Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Non-decarbonylative photochemical versus thermal activation of Bu4N[Fe(CO)3(NO)] – the Fe-catalyzed Cloke–Wilson rearrangement of vinyl and arylcyclopropanes†
Che-Hung
Lin‡
a,
Dominik
Pursley‡
a,
Johannes E. M. N.
Klein‡
a,
Johannes
Teske‡
a,
Jennifer A.
Allen
b,
Fabian
Rami
ac,
Andreas
Köhn
c and
Bernd
Plietker
*a
aInstitut für Organische Chemie, Universität Stuttgart, Pfaffenwaldring 55, DE-70569 Stuttgart, Germany. E-mail: bernd.plietker@oc.uni-stuttgart.de
bMettler-Toledo GmbH, Ockerweg 3, D-35396 Giessen, Germany
cInstitut für Theoretische Chemie, Universität Stuttgart, Pfaffenwaldring 55, DE-70569 Stuttgart, Germany
First published on 3rd September 2015
Abstract
The base metal complex Bu4N[Fe(CO)3(NO)] (TBA[Fe]) catalyzes the rearrangement of vinyl and arylcyclopropanes both under thermal or photochemical conditions to give the corresponding vinyl or aryldihydrofurans in good to excellent yields. Under photochemical conditions the reaction is performed at room temperature. Spectroscopic investigations show that the metal carbonyl catalyst is not decarbonylated. The best performance was observed at a wavelength of 415 nm. icMRCI+Q analysis of the excited singlet and triplet states of the [Fe(CO)3(NO)] anion was performed and used to calculate the vertical excitation energies which are in good agreement with the experimental data. CASSCF analysis indicates that the Fe center in all excited states of the ferrate becomes more electrophilic while adopting a distorted tetrahedral configuration. Both aspects have a positive synergistic effect on the formation of the initial π-complex with the incoming organic substrate.
Introduction
The photochemical activation of Fe complexes is ubiquitous in nature and has attracted significant research interest, in particular in the field of water splitting or proton reductions.1 In the field of non-biomimetic photocatalysis using iron–carbonyl complexes, landmark contributions from the groups of Beller2 and Darcel3 were most recently communicated. In these reports, Fe(CO)5− or (NHC)Fe(CO)4− catalysts (N-heterocyclic carbene) are activated through photochemical decarbonylation. Although quite common in organometallic chemistry, the exact mechanism of the photochemical activation-decarbonylation is not well understood. Important insights were gained through combined flash photolysis–ultrafast IR spectroscopy,4 ultrafast electron diffraction,5 and more recently femtosecond X-ray spectroscopy.6,7 From these data it was suggested that a decarbonylation of Fe(CO)5 results in the formation of an excited singlet Fe(CO)4, which can undergo intersystem crossing to the corresponding triplet Fe(CO)4. The analysis of spin-density indicated the decarbonylation to be a complex interplay of antibonding and bonding orbitals plus electron spin. In sharp contrast to the well-established photodecarbonylative activation of metal–carbonyl complexes, examples of a non-decarbonylative photochemical activation of a metal–carbonyl catalyst are not known in the literature.For a number of years our group has been interested in base metal catalysis using the stable and readily accessible electron-rich complex Bu4N[Fe(CO)3(NO)] (TBA[Fe]).8 Aiming to understand the interplay between electronic ground state structure and reactivity, a combined in-depth spectroscopic and experimental study indicated that the metal center should be regarded as being zero-valent while the negative charge is located at the NO-ligand. Importantly, CASSCF- and IBO-analysis independently showed that the Fe–NO-moiety should be considered as a singlet ground-state complex with a triplet Fe(0) atom being antiferromagnetically coupled to a triplet NO-anion via two non-polar covalent Fe–N(d,p)-π-bonds.9 In a subsequent theoretical investigation of the mechanism of the TBA[Fe]-catalyzed Cloke–Wilson rearrangement,10 a reaction that was observed during our studies on the Fe-catalyzed activation of allyl C–C bonds,11 it was found that only one out the two Fe–N–π-bonds is involved in cleaving allylic activated atom bonds via a ligand-centered two-electron oxidation.12 The oxidation state of the metal center remains as zero. Moreover, the theoretical results indicated that the Fe-catalyzed activation of a vinylcyclopropane could follow both an SN2′-but also an SN2-pathway (Fig. 1).
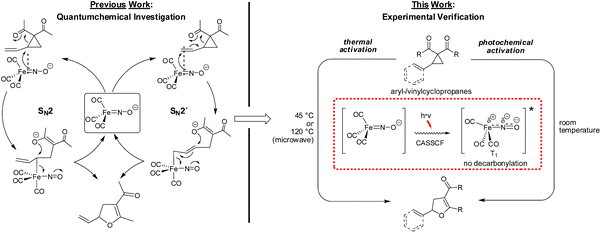 | ||
| Fig. 1 SN2- versus SN2́-anti-mechanism in the TBA[Fe]-catalyzed Cloke-Wilson rearrangement of vinyl- and arylcyclopropanes.13,14 | ||
With regard to these previous findings we initiated a research project to investigate whether
• The ferrate complex in its unusual electronic ground-state could be activated for catalytic transformations upon irradiation with light.
• The ferrate complex remains intact or undergoes a decarbonylation upon irradiation with UV-light.
Results and discussion
The Fe-catalyzed Cloke–Wilson rearrangement was found as a major side-reaction during our studies on Fe-catalyzed allylic C–C bond activations.11 At the outset of our studies the reaction was systematically optimized (Table 1). As already observed during our investigations on carbene-transfer catalysis the addition of ligands led to a significant decrease of the conversion. For the thermal reaction the use of only 1 mol% of TBA[Fe] in dichloromethane as the solvent at a temperature of 45 °C resulted in a quantitative conversion of vinylcyclopropane 1 (entry 6, Table 1). In sharp contrast the same reaction albeit at room temperature showed no conversion (entry 7, Table 1). With this reference for the thermal activation in hand we set out to investigate the influence of photochemical activation. Indeed, irradiation of the reaction mixture using a 180 W Hg-lamp at room temperature in dichloromethane gave the desired product in low yield (entry 8, Table 1). Changing the solvent to the more polar acetonitrile and increasing the catalyst loadings to 2.5 mol% resulted in a clean dihydrofuran formation at room temperature (entry 11, Table 1). Importantly, the reaction did not proceed without irradiation under otherwise identical reaction conditions.| Entry | Cat. [mol%] | Solvent | Ligand | T [°C] | Light | Conv.b [%] |
|---|---|---|---|---|---|---|
| a 14 h. b Determined using NMR-integration using mesitylene as the internal standard. c 3 h. d Isolated yield. | ||||||
| 1a | 2.5 | THF | SIMes | 50 | — | 72 |
| 2a | 2.5 | THF | PPh3 | 50 | — | 6 |
| 3a | 2.5 | Benzene | PPh3 | 50 | — | <5 |
| 4a | 2.5 | CH2Cl2 | PPh3 | 50 | — | 7 |
| 5a | 2.5 | CH2Cl2 | — | 50 | — | >98 |
| 6a | 1 | CH2Cl2 | — | 45 | — | >98 |
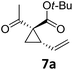
| 7a | 1 | CH2Cl2 | — | 20 | — | — |
| 8a | 1 | CH2Cl2 | — | 20 | 180 W (Hg) | 8 |
| 9a | 1 | CH3CN | — | 20 | 180 W (Hg) | 16 |
| 10c | 2.5 | CH2Cl2 | — | 20 | 180 W (Hg) | 85 |
| 11c | 2.5 | CH3CN | — | 20 | 180 W (Hg) | 96d |
| 12c | 2.5 | CH3CN | — | R.T. | 75 W (Xe) | 93 |
| 13c | 2.5 | CH3CN | — | R.T. | 23 W | 92d |
To exclude the disturbing heating effect caused by the strong UV-lamp an eventual temperature–time correlation of the reaction mixture was analyzed using in-operando-IR spectroscopy/thermometry.15 Starting at room temperature (20 °C) an increase of the temperature to about 28 °C was recorded, however this temperature increase correlates with the conversion rate and was attributed to the exothermicity of the reaction. Importantly, the Cloke–Wilson rearrangement did not take place at 28 °C without irradiation. Knowing that the rearrangement proceeds under photochemical conditions using a 180 W Hg-lamp we were wondering whether less-intense UV-light or even visible light irradiation could activate the complex. We were pleased to find that the use of a 75 W Xe-UV-lamp (entry 12, Table 1) and even a commercial 23 W household lamp led to the formation of dihydrofuran 2 in excellent isolated yields (entry 13, Table 1).
With these encouraging results in hand we subsequently set out to compare the reaction scope of the thermal versus the photochemical Fe-catalyzed Cloke–Wilson rearrangement (Table 2).
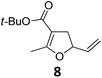
| Entry | Substrate | Product | Cond.a | Yieldb [%] | Entry | Substrate | Product | Cond.a | Yieldb [%] |
|---|---|---|---|---|---|---|---|---|---|
| a Conditions A: 0.5 mmol of substrate, 1 mol% of TBA[Fe], 1 mL (abs.) of CH2Cl2, 45 °C, 16 h; conditions B: 0.4 mmol of substrate, 2.5 mol% of TBA[Fe], 1 mL (abs.) of CH3CN, 180 W (Hg-lamp), 20 °C, 3 h. b Isolated yields. c 5 mol% of TBA[Fe]. d 10 mol% of TBA[Fe] in 1 mL (abs.) of THF, 24 h. e 75 W (Xe-lamp). f 23 W compact fluorescence lamp. g 6 h. | |||||||||
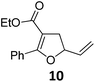
| 1 |
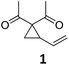
|
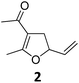
|
A | 94 | 17c |
|
|
A | 87 |
| 2 | B | 96, 93e, 92f | 18c,g | B | 87 | ||||
| 3 |
|
|
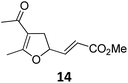
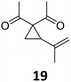
A | 92 | 19c |
|
|
A | 96 |
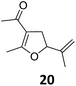
| 4 | B | 93 | 20c,g | B | 89 | ||||
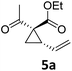
| 5 |
|
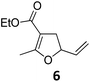
|
A | 93 | 21c |
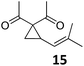
|
|
A | 54 |
| 6 | B | 94, 93f | 22c,g | B | 75, 76f | ||||
| 7 |
|
A | 92 | 23c |
|
|
A | — | |
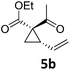
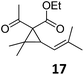
| 8 | B | 92, 92f | 24d | B | 98, 96e, 19f | ||||
| 9 |
|
|
A | 94 | 25c |
|
|
A | 96 |
| 10 | B | 85 | 26 | B | 97 | ||||
| 11 |
|
A | 60 | 27c |
|
|
A | 98 | |
| 12 | B | 91 | 28c,g | B | 98 | ||||
| 13c |
|
|
A | 87 | 29 |
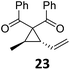
|
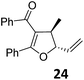
|
A | 72 |
| 14 | B | 91 | 30 | B | 92 | ||||
| 15 |
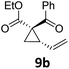
|
A | 86 | ||||||
| 16 | B | 92 | |||||||
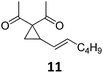
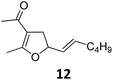
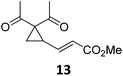
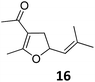
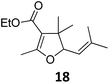
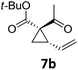
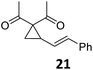
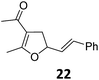
Both protocols proved to be broadly applicable, however certain differences indicate that they do have complementary advantages/disadvantages. Stereocenters within the substrate do have a profound effect on the reaction course. Whereas both diastereomers of ethyl acetoacetate derived VCP 5 react equally well both under thermal and photochemical conditions (entries 5–8, Table 2), the corresponding tert-butyl acetoacetate derived VCP 7 reacts at significantly different reaction rates under thermal conditions (entries 9–16, Table 2). For the cis-oriented ester and vinyl group a decrease of the overall yield was observed, a result that indicates the sterically demanding tert-butyl ester to block the SN2′- (or SN2-) anti trajectory of the incoming nucleophilic catalyst. In contrast, both diastereomers are equally reactive under photocatalytic conditions (entries 9–12, Table 2). In order to shed more light into this unexpected result, a test reaction in which a mixture of 25 mol% of the less reactive substrate 7b and 75 mol% of the more reactive diastereomer 7a was subjected to the thermal reaction conditions and the conversion was analyzed after 5 h. The more reactive substrate reacted at a significantly lower rate as compared to the same reaction using diastereomerically pure VCP 7a (18% conversion for the mixture of 7a/7b, 7a was recovered in 75% yield; 7b was recovered in 98% yield) versus 28% conversion for pure 7a (7a was recovered in 65% yield; reaction conditions: 0.5 mmol of 7a or 0.4 mmol of 7a/0.1 mmol 7b, 1 mol% of TBA[Fe], CH2Cl2, 45 °C). Apart from the steric arguments, one of the reviewers suggested an attractive orbital overlap between the incoming ferrate catalyst and the electron-withdrawing ketone and hence a favoured elongate formation to account for the inhibiting effect of the less reactive diastereomer. Future work will focus on this interesting stereoelectronic discrimination under thermal conditions. On the contrary, the lack of this discrimination might be the result of the change in complex configuration upon irradiation (vide infra), which results in an overall reduction of the metal-centered electron density with the adoption of a formally coordinatively unsaturated trigonal-bipyramidal configuration. The combination of electrophilicity at the metal center and a higher accessibility of the Fe center might explain the observed high reactivity to both VCPs 7a and 7b (vide infra). Furthermore, a correlation of the yield and the power of the light source was observed (entry 24, Table 2). The thermally unreactive vinylcyclopropane 17 and TBA[Fe] could be activated at room temperature using a 23 W household lamp to give the desired product in about 19% yield. Using a 75 W Xe-lamp, full conversion and an isolated yield of 96% was obtained (entry 24, Table 2).
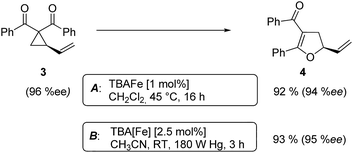
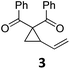
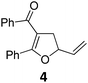
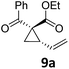
At this point we analyzed the stereoselective course of the TBA[Fe]-catalyzed Cloke–Wilson rearrangement (Scheme 1). Starting from the enantiomerically enriched (ee > 95%) vinylcyclopropane 3 the corresponding enantiomerically enriched product 4, in which the new C–O bond was formed with retention of configuration, was isolated in an excellent yield and with near-complete transfer of enantiopurity both under thermal and photocatalytic conditions (Scheme 1). Importantly, the absolute configuration was unambiguously assigned using X-ray spectroscopy.15 This result indicates that a double SN2′- (or SN2-) mechanism is operative. Previous work from our group showed that the TBA[Fe]-catalyzed allylic substitution of allylic carbonates occurs with high regioselectivity and retention of configuration. We proposed a σ-enyl-type mechanism with a slow σ–π–σ equilibrium of the allyl-Fe complex to account for this unusual observation.8h–j More recently, however, we reported a quantum chemical study of the Cloke–Wilson rearrangement and noted that for this type of reaction both the SN2′- and the SN2-pathways could be operative.12
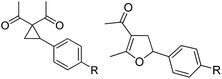
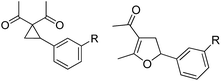
Whereas the VCP-rearrangement can follow both mechanisms, the situation changes drastically for the rearrangement of arylcyclopropanes (ACP). In this case the SN2-mechanism should be favourable. Consequently, TBA[Fe] was subsequently employed in the rearrangement of arylcyclopropanes, both under thermal as well as photochemical conditions. We were pleased to find the rearrangement of ACP 25 to proceed using substoichiometric amounts of the nucleophilic ferrate both under thermal conditions (120 °C, microwave, 200 W, 2 h, DMF) as well as under photochemical conditions (180 W Hg-lamp, 20 °C, DMF, 24 h). The thermal conditions are rather harsh, however, shorter reaction times and lower catalyst loadings as compared to the photochemical conditions are required (Table 3). Interestingly, DMF as a more polar solvent proved to be necessary in order to get the conversion both under thermal and photochemical conditions. In DMF, TBA[Fe] might exist as a solvent-separated ion pair with significantly increased metal-centered nucleophilicity.8h Different functional group compatibilities were observed. In particular halide atoms in the para-position of the aromatic unit had a significant effect on the rearrangement under photochemical conditions (entries 7–12, Table 3). Whereas the respective arylcyclopropanes undergo a clean rearrangement to the desired product under thermal conditions (entries 9 and 11, Table 3), lower yields for the reaction of p-chloro substituted ACP 39 and no conversion for p-bromo substituted ACP 41 were observed (entries 10 and 12, Table 3). The same effect was observed for the corresponding meta-substituted ACPs (entries 15–18, Table 3). Although we did not observe the product of protodehalogenation, we can not exclude this side-reaction as being operative and as leading to catalyst decomposition.
| Entry | Substrate | Product | Cond.a | Yieldb [%] | Entry | Substrate | Product | Cond.a | Yieldb [%] |
|---|---|---|---|---|---|---|---|---|---|
| a Conditions A: 0.25 mmol of substrate, 5 mol% of TBA[Fe], 1 mL (abs.) of DMF, 120 °C, MW (200 W), 2 h; conditions B: 0.4 mmol of substrate, 10 mol% of TBA[Fe], 1 mL (abs.) of DMF, 180 W Hg-lamp, 20 °C, 24 h. b Isolated yield. c 75 W Xe-lamp. d 23 W compact fluorescence lamp. | |||||||||
|
|
||||||||
| 1 | 25 (R = H) | 26 | A | 99 | 13 | 37 (R = OMe) | 38 | A | 85 |
| 2 | B | 82, 57c, 5d | 14 | B | 93 | ||||
| 3 | 27 (R = Me) | 28 | A | 99 | 15 | 39 (R = Cl) | 40 | A | 90 |
| 4 | B | 85 | 16 | B | 72 | ||||
| 5 | 29 (R = t-Bu) | 30 | A | 75 | 17 | 41 (R = Br) | 42 | A | 84 |
| 6 | B | 77 | 18 | B | — | ||||
| 7 | 31 (R = F) | 32 | A | 76 | 19 |
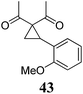
|
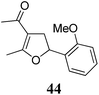
|
A | 83 |
| 8 | B | 62 | 20 | B | 41 | ||||
| 9 | 33 (R = Cl) | 34 | A | 92 | 20 |
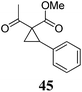
|
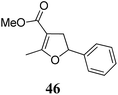
|
A | 74 |
| 10 | B | 75 | 21 | B | 81 | ||||
| 11 | 35 (R = Br) | 36 | A | 97 | 22 |
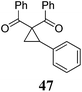
|
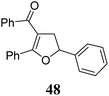
|
A | 93 |
| 12 | B | — | 23 | B | 40 | ||||
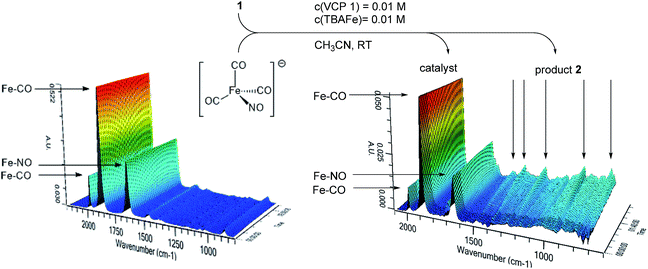
In principle, different scenarios for the photochemical activation of Bu4N[Fe(CO)3(NO)] (TBA[Fe]) are possible. In order to get first insights into a probable mode of activation, an acetonitrile solution of TBA[Fe] was irradiated at room temperature with the 180 W Hg-lamp employed in the catalysis. The carbonyl and nitrosyl signals were observed using in situ IR-ATR spectroscopy (Fig. 2). However, even after extended irradiation times no significant change in the IR-spectrum was observed. Furthermore, repetition of the same experiment with the addition of Ph3P as a suitable ligand did not result in ligand exchange.15 Moreover, when VCP 1 was added to the mixture and the reaction course was monitored using in situ IR spectroscopy, no change in the TBA[Fe] signals even after full conversion was detected (Fig. 2). Hence, at the current stage of the research it seems as if a photochemically induced decarbonylation of TBA[Fe] might not be the activating step.
In the case of the VCP-rearrangement, irradiation using a 23 W lamp was sufficient for the activation of TBA[Fe]. Both the 180 W Hg- and the 75 W Xe-lamps have strong emissions in the UV but also in the visible light region (Fig. 3).16 The complex has absorption maxima at 242, 263, 292, and 370 nm. Furthermore, there is a saddle point at 410 nm. Very much to our surprise, a conversion–wavelength correlation of the TBA[Fe]-catalyzed rearrangement of 1 indicated that the complex has the highest activity at 415 nm (Fig. 3).
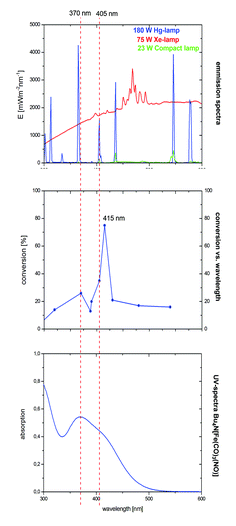 | ||
| Fig. 3 Overlay of the emission and absorption spectra with the conversion–wavelength correlation in the rearrangement of VCP 1. | ||
Interestingly, a significant drop in the conversion was observed at wavelengths below 405 and above 370 nm. Apparently, the Hg-emission line at 405 nm that was observed for the 23 W household lamp is responsible for the observed activity. At the current stage of the research we believe that this higher photon flux accounts for the increase in reactivity, e.g. in the rearrangement of ACPs. Based on our assumption that TBA[Fe] is the active catalyst and in order to get a deeper insight into the electronic structure of the potential activated species, quantum-chemical investigations were carried out.15
The ferrate, [Fe(CO)3(NO)], is an anionic complex, however, computations indicate that the solvated anion will not be oxidized by irradiation up to 3.5 eV (354 nm). The predicted electron affinity of the isolated neutral [Fe(CO)3(NO)] complex is 2.8 eV with DFT (PBE functional17), while MRCI+Q18 calculations result in 1.8 eV. Solvation effects stabilize the anion significantly, by 1 eV in apolar solvents and up to 2 eV in polar solvents, as estimated using a continuum solvation model.19 While a more accurate determination of the electron affinity will require more rigorous calculations, we can safely estimate that the anionic species will be stable under the herein considered experimental conditions and the catalytic activity will only be due to an excited anionic ferrate species.
The DFT-calculated equilibrium structure of the anionic species has C3v point-group symmetry and the structure parameters are similar to the ones reported in previous works.9 Starting from this structure, we subsequently calculated the vertical excitation spectrum of [Fe(CO)3(NO)] using MRCI+Q (Table 4).
| Singlet excitations | Triplet excitations | |||||
|---|---|---|---|---|---|---|
| State (sym.) | ΔE [eV] | λ [nm] | f | State (sym.) | ΔE [eV] | λ [nm] |
| a icMRCI+Q/def2-TZVPP calculations at the PBE/def2-TZVPP′ optimized structure. b Oscillator strength (length gauge) based on the MRCI transition density. c Average over slightly symmetry broken MRCI+Q results for the two columns of the E representation (deviations are <0.04 eV). | ||||||
| S1 (1A2) | 3.26 | 381 | 0 | T1 (3A1) | 2.32 | 534 |
| S2 (1E)c | 3.53 | 351 | 0.008 | T2 (3E)c | 2.96 | 420 |
| S3 (1A1) | 3.64 | 340 | 0.013 | T3 (3A2) | 3.15 | 394 |
| S4 (1E)c | 4.16 | 298 | 0.002 | T4 (3A1) | 3.79 | 327 |
| S5 (1A2) | 4.34 | 286 | 0 | T5 (3E)c | 3.99 | 311 |
| S6 (1A1) | 4.75 | 261 | <0.001 | T6 (3A2) | 4.31 | 287 |
As expected from the orbital structure (both HOMO and LUMO are degenerate) we obtain four close-lying singlet excited states in the region of 3.3 to 3.6 eV of A2, E and A1 symmetry. CASSCF-based structure optimizations15,20 of the excited states indicate that these states undergo strong structural deformations with reorganization energies in the order of 1 eV. This explains the broad band observed in the experimental spectrum, ranging from 2.8 eV to 3.9 eV. We note that the transition to the lowest singlet A2 state is symmetry forbidden but non-vanishing absorption strength may occur in the spectrum due to vibronic coupling.
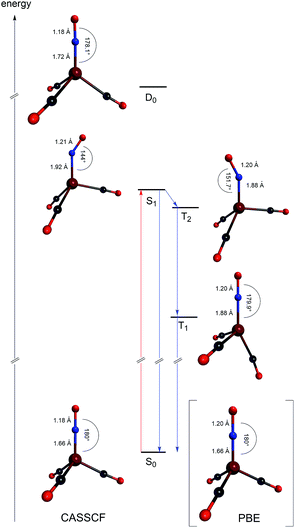
The excited state structures calculated at the CASSCF level are shown in Fig. 4, along with a schematic energy level scheme derived from MRCI+Q and CASSCF calculations.
We find that the S1 and T2 states are close in energy, in particular at the relaxed S1 structure where the S1 energy is even 0.03 eV below the T2 energy at the CASSCF level. This is in accordance with the S1 structure, which shows an Fe–N–O bond angle of 144°, indicative of the loss of π-bond character and the reduction of the NO-ligand.15 Electronically, two unpaired electrons reside on both Fe and NO and couple only weakly, such that the singlet and triplet are nearly isoenergetic. Even though only a weak spin–orbit coupling between S1 and T2 is calculated (6 cm−1 at the CASSCF level), the near-degeneracy close to the S1 equilibrium structure will drive efficient intersystem crossing to T2, followed by fast internal conversion to T1. Hence, we expect that the activated [Fe(CO)3(NO)] species is in the T1 state. In this state, the complex adopts an almost trigonal-bipyramidal configuration with an Fe–N–O angle of close to 180°, in which the metal center might be considered to possess an open binding site. Consequently, this complex geometry might facilitate the coordination of the olefinic substrate as the initial step of the catalytic reaction. The fast intersystem crossing from S1 to T2 and the fast internal conversion prevents a detection of the Fe–NO-species with bent NO-ligands using the spectroscopic methods used in this work. Research aiming to detect these short-lived intermediates is ongoing. The NAO charges of the lowest [Fe(CO)3(NO)] states are listed in Table 5.
| S0b {Fe8.00(NO)}10 | S1 {Fe7.34(NO)}10 | T1 {Fe7.48(NO)}10 | T2 {Fe7.36(NO)}10 | |
|---|---|---|---|---|
| a Natural atomic orbitals (NAOs) were obtained from the state-averaged CASSCF/def2-TZVPP calculations. The corresponding CASSCF/def2-TZVPP′ equilibrium structures were used. All charges are multiples of the elementary charge e0. b The NAO charges obtained using the PBE-derived equilibrium structure are shown in parentheses. | ||||
| Fe | 0.00 (−0.07) | 0.66 | 0.52 | 0.64 |
| N | −0.10 (−0.05) | −0.35 | −0.32 | −0.37 |
| O | −0.42 (−0.41) | −0.43 | −0.41 | −0.39 |
| C | −0.48 (−0.47) | −0.87 | −0.79 | −0.88 |
The NAO analysis shows that the Fe center after excitation has a positive partial charge of about 0.5e0 while significant charge transfer into the ligand backbonds is observed. The nitrogen atom of the NO-ligand is reduced by about 0.3e0. Furthermore, a significant reduction of the CO-ligands is observed for all excited states.
Experimental section
General remarks
The photochemical reactions were performed in borosilicate glassware. The distance between the glassware and lamp was 15 cm.General procedure for the thermal VCP rearrangement
TBA[Fe] (10.3 mg, 0.025 mmol) was weighed into a dried Schlenk tube. Anhydrous CH2Cl2 (5 mL) was added and the mixture was stirred until all solids dissolved. 1 mL (0.005 mmol TBA[Fe]) of this solution was transferred into a separate dried Schlenk tube which was subsequently charged with the appropriate vinylcyclopropane (0.5 mmol). The Schlenk tube was sealed under an atmosphere of dry nitrogen and heated to 45 °C for 16 h. The solvent was removed under reduced pressure and the residue was subjected to column chromatography on silica gel.General procedure for the photochemical VCP rearrangement
A 10 mL Schlenk tube was charged with vinylcyclopropane (0.40 mmol, 1 equiv.), TBA[Fe] (0.001 mmol, 0.025 equiv.), and CH3CN (1 mL) under N2. The reactions were carried out at room temperature under irradiation using the respective light source for 3 h. The reaction was quenched with diethyl ether and concentrated in vacuo. Purification using silica column chromatography afforded the desired dihydrofuran product.General procedure for the thermal ACP rearrangement
TBA[Fe] (5.2 mg, 0.0125 mmol, 0.05 equiv.) and the corresponding arylcyclopropane (0.25 mmol, 1 equiv.) were weighed into a dried 10 mL microwave tube. Anhydrous DMF (1 mL) was added and the tube was sealed under an atmosphere of dry nitrogen. The reaction mixture was stirred for 2 h at 120 °C under microwave conditions. The pure product was obtained after chromatography on silica gel.General procedure for the photochemical ACP rearrangement
A 10 mL Schlenk tube was charged with arylcyclopropane (0.40 mmol, 1 equiv.), TBA[Fe] (0.04 mmol, 0.1 equiv.), and DMF (1 mL) under N2. The reactions were carried out at room temperature under light irradiation for 24 h. The reaction was quenched with diethyl ether and concentrated in vacuo. Purification using silica column chromatography afforded the desired dihydrofuran product.Computational details
All computations were carried out with the Molpro 2012.1 program package.21 Molecular structures were determined at the DFT level of theory using the PBE and PBE0 functionals17 and at the state-averaged CASSCF level of theory.20 We employed the def2-TZVPP basis set,22 omitting the g-functions however, due to restrictions in the state-averaged CASSCF gradient code (the basis will be called def2-TZVPP′ in this work). Both the DFT closed-shell and open-shell calculations relied on a restricted Kohn–Sham scheme. Solvent effects were estimated using COSMO19 with standard parameters for the cavity construction and two values for the dielectric constant: ε = 2.5 to model apolar solvents and ε = ∞ to model strongly polar solvents.15 The CASSCF calculations were run with averaging over five states and four states in the singlet and triplet manifold of anionic [Fe(CO)3(NO)], respectively, and with averaging over four states for the neutral species. The active space consisted of all Fe d orbitals and the NO π and π* orbitals (12 electrons in 9 orbitals). Cs point-group symmetry of the molecule was exploited in all calculations (the actual point group of anionic [Fe(CO)3(NO)] is C3v but Jahn–Teller distortions occurred for all excited and ionized states).Vertical excitation energies were computed with internally contracted MRCI18 and a modified Davidson correction for disconnected quadruple excitations (icMRCI+Q).18a Orbitals were taken from the state-averaged CASSCF calculations, using the same active space as described above. The 1s orbitals of C, N, and O and the (1s2s2p3s3p) orbitals of Fe were excluded from the correlation treatment.
Natural atomic orbitals (NAOs) were determined from the CASSCF densities.23
Conclusions
Herein we describe a comparative study of the use of Bu4N[Fe(CO)3(NO)] (TBA[Fe]) in the thermal versus photochemical Cloke–Wilson rearrangement of vinyl- and arylcyclopropanes. The catalyst shows good reactivity under both conditions and allows the preparation of a variety of substituted dihydrofurans under mild conditions. Upon irradiation the catalytic transformation can be performed at room temperature. In-operando spectroscopic investigations suggest that the Fe-carbonyl catalyst is probably not decarbonylated under photochemical conditions. Detailed studies on the correlation of the wavelength versus conversion showed that the best conversion rate is achieved at 415 nm. This value is close to a saddle point within the absorption curve of the ferrate. Quantum chemical investigations of the potential activated singlet and triplet states of the ferrate catalyst gave insight into the vertical excitation energies and show that the ferrate has an S1-state, from which the activated species can undergo intersystem crossing into the nearly isoenergetic triplet state, T2, from which the energetically favorable T1 state is accessible via internal conversion. The deviations of the Fe–N–O bond angle from 180° for S1 and T2 are indicative of a significant charge transfer into the backbond of the NO- and CO-ligands, leading to a polarization of the complex in which the metal center is partially positively charged. A significant Jahn–Teller-distortion is observed, which leads to a change of the coordination mode from the former tetrahedral 18-electron complex in the ground state S0 into a formally unsaturated trigonal-bipyramidal complex in the triplet state T1. This coordination mode and the partial positive charge of the metal center facilitate the formation of a reactive π-complex as the initial step within the proposed catalytic cycle (Fig. 5).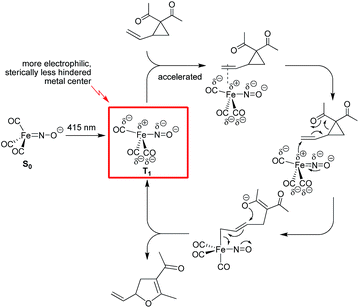 | ||
| Fig. 5 Mechanistic proposal for the photochemical Fe-catalyzed Cloke–Wilson rearrangement of VCPs following an SN2′-anti mechanism. | ||
The electron affinity is reduced in the excited state and the electron will be more readily transferred to the substrate in an SN2′-anti fashion. The nitrogen and the carbon atoms of the CO-ligand act as electron-donating centers that pump electrons into the antibonding π*C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C-orbital of the C
C-orbital of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond within the substrate. The allyl-Fe complex, which is formed upon electron transfer reacts with the O-nucleophile in an SN2′-anti fashion to regenerate the Fe complex in the T1 state.
C bond within the substrate. The allyl-Fe complex, which is formed upon electron transfer reacts with the O-nucleophile in an SN2′-anti fashion to regenerate the Fe complex in the T1 state.
On the contrary, the adoption of a trigonal-bipyramidal configuration in the T1-state allows the electron-rich, formally unsaturated and sterically less hindered Fe complex to access the σ*C–C-orbital of the tertiary benzylic (or allylic) C atom in aryl- or vinylcyclopropanes with concomitant C–C bond activation (Fig. 6). Reaction with the formed O-nucleophile in an SN2-fashion closes the catalytic cycle with the formation of the respective dihydrofuran products.
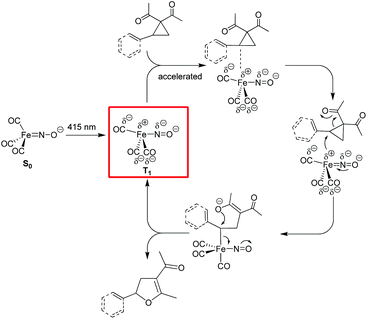 | ||
| Fig. 6 Mechanistic proposal for the photochemical Fe-catalyzed Cloke–Wilson rearrangement of VCPs/ACPs following an SN2-anti-mechanism. | ||
These results open up a new research direction using Bu4N[Fe(CO)3(NO)] as a stable catalyst that is selectively activated at 415 nm, a region in which common (non-catalytic) photochemical organic (side) reactions are not operative. Studies aiming to use this concept for the activation of less reactive C–H and C–C bonds are currently being carried out in our laboratories.
Abbreviations
| ACP | Arylcyclopropane |
| VCP | Vinylcyclopropane |
Acknowledgements
Financial support from the Deutsche Forschungsgemeinschaft is gratefully acknowledged. We are grateful to Dr Wolfgang Frey for X-ray analysis.References
- (a) J. P. McEvoy and G. W. Brudvig, Chem. Rev., 2006, 106, 4455–4483 CrossRef CAS PubMed; (b) A. Guskov, J. Kern, A. Gabdulkhakov, M. Broser, A. Zouni and W. Saenger, Nat. Struct. Mol. Biol., 2009, 16, 334–342 CrossRef CAS PubMed.
- (a) A. Boddien, B. Loges, F. Gärtner, C. Torborg, K. Fumino, H. Junge, R. Ludwig and M. Beller, J. Am. Chem. Soc., 2001, 132, 8924–8934 CrossRef PubMed; (b) F. Gärtner, A. Boddien, E. Barsch, K. Fumino, S. Losse, H. Junge, D. Hollmann, A. Brückner, R. Ludwig and M. Beller, Chem.–Eur. J., 2011, 17, 6425–6436 CrossRef PubMed.
- H. Li, C. M. Castro, J. Zheng, T. Roisnel, V. Dorcet, J.-B. Sortais and C. Darcel, Angew. Chem., Int. Ed., 2013, 52, 8045–8049 CrossRef CAS PubMed.
- (a) P. T. Snee, C. K. Payne, K. T. Kotz, H. Yang and C. B. Harris, J. Am. Chem. Soc., 2001, 123, 2255–2264 CrossRef CAS PubMed; (b) P. T. Snee, C. K. Payne, S. D. Mebane, K. T. Kotz and C. B. Harris, J. Am. Chem. Soc., 2001, 123, 6909–6915 CrossRef CAS.
- H. Ihee, J. Cao and A. H. Zewail, Angew. Chem., Int. Ed., 2001, 40, 1532–1536 CrossRef CAS.
- P. Wernet, K. Kunnus, I. Josefsson, I. Rajkovic, W. Quevedo, M. Beye, S. Schreck, S. Grübel, M. Scholz, D. Nordlund, W. Zhang, R. W. Hartsock, W. F. Schlotter, J. J. Turner, B. Kennedy, F. Hennies, F. M. F. deGroot, K. J. Gaffney, S. Techert, M. Odelius and A. Föhlisch, Nature, 2015, 520, 78–81 CrossRef CAS PubMed.
- S. A. Trushin, W. Fuss, K. L. Kompa and W. E. Schmid, J. Phys. Chem. A, 2000, 104, 1997–2006 CrossRef CAS.
- For first reports on the [Fe(CO)3(NO)] anion see: (a) W. O. Hieber and K. Z. Beutner, Z. Naturforsch., B: J. Chem. Sci., 1960, 15, 323–324 Search PubMed; (b) M. J. Hogsed and E. I. Du Pont de Nemours, US 2865707, 1958Chem. Abstr.1959:539592; selected examples of allylic substitutions; (c) M. Jegelka and B. Plietker, ChemCatChem, 2012, 4, 329–332 CrossRef CAS PubMed; (d) A. P. Dieskau, M. S. Holzwarth and B. Plietker, Chem.–Eur. J., 2012, 18, 2423–2429 CrossRef CAS PubMed; (e) M. Jegelka and B. Plietker, Chem.–Eur. J., 2011, 17, 10417–10430 CrossRef CAS PubMed; (f) M. S. Holzwarth, W. Frey and B. Plietker, Chem. Commun., 2011, 47, 11113–11115 RSC; (g) M. S. Holzwarth, A. P. Dieskau, M. Tabassam and B. Plietker, Angew. Chem., Int. Ed., 2009, 48, 7251–7255 CrossRef CAS PubMed; (h) B. Plietker, A. P. Dieskau, K. Möws and A. Jatsch, Angew. Chem., Int. Ed., 2008, 47, 198–201 CrossRef CAS PubMed; (i) B. Plietker, Angew. Chem., Int. Ed., 2006, 45, 6053–6056 CrossRef CAS PubMed; (j) B. Plietker, Angew. Chem., Int. Ed., 2006, 45, 1469–1473 CrossRef CAS PubMed ; for carbene transfer reactions see; (k) M. S. Holzwarth, I. Alt and B. Plietker, Angew. Chem., Int. Ed., 2012, 51, 5351–5354 CrossRef CAS PubMed.
- J. E. M. N. Klein, B. Miehlich, M. S. Holzwarth, M. Bauer, M. Milek, M. M. Khusniyarov, M. G. Knizia, H.-J. Werner and B. Plietker, Angew. Chem., Int. Ed., 2014, 53, 1790–1794 CrossRef CAS PubMed.
- (a) J. B. Cloke, J. Am. Chem. Soc., 1929, 51, 1174–1187 CrossRef CAS; (b) C. L. Wilson, J. Am. Chem. Soc., 1947, 69, 3002–3004 CrossRef CAS.
- (a) A. P. Dieskau, M. S. Holzwarth and B. Plietker, J. Am. Chem. Soc., 2012, 134, 5048–5051 CrossRef CAS PubMed; (b) D. Pursley and B. Plietker, Synlett, 2014, 25, 2316–2318 CrossRef.
- J. E. M. N. Klein, G. Knizia, B. Miehlich, J. Kästner and B. Plietker, Chem.–Eur. J., 2014, 20, 7254–7257 CrossRef CAS PubMed.
- For transition metal catalyzed Cloke–Wilson-rearrangements see: (a) R. K. Bowman and J. S. Johnson, Org. Lett., 2006, 8, 573–576 CrossRef CAS PubMed; (b) U. M. Dzhemilev, R. I. Khusnutdinov, N. A. Shchadneva and G. A. Tolstikov, Russ. Chem. Bull., 1990, 39, 1447–1450 CrossRef; (c) A. A. Vardapetyan, D. S. Khachatryan, G. A. Panosyan and N. M. Morlyan, Journal of Organic Chemistry of the USSR, 1986, 22, 2034–2037 Search PubMed.
- Arylcyclopropanes have found frequent use in ring-enlargement reactions. For Lewis and Brønsted acid-catalyzed conditions. (a) M. Honda, T. Naitou, H. Hoshino, S. Takagi, M. Segi and T. Nakajima, Tetrahedron Lett., 2005, 46, 7345–7348 CrossRef CAS PubMed; (b) R. Zhang, Y. Liang, G. Zhou, K. Wang and D. Dong, J. Org. Chem., 2008, 73, 8089–8092 CrossRef CAS PubMed; (c) D. V. Patil, L. H. Phun and S. France, Org. Lett., 2010, 12, 5684–5687 CrossRef CAS PubMed; (d) H. Wang, J. R. Denton and H. M. L. Davies, Org. Lett., 2011, 13, 4316–4319 CrossRef CAS PubMed ; For thermal conditions; (e) D. E. McGreer and J. W. McKinley, Can. J. Chem., 1973, 51, 1487–1493 CrossRef CAS; (f) M. E. Alonso, A. W. Chitty, S. Pekerar and M. de L. Borgo, J. Chem. Soc., Chem. Commun., 1984, 1542–1543 RSC; (g) C. K. Ghosh and S. Sahana, Tetrahedron, 1993, 49, 4135–4140 CrossRef CAS; (h) A. M. Bernard, A. Frongia, P. P. Piras, F. Secci and M. Spiga, Org. Lett., 2005, 7, 4565–4568 CrossRef CAS PubMed; (i) P. Huang, R. Zhang, Y. Liang and D. Dong, Org. Lett., 2012, 14, 5196–5199 CrossRef CAS PubMed ; For base-catalyzed condition; (j) R.-Z. Zhang, C.-Y. Meng, J.-W. Xie, M.-L. Xu and W.-D. Zhu, Eur. J. Org. Chem., 2014, 3104–3107 CrossRef CAS PubMed ; For metal-catalyzed condition; (k) A.-E. Bosnidou, D. Kalpogiannaki, S. Karanestora, J. A. Nixas and L. P. Hadjiarapoglou, J. Org. Chem., 2015, 80, 1279–1283 CrossRef CAS PubMed.
- For details see ESI†.
- The conversion–wavelength correlation was recorded using a 75 W Xe-lamp equipped with a monochromator, the emission spectra of the three different lamps were recorded at Heraeus Hanau (Germany) under ISO-certified conditions.
- (a) J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS; (b) J. P. Perdew, M. Ernzerhof and K. Burke, J. Chem. Phys., 1996, 105, 9982–9985 CrossRef CAS PubMed.
- (a) H.-J. Werner and P. J. Knowles, J. Chem. Phys., 1988, 89, 5803–5814 CrossRef CAS PubMed; (b) P. J. Knowles and H.-J. Werner, Chem. Phys. Lett., 1988, 145, 514–522 CrossRef CAS; (c) P. J. Knowles and H.-J. Werner, Theor. Chim. Acta, 1992, 84, 95–103 CrossRef CAS.
- A. Klamt and G. Schüürmann, J. Chem. Soc., Perkin Trans. 2, 1993, 799–805 RSC.
- (a) H.-J. Werner and P. J. Knowles, J. Chem. Phys., 1985, 82, 5053–5063 CrossRef CAS PubMed; (b) P. J. Knowles and H.-J. Werner, Chem. Phys. Lett., 1985, 115, 259–267 CrossRef CAS; (c) T. Busch, A. Degli Esposti and H.-J. Werner, J. Chem. Phys., 1991, 94, 6708–6715 CrossRef CAS PubMed.
- (a) H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, WIREs Computational Molecular Science, 2012, 2, 242–253 CrossRef CAS PubMed; (b) MOLPRO, version 2012.1, a package of ab initio programs, see http://www.molpro.net Search PubMed.
- F. Weigend and R. Ahlrichs, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1409311–1409314. For ESI and crystallographic data in CIF or other electronic formats see DOI: 10.1039/c5sc02342d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |