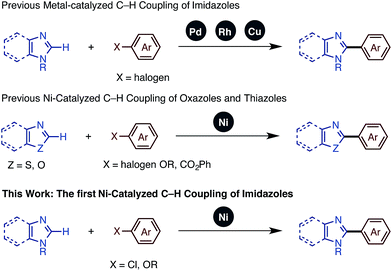
In recent years, the transition metal-catalyzed C–H functionalization approach has attracted attention as it enables rapid and straightforward synthesis of various functional heteroarenes.4 Within this class of reactions, C–H arylations and alkenylations of imidazoles using transition-metal catalysts have been reported, mainly involving palladium5 and rhodium.6 In 2009, Daugulis and coworkers discovered the Cu-catalyzed C–H arylation of imidazoles, which allowed the use of an inexpensive transition metal catalyst.7
In our studies of catalytic C–H functionalization,8 we have developed several unique nickel catalysts9 that facilitate the C–H arylation of 1,3-azoles, such as oxazoles and thiazoles, with haloarenes (C–H/C–X coupling),10 phenol derivatives (C–H/C–O coupling),11 and arenecarboxylates (decarboxylative C–H coupling).12 The advantages of our recent nickel-based catalytic systems11 are not only their low cost, but also their ability to activate and couple phenol derivatives (C–O electrophiles).13,14 However, imidazoles and benzimidazoles still remained challenging substrates for our nickel-catalyzed C–H coupling campaign. Herein, we report the first general protocol for Ni-catalyzed C–H arylation as well as alkenylation of imidazoles (Fig. 2).
Results and discussion
C–H arylation of imidazoles with phenol derivatives
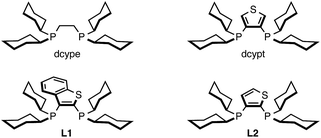
Among the myriad of nickel catalysts for C–H arylation reported over the past decade from our group,10–12 Miura,15 and Chatani,16 our Ni(cod)2/dcype (cod = 1,5-cyclooctadiene; dcype = 1,2-bis(dicyclohexylphosphino)ethane) catalyst with Cs2CO3 as the base in 1,4-dioxane as the solvent is well suited for the direct coupling of 1,3-azoles and phenol derivatives (C–H/C–O coupling). The selection of an appropriate ligand is crucial, as this reaction proceeds only when dcype is used. In contrast, this catalytic system was not affected by the choice of base and solvent since C2-functionalized azoles could be formed in the absence of base. Through several mechanistic investigations including the isolation of a catalytic intermediate, kinetic studies,11c and theoretical calculations,11d we have previously identified the ligand effect and a plausible catalytic cycle for the reaction. In particular, DFT calculations provided significant insight regarding the mechanism of the C–H nickelation steps: the formation of a Cs–Ni cluster was found to play a key roll in accelerating C–H nickelation. However, experimentally and computationally, it has also been revealed that new catalytic conditions need to be developed for the C–H coupling of imidazoles.With these considerations in mind, we focused on the investigation of base and solvent effects by using N-methylbenzimidazole (1A) and phenyl carbamate 2a as model substrates (Table 1). Our previous Ni(cod)2/dcype-based catalytic conditions, employing Cs2CO3 and 1,4-dioxane, afforded no coupling products (entry 1). An intensive and thorough investigation led to the discovery that the combination of K3PO4 and t-amyl alcohol (t-amylOH) could facilitate the C–H arylation of 1A to afford 3Aa in 83% yield (entry 2). The replacement of t-amylOH with t-BuOH gave 3Aa in slightly lower yield (entry 3). When the base and/or the solvent were changed, the reaction efficiency diminished. For example, the use of aprotic solvents and secondary alcohol solvents was completely ineffective for this reaction (entries 4–6). Additionally, the reaction in the presence of Cs2CO3 or LiOt-Bu resulted in lower yields than with K3PO4 (entries 7 and 8). While we initially thought that in situ-generated potassium tertiary alkoxides might be the reactive species, this seems not to be the case; the use of KOt-Bu completely shut down the catalytic activity (entry 9). Regarding the ligand effect, PCy3 and an N-heterocyclic carbene ligand were inactive for the present reaction (entries 10 and 11). Our thiophene-based diphosphine ligand, dcypt (3,4-bis(dicyclohexylphosphino)thiophene),17 as well as other dcype derivatives such as L1 and L2 furnished 3Aa in moderate yields (entries 12–14). To our delight, the replacement of Ni(cod)2 with Ni(OTf)2 as the pre-catalyst maintained the catalytic activity (entry 15). Considering the significant advantage of using air-stable Ni(OTf)2, we decided to use the Ni(OTf)2/dcype catalyst and K3PO4 in t-amylOH for further studies.
| Entry | Ligand | Base | Solvent | 3Aa (%) |
|---|---|---|---|---|
| a Unless otherwise noted, the reaction conditions were as follows: 1A (0.40 mmol), 2a (1.5 equiv.), Ni(cod)2 (10 mol%), ligand (bidentate: 12 mol%, monodentate: 24 mol%), base (3.0 equiv.), solvent (1.6 mL), 110 °C, and 12 h. b GC yield. c Ni(OTf)2 (10 mol%) was used. | ||||
| 1 | dcype | Cs2CO3 | 1,4-Dioxane | 0 |
| 2 | dcype | K3PO4 | t-AmylOH | 83 |
| 3 | dcype | K3PO4 | t-BuOH | 70 |
| 4 | dcype | K3PO4 | 1,4-Dioxane | 0 |
| 5 | dcype | K3PO4 | DMF | 0 |
| 6 | dcype | K3PO4 | i-PrOH | 0 |
| 7 | dcype | Cs2CO3 | t-AmylOH | 44 |
| 8 | dcype | LiOt-Bu | t-AmylOH | 59 |
| 9 | dcype | KOt-Bu | t-AmylOH | 0 |
| 10 | PCy3 | K3PO4 | t-AmylOH | 0 |
| 11 | IPr·HCl | K3PO4 | t-AmylOH | 0 |
| 12 | dcypt | K3PO4 | t-AmylOH | 55 |
| 13 | L1 | K3PO4 | t-AmylOH | 53 |
| 14 | L2 | K3PO4 | t-AmylOH | 64 |
| 15c | dcype | K3PO4 | t-AmylOH | 82 |
|
||||
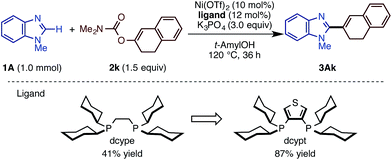
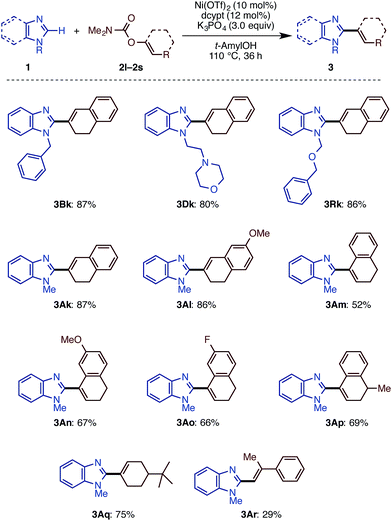
We then examined the substrate scope of the Ni(OTf)2/dcype-catalyzed C–H arylation (C–H/C–O coupling) of imidazoles 1 with carbamates of phenol derivatives 2 (Scheme 1). Several N-substituted benzimidazoles such as N-benzyl (1B), phenyl (1C), morpholinoethyl (1D), and methoxymethyl benzimidazoles (1E) underwent C–H/C–O coupling to afford the corresponding products in moderate to excellent yields. In addition to benzimidazoles, imidazoles were also successfully coupled with phenol derivatives 2, although it was necessary to use Ni(cod)2 as the catalyst precursor as well as a longer reaction time. In general, the coupling proceeded smoothly with the substrates having electron-withdrawing groups on the phenyl rings at the C5 position of the imidazole ring. For example, the reaction of trifluoromethyl-substituted 5-arylimidazole furnished triaryl 3La in a superior yield (>95%) than that for the methyl-substituted triaryl 3Ka (65%). Although the reason remains unclear at this stage, the dcypt ligand gave better results for the coupling of C4-substituted imidazoles (1N, 1O, and 1P). Notably, triazole 1M also underwent C–H/C–O coupling with 2a to afford 5-phenyl N-methyl-1,2,4-triazole (3Ma) in 82% yield. Regarding aryl electrophiles, a broad functional group tolerance was observed. An amino group or nitrogen heterocycle, which often behaves as a catalyst-deactivating group, did not inhibit the reaction; 3Da, 3Ag, and 3Ah were obtained in good to excellent yields. Although transesterification took place with t-amylOH when the ethoxycarbonyl-substituted phenol electrophile 2f was used, the product 3Af was generated in good yield. Delightfully, we could directly functionalize pilocarpine (1Q), which is a drug for the treatment of a dry mouth,18 in 69% yield without any lactone opening or epimerization at the α-position of the carbonyl group. It should be emphasized that this coupling reaction proceeds with high regioselectivity at the C2 position of the imidazole rings.
C–H alkenylation of imidazoles with enol derivatives
Following our previous success of the Ni-catalyzed C–H alkenylation of oxazoles with enol derivatives,11b,19 we envisioned that alkenylation of imidazoles would be feasible under a Ni(OTf)2/diphosphine/K3PO4/t-amylOH system. The use of C–O alkenyl electrophiles for coupling reactions is advantageous because they can be easily prepared from the corresponding ketones and aldehydes. Motivated by the fact that the alkenyl group is a versatile platform in organic synthesis, we next embarked on the development of the C–H/C–O alkenylation of imidazoles (Scheme 2). Although the coupling reaction of 1A and 2k under Ni(OTf)2/dcype catalysis is feasible, it provided the alkenylated product 3Ak in only 41% yield. While changes in base and solvent did not lead to the improvement of the reaction yield, the Ni(OTf)2/dcypt catalyst dramatically boosted the reaction efficiency, providing the coupling product 3Ak in 87% yield.It turns out that this newly discovered Ni(OTf)2/dcypt catalytic system can effectively facilitate the imidazole C–H alkenylation with broad scope (Scheme 3). As with the case of biaryl couplings, several N-alkylated benzimidazoles (such as 1D and 1R) could couple with enol carbamates. Both of the enol carbamates synthesized from α- and β-tetralones were reactive with the Ni(OTf)2/dcypt catalyst to afford the coupling products 3Al–3AP. Cyclohexenyl benzimidazoles could be synthesized from cyclohexanone derived electrophiles. The aldehyde-derived enol derivative 2r also coupled with 1A, but its rather fast decomposition under the reaction conditions turned out to be somewhat problematic. It is known that C–OMe14a,e and C–F bonds20 can be cleaved with nickel complexes, but these groups were completely tolerated in the present Ni-catalyzed coupling reaction.
C–H arylation of imidazoles with chloroarenes
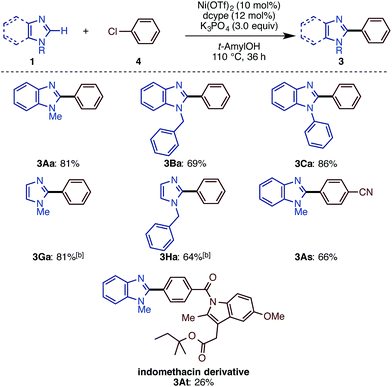
While we mainly focused on the use of C–O electrophiles in this study, it was found that the newly developed Ni(OTf)2/dcype/K3PO4/t-amylOH system is also effective for imidazole arylation with chloroarenes. As shown in Scheme 4, a range of imidazoles and benzimidazoles cross-coupled with chlorobenzene derivatives under the standard conditions. N-Methyl, -phenyl, and -benzyl benzimidazoles underwent C–H arylation with chlorobenzene (4a) to deliver phenylated imidazoles in good yield. N-Methyl and -benzyl imidazoles also reacted as well. Nitrile-substituted aryl chloride 4s furnished the corresponding product 3As in good yield. Although the reaction yield was low (26%), we could apply the indomethacin derivative 4t to the reaction to give 3At, but with significant amounts of a homodimerization by-product. Very interestingly, the reactions of aryl iodides and bromides in turn resulted in poor or zero yields of product.C–H arylation and alkenylation of other 1,3-azoles
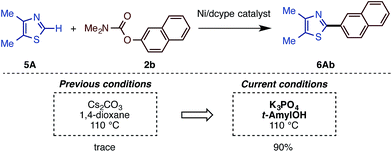
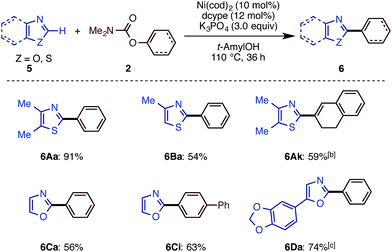
The present catalytic protocols were found to be applicable not only for imidazoles, but also for thiazoles and oxazoles. While our previous catalyst, employing Ni(cod)2/dcype/Cs2CO3 in 1,4-dioxane, was effective for oxazoles and thiazoles (in particular for benzo-fused substrates),11 coupling was not efficient for relatively electron-rich azoles. For example, the reaction of 4,5-dimethylthiazole (5A) with naphthyl carbamate 2b under the previous conditions furnished no coupling product. Thus, we applied our new protocol with K3PO4 and t-amylOH to the reaction of the previously unreactive azole 5A. Gratifyingly, 5A and 2b cross-coupled very smoothly under the present conditions to furnish 6Ab in 90% yield (Scheme 5).Depicted in Scheme 6 are the results of the Ni-catalyzed reactions of the other 1,3-azoles. In addition to thiazoles, oxazoles were also found to be good substrates, generating the corresponding coupling products in good yields. Although previous alkenylation reactions with C–O electrophiles were limited to the reaction of oxazoles, the present Ni-catalyzed reaction in t-amylOH was also applicable to thiazoles.
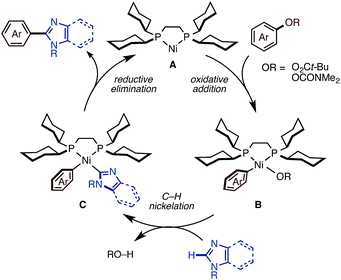
Plausible mechanism
We succeeded in developing new catalytic systems to significantly expand the C–H/C–O coupling of 1,3-azoles with phenol/enol-based electrophiles, but we feel that the basic catalytic cycle with Ni(OTf)2/diphosphine/K3PO4/t-amylOH should be similar to those operating under the previous conditions. First, Ni(OTf)2 should be reduced to a nickel(0) species by the action of diphosphine (dcype or dcypt) and/or an imidazole substrate to initiate a Ni(0)/Ni(II) redox cycle as shown in Fig. 3. Oxidative addition of the C–O or C–Cl bond of the electrophile to Ni(0) A affords intermediate B. Then, base-promoted C–H nickelation of imidazoles, followed by reductive elimination would furnish the coupling products with regeneration of the active Ni(0) species. Previously, we successfully isolated and characterized intermediate B by using naphthalen-2-yl-pivalate as a C–O electrophile,11c which supports our hypothesized catalytic cycle. The remaining question is how the new conditions (particularly the activation modes of K3PO4 and t-amylOH) allow imidazoles to participate in this catalytic cycle. We are currently focusing on uncovering these phenomena experimentally and theoretically.Conclusions
In summary, we have established a general protocol for the nickel-catalyzed C–H coupling of imidazoles. The newly discovered conditions, employing a catalytic amount of Ni(OTf)2/dcype or Ni(OTf)2/dcypt and K3PO4 in t-amylOH, enable direct C–C bond-forming reactions of imidazoles including C–H/C–O arylations and alkenylations. The C–H arylation of imidazoles with chloroarenes as well as that of thiazoles and oxazoles with phenol/enol derivatives can also be achieved with this catalytic system. The key to the success of the new nickel-catalyzed system is the choice of a tertiary alcohol as solvent, as neither aprotic solvents nor secondary alcohols were effective. We believe that the present method provides significant opportunities to synthesize and derivatize valuable functionalized imidazoles.Acknowledgements
This work was supported by the ERATO program from JST (K. I.), KAKENHI (25708005 to J. Y.) from MEXT, and a JSPS research fellowship for young scientists (to K. M.). We thank Ryosuke Takise (Nagoya University) for providing phosphine ligands. ITbM is supported by the World Premier International Research Center (WPI) Initiative, Japan.Notes and references
- L. D. Luca, Curr. Med. Chem., 2006, 13, 1 Search PubMed.
- M. R. Grimmett, Imidazole and Benzimidazole Synthesis, Academic Press, London, 1997 Search PubMed.
- (a) M. Kosugi, M. Koshiba, A. Atoh, H. Sano and T. Migita, Bull. Chem. Soc. Jpn., 1986, 59, 677 CrossRef CAS; (b) A. S. Bhanu Prasad, T. M. Stevenson, J. R. Citineni, V. Nyzam and P. Knochel, Tetrahedron, 1997, 53, 7237 CrossRef.
- For reviews, see: (a) L. Ackermann, R. N. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 48, 9792 Search PubMed; (b) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed; (c) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS PubMed; (d) J. Wencel-Delord and F. Glorius, Nat. Chem., 2013, 5, 369 CrossRef CAS PubMed; (e) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef CAS PubMed; (f) Y. Segawa, T. Maekawa and K. Itami, Angew. Chem., Int. Ed., 2014, 54, 66 CrossRef PubMed.
- For Pd-catalyzed C–H coupling of imidazoles, see: (a) J. M. Joo, B. B. Touré and D. Sames, J. Org. Chem., 2010, 75, 4911 CrossRef CAS PubMed; (b) L.-C. Campeau, D. R. Stuart, J.-P. Leclerc, M. Bertrand-Laperle, E. Villemure, H.-Y. Sun, S. Lasserre, N. Guimond, M. Lecavallier and K. Fagnou, J. Am. Chem. Soc., 2009, 131, 3291 CrossRef CAS PubMed; (c) D. Zhao, W. Wang, S. Lian, F. Yang, J. Lan and J. You, Chem.–Eur. J., 2009, 15, 13370 Search PubMed.
- For Rh-catalyzed C–H coupling of imidazoles, see: (a) J. C. Lewis, S. H. Wiedemann, R. G. Bergman and J. A. Ellman, Org. Lett., 2003, 6, 35 CrossRef PubMed; (b) J. C. Lewis, J. Y. Wu, R. G. Bergman and J. A. Ellman, Angew. Chem., Int. Ed., 2006, 45, 1589 CrossRef CAS PubMed; (c) J. C. Lewis, A. M. Berman, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2008, 130, 2493 CrossRef CAS PubMed; (d) J. C. Lewis, R. G. Bergman and J. A. Ellman, Acc. Chem. Res., 2008, 41, 1013 CrossRef CAS PubMed.
- For Cu-catalyzed C–H coupling of imidazoles, see: (a) H.-Q. Do and O. Daugulis, J. Am. Chem. Soc., 2007, 129, 12404 CrossRef CAS PubMed; (b) H.-Q. Do, R. M. K. Khan and O. Daugulis, J. Am. Chem. Soc., 2008, 130, 15185 CrossRef CAS PubMed; (c) D. Zhao, W. Wang, F. Yang, J. Lan, L. Yang, G. Gao and J. You, Angew. Chem., Int. Ed., 2009, 48, 3296 CrossRef CAS PubMed; (d) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef CAS PubMed.
- (a) S. Yanagisawa, T. Sudo, R. Noyori and K. Itami, J. Am. Chem. Soc., 2006, 128, 11748 CrossRef CAS PubMed; (b) B. Join, T. Yamamoto and K. Itami, Angew. Chem., Int. Ed., 2009, 48, 3644 CrossRef CAS PubMed; (c) S. Yanagisawa, K. Ueda, H. Sekizawa and K. Itami, J. Am. Chem. Soc., 2009, 131, 14622 CrossRef CAS PubMed; (d) K. Ueda, S. Yanagisawa, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2010, 49, 8946 CrossRef CAS PubMed; (e) S. Kirchberg, S. Tani, K. Ueda, J. Yamaguchi, A. Studer and K. Itami, Angew. Chem., Int. Ed., 2011, 50, 2387 CrossRef CAS PubMed; (f) K. Mochida, K. Kawasumi, Y. Segawa and K. Itami, J. Am. Chem. Soc., 2011, 133, 10716 CrossRef CAS PubMed; (g) D. Mandal, A. D. Yamaguchi, J. Yamaguchi and K. Itami, J. Am. Chem. Soc., 2011, 133, 19660 CrossRef CAS PubMed; (h) K. Kawasumi, Q. Zhang, Y. Segawa, L. T. Scott and K. Itami, Nat. Chem., 2013, 5, 739 CrossRef CAS PubMed; (i) S. Tani, T. N. Uehara, J. Yamaguchi and K. Itami, Chem. Sci., 2014, 5, 123 RSC; (j) K. Ueda, K. Amaike, R. M. Maceiczyk, K. Itami and J. Yamaguchi, J. Am. Chem. Soc., 2014, 136, 13226 CrossRef CAS PubMed; (k) A. D. Yamaguchi, K. M. Chepiga, J. Yamaguchi, K. Itami and H. M. L. Davies, J. Am. Chem. Soc., 2015, 137, 644 CrossRef CAS PubMed; (l) T. Kawakami, K. Murakami and K. Itami, J. Am. Chem. Soc., 2015, 137, 2460 CrossRef CAS PubMed; (m) Y. Saito, Y. Segawa and K. Itami, J. Am. Chem. Soc., 2015, 137, 5193 CrossRef CAS PubMed; (n) S. Suzuki, Y. Segawa, K. Itami and J. Yamaguchi, Nat. Chem., 2015, 7, 227 CrossRef CAS PubMed; (o) K. Ozaki, K. Kawasumi, M. Shibata, H. Ito and K. Itami, Nat. Commun., 2015, 6, 6251 CrossRef CAS PubMed.
- For recent reviews on nickel-catalyzed reactions, see: (a) S. Z. Tasker, E. A. Standley and T. F. Jamison, Nature, 2014, 509, 299 CrossRef CAS PubMed; (b) J. Yamaguchi, K. Muto and K. Itami, Eur. J. Org. Chem., 2013, 19 CrossRef CAS PubMed.
- (a) J. Canivet, J. Yamaguchi, I. Ban and K. Itami, Org. Lett., 2009, 11, 1733 CrossRef CAS PubMed; (b) T. Yamamoto, K. Muto, M. Komiyama, J. Canivet, J. Yamaguchi and K. Itami, Chem.–Eur. J., 2011, 17, 10113 CrossRef CAS PubMed.
- (a) K. Muto, J. Yamaguchi and K. Itami, J. Am. Chem. Soc., 2012, 134, 169 CrossRef CAS PubMed; (b) L. Meng, Y. Kamada, K. Muto, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2013, 52, 10048 CrossRef CAS PubMed; (c) K. Muto, J. Yamaguchi, A. Lei and K. Itami, J. Am. Chem. Soc., 2013, 135, 16384 CrossRef CAS PubMed; (d) H. Xu, K. Muto, J. Yamaguchi, C. Zhao, K. Itami and D. G. Musaev, J. Am. Chem. Soc., 2014, 136, 14834 CrossRef CAS PubMed.
- K. Amaike, K. Muto, J. Yamaguchi and K. Itami, J. Am. Chem. Soc., 2012, 134, 13573 CrossRef CAS PubMed.
- Reviews on C–O activation: (a) B.-J. Li, D.-G. Yu, C.-L. Sun and Z.-J. Shi, Chem.–Eur. J., 2011, 17, 1728 CrossRef CAS PubMed; (b) B. M. Rosen, K. W. Quasdorf, D. A. Wilson, N. Zhang, A.-M. Resmerita, N. K. Garg and V. Percec, Chem. Rev., 2011, 111, 1346 CrossRef CAS PubMed; (c) D.-G. Yu, B.-J. Li and Z.-J. Shi, Acc. Chem. Res., 2010, 43, 1486 CrossRef CAS PubMed; (d) T. Mesganaw and N. K. Garg, Org. Process Res. Dev., 2013, 17, 29 CrossRef CAS; (e) S. I. Kozhushkov, H. K. Potukuchi and L. Ackermann, Catal. Sci. Technol., 2013, 3, 562 RSC; (f) M. Tobisu and N. Chatani, Acc. Chem. Res., 2015, 48, 1717 CrossRef CAS PubMed.
- For representative examples of C–O activation, see: (a) M. Tobisu, T. Shimasaki and N. Chatani, Angew. Chem., Int. Ed., 2008, 47, 4866 CrossRef CAS PubMed; (b) B.-T. Guan, Y. Wang, B.-J. Li, D.-G. Yu and Z.-J. Shi, J. Am. Chem. Soc., 2008, 130, 14468 CrossRef CAS PubMed; (c) K. W. Quasdorf, X. Tian and N. K. Garg, J. Am. Chem. Soc., 2008, 130, 14422 CrossRef CAS PubMed; (d) K. W. Quasdorf, A. Antoft-Finch, P. Liu, A. L. Silberstein, A. Komaromi, T. Blackburn, S. D. Ramgren, K. N. Houk, V. Snieckus and N. K. Garg, J. Am. Chem. Soc., 2011, 133, 6352 CrossRef CAS PubMed; (e) C. Zarate and R. Martin, J. Am. Chem. Soc., 2014, 136, 2236 CrossRef CAS PubMed; (f) C. Zarate, R. Manzano and R. Martin, J. Am. Chem. Soc., 2015, 137, 6754 CrossRef CAS PubMed.
- (a) H. Hachiya, K. Hirano, T. Satoh and M. Miura, Org. Lett., 2009, 11, 1737 CrossRef CAS PubMed; (b) H. Hachiya, K. Hirano, T. Satoh and M. Miura, Angew. Chem., Int. Ed., 2010, 49, 2202 CrossRef CAS PubMed; (c) H. Hachiya, K. Hirano, T. Satoh and M. Miura, ChemCatChem, 2010, 2, 1403 CrossRef CAS PubMed.
- For review, see: (a) L. C. M. Castro and N. Chatani, Chem. Lett., 2015, 44, 410 CrossRef CASFor representative examples, see: (b) M. Tobisu, I. Hyodo and N. Chatani, J. Am. Chem. Soc., 2009, 131, 12070 CrossRef CAS PubMed; (c) Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2013, 135, 5308 CrossRef CAS PubMed; (d) Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2014, 136, 898 CrossRef CAS PubMed; (e) Y. Aihara, M. Tobisu, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2014, 136, 15509 CrossRef CAS PubMed.
- (a) R. Takise, K. Muto, J. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2014, 53, 6791 CrossRef CAS PubMed; (b) E. Koch, R. Takise, A. Studer, J. Yamaguchi and K. Itami, Chem. Commun., 2015, 51, 855 RSC; (c) K. Muto, J. Yamaguchi, D. G. Musaev and K. Itami, Nat. Commun., 2015, 6, 7508 CrossRef CAS PubMed.
- P. C. Fox, Arch. Intern. Med., 1991, 151, 1149 CrossRef CAS PubMed.
- (a) Y. Nakao, K. S. Kanyiva, S. Oda and T. Hiyama, J. Am. Chem. Soc., 2006, 128, 8146 CrossRef CAS PubMed; (b) K. S. Kanyiva, F. Löbermann, Y. Nakao and T. Hiyama, Tetrahedron Lett., 2009, 50, 3463 CrossRef CAS PubMed; (c) Y. Nakao, K. S. Kanyiva and T. Hiyama, J. Am. Chem. Soc., 2008, 130, 2448 CrossRef CAS PubMed; (d) C.-C. Tsai, W.-C. Shih, C.-H. Fang, C.-Y. Li, T.-G. Ong and G. P. A. Yap, J. Am. Chem. Soc., 2010, 132, 11887 CrossRef CAS PubMed.
- For representative examples of C–F activation by Ni catalysts, see: (a) N. Yoshikai, H. Matsuda and E. Nakamura, J. Am. Chem. Soc., 2009, 131, 9590 CrossRef CAS PubMed; (b) M. Tobisu, T. Xu, T. Shimasaki and N. Chatani, J. Am. Chem. Soc., 2011, 133, 19505 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc02942b |
| This journal is © The Royal Society of Chemistry 2015 |