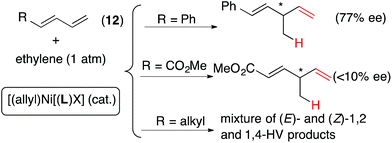Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cobalt-catalysed asymmetric hydrovinylation of 1,3-dienes†
Yam N.
Timsina
,
Rakesh K.
Sharma‡
and
T. V.
RajanBabu
*
Department of Chemistry and Biochemistry, The Ohio State University, 100 West 18th Avenue, Columbus, Ohio 43210, USA. E-mail: rajanbabu.1@osu.edu; Fax: +1 614 292 1685; Tel: +1 614 688 3543
First published on 23rd April 2015
Abstract
In the presence of bidentate 1,n-bis-diphenylphosphinoalkane-CoCl2 complexes {Cl2Co[P ∼ P]} and Me3Al or methylaluminoxane, acyclic (E)-1,3-dienes react with ethylene (1 atmosphere) to give excellent yields of hydrovinylation products. The regioselectivity (1,4- or 1,2-addition) and the alkene configuration (E- or Z-) of the resulting product depend on the nature of the ligand and temperature at which the reaction is carried out. Cobalt(II)-complexes of 1,1-diphenylphosphinomethane and similar ligands with narrow bite angles give mostly 1,2-addition, retaining the E-geometry of the original diene. Complexes of most other ligands at low temperature (−40 °C) give almost exclusively a single branched product, (Z)-3-alkylhexa-1,4-diene, which arises from a 1,4-hydrovinylation reaction. A minor product is the linear adduct, a 6-alkyl-hexa-1,4-diene, also arising from a 1,4-addition of ethylene. As the temperature is increased, a higher proportion of the major branched-1,4-adduct appears as the (E)-isomer. The unexpectedly high selectivity seen in the Co-catalysed reaction as compared to the corresponding Ni-catalysed reaction can be rationalized by invoking the intermediacy of an η4-[(diene)[P ∼ P]CoH]+-complex and its subsequent reactions. The enhanced reactivity of terminal E-1,3-dienes over the corresponding Z-dienes can also be explained on the basis of the ease of formation of this η4-complex in the former case. The lack of reactivity of the X2Co(dppb) (X = Cl, Br) complexes in the presence of Zn/ZnI2 makes the Me3Al-mediated reaction different from the previously reported hydroalkenylation of dienes. Electron-rich phospholanes, bis-oxazolines and N-heterocyclic carbenes appear to be poor ligands for the Co(II)-catalysed hydrovinylation of 1,3-dienes. An extensive survey of chiral ligands reveals that complexes of DIOP, BDPP and Josiphos ligands are quite effective for these reactions even at −45 °C and enantioselectivities in the range of 90–99% ee can be realized for a variety of 1,3-dienes. Cobalt(II)-complex of an electron-deficient Josiphos ligand is especially active, requiring only <1 mol% catalyst to effect the reactions.
Introduction
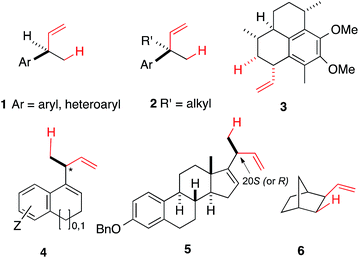
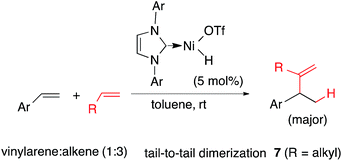
Heterodimerization reactions involving ethylene and other alkenes catalysed by late transition metals including iron,1–3 cobalt,4–8 nickel,9–13 rhodium,14 palladium15 and ruthenium14a,16 had a long history17 even before the more recent discoveries of applications of related chemistry in olefin polymerization reactions emerged.18–21 Among these, palladium,15 nickel and cobalt, and to a limited extent, ruthenium catalysed reactions22–29 have attracted the most attention in the stereoselective synthesis of small molecules. Through optimizations of ligands, promoters and reaction conditions, the Ni-catalysed asymmetric hydrovinylations (eqn (1)) of vinylarenes,30–36 selected dienes,37–39 and strained alkenes40,41 have been accomplished with excellent regio- and stereoselectivities giving highly valuable intermediates. Prototypical examples of the products of these reactions (1–6) are shown in Fig. 1. In a related reaction, Ho et al. reported42 dimerization of vinylarenes and 1-alkenes catalysed by N-heterocyclic carbene complexes of Ni(II)-salts to give uncommon tail-to-tail dimerization products (7, eqn (2)).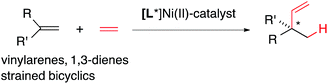 | (1) |
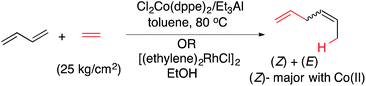
In sharp contrast, stereoselective dimerization reactions catalysed by cobalt-complexes are much less developed,43,44 even though a high pressure reaction between 1,3-butadiene and ethylene in the presence of Cl2Co(dppe)2 [dppe = 1,2-bis-diphenylphosphinoethane] and Et3Al to give hexadiene isomers (eqn (3)) has been known since 1967.45
 | (2) |
 | (3) |
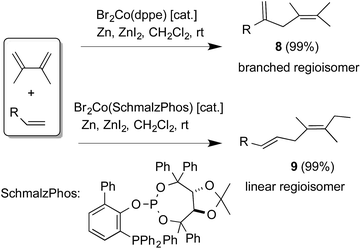
In 2001 Hilt et al. reported46–48 a remarkable hydroalkenylation reaction between 2,3-dimethylbuta-1,3-diene and a terminal alkene in the presence of Br2Co(dppe)/Zn/ZnI2 to give 1,4-addition products in very high yields and selectivities. Expansion of the scope of this reaction and several applications of this and related heterodimerization reactions have since been reported.49–52 A notable result in this area relevant to the present discussion is the ligand control of regio- and stereoselectivity in the reactions of substituted butadienes with terminal alkenes to give either the branched (8) or linear (9) adduct (Scheme 1).48,53
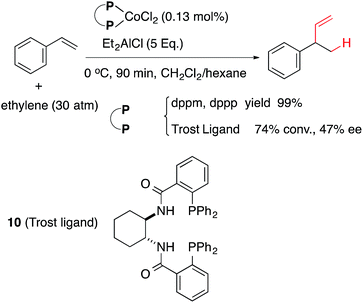
Expanded scope of substrates in the cobalt-catalysed hydrovinylation (HV, addition of ethylene) of alkenes and related reactions have been the subject of several recent publications. In 2006 Vogt reported the first example of a Co-catalysed asymmetric hydrovinylation of styrene using a Co(II)-complex of the Trost ligand 10 giving modest yield and selectivity (eqn (4)).54,55 Similarly norbornene has been reported to undergo a highly efficient alkylation–hydrovinylation catalysed by a pyridine–imine cobalt complex 11 (eqn (5)).56 Incidentally, styrene does not undergo HV under these conditions.
 | (4) |
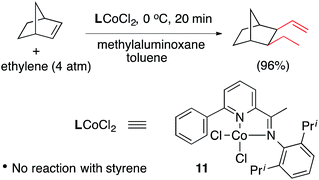 | (5) |
 | (6) |
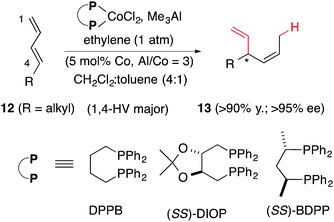
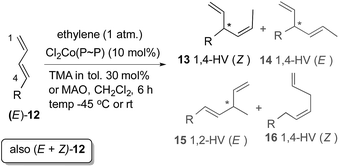
In 2010 we reported a novel protocol for the cobalt-catalysed asymmetric hydrovinylation of unactivated linear 1,3-dienes (12) under one atmosphere of ethylene and temperatures in the range of −45 °C to 25 °C (eqn (6)).57a This class of substrates gave unacceptable results in all previously reported HV reactions using iron,3 ruthenium,25 and nickel.13,58 We have since found that ligands and promoters play significant and, perhaps more importantly, predictable roles in the control of regio- and stereoselectivities of these reactions. Some expansion of the scope of this reaction57b and remarkable reactivity differences between (Z)- and (E)-terminal 1,3-dienes have also been reported.57c With some modifications, the new protocols for the cobalt-catalysed reactions are more broadly applicable and more substrates than originally reported undergo the reaction under moderate conditions. The full details of these studies are reported in this paper.
Results
Hydrovinylation of linear 1,3-dienes
1,3-Dienes are among the most readily available59 yet under-utilized precursors in enantioselective carbon–carbon bond-forming reactions. Thanks to advances in Wittig reactions and its alternatives, elimination, cross-coupling and cross-metathesis reactions, many structural and stereochemical variations in the dienes are possible. However, except for Diels–Alder reactions,60 few useful asymmetric-catalysed intermolecular C–C bond-forming reactions involving these substrates are known. In these instances synthetically acceptable enantioselectivities have been achieved only for limited reaction types [e.g., cyclopropanation,61,62 and hydroformylation,63,64] and that too for a limited set of substrates. Ni(II)-33,37 and Ru(II)25-catalysed hydrovinylation has been moderately successful in 1-arylbutadiene and methyl 2,4-pentadienoate, yet the enantioselctivities for these substrates remain unacceptably low (Scheme 2). Attempts to carry out the Ni-catalysed asymmetric hydrovinylation of an unactivated 1,3-diene (R = alkyl in 12, Scheme 2) such as (E)-1,3-octadiene under a variety of conditions led to a mixture of several products including 1,2- and 1,4-HV products.A careful review of the extensive literature on the codimerization of butadiene and ethylene1,5,10,14,65,66 revealed that while most reactions were carried out at higher temperatures (typically >60 °C) and pressures (>20 kg cm−3), remarkable selectivity for the formation of the Z- or E-1,4-hexadiene can be achieved by selection of the appropriate metal/ligand combinations. While highest selectivity for the E-1,4-hexadiene was obtained with [(ethylene)2RhCl]2 in alcoholic medium, Cl2Co(II) (P ∼ P) [(P ∼ P) = 1,2-bis-diphenylphosphinoethane) provided the best selectivity for the Z-1,4-hexadiene (eqn (3)). The most selective catalysts appeared to be those based on Fe(III)65 and Co(II)5,7 and we decided to concentrate our efforts on these two metals in our initial explorations.
While we have convincingly demonstrated that the Ni(II)-catalysed hydrovinylation of vinylarenes was completely inhibited by chelating bis-phosphines,24,67 many experiments in the literature suggested that chelating ligands can be used in the cobalt- or iron-mediated reactions since these metals are capable of higher coordination numbers.54 Our investigations started with an examination of 1,n-bis-diphenylphosphinoalkane-complexes of cobalt(II) X2Co[(Ph2P–(CH2)n–PPh2)] as catalysts in the presence of various additives in the reactions of ethylene with prototypical 1,3-dienes. Based on our work on the mechanism of the Ni(II)-catalysed HV,68 we set out to generate a cobalt-hydride or an equivalent species in solution (more on this later) which could serve as a catalyst in these reactions. Several cobalt hydride species carrying bisphosphines (e.g. (dppe)2CoH,69,70 [HCo(dppe)2(CH3CN)]+)71], which could serve such a role have been fully characterized. Further we wondered whether the expanded coordination sites could have other advantages such as reducing the conformational mobility of a 1,3-diene via an η4-coordination at critical stages in the catalytic cycle (vide infra), and if so, could this contribute to increased selectivity in the reactions of the 1,3-dienes.
Synthesis of the X2Co(P ∼ P)
The bis-phosphine complexes were readily prepared by modification of a procedure described in the literature.70,72 We57 and others55 have previously described the full characterization of the several Cl2Co(P ∼ P) complexes including their solid-state structures. Several more have been characterized recently.73 Anhydrous CoCl2 dissolved in freshly distilled THF (12 mL per mmol) was treated with 1.05 equivalents of the bis-phosphine for 15 minutes under argon to get a blue solution to which was added 12 mL mmol−1 of degassed ether to form a turbid solution. The mixture was stirred for 12–24 h and excess degassed hexane was added to precipitate the complex, which was collected and washed with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ether/hexane. This solid was used directly for the subsequent hydrovinylation reactions.
1 ether/hexane. This solid was used directly for the subsequent hydrovinylation reactions.
Optimization of a protocol for Co-catalysed hydrovinylation. Effect of ligands
For our initial studies we chose (E)-1,3-nonadiene (12a) as a prototypical, unactivated 1,3-diene substrate. After an initial series of experiments in which the reaction parameters such as temperature, time, solvents and sequence of addition of various reagents were systematically varied, we settled on a general protocol that is outlined in eqn (7).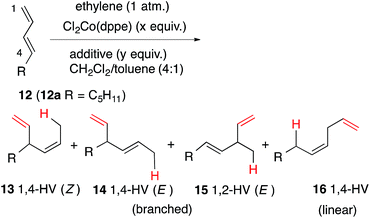 | (7) |
The reaction conditions and the distribution of products obtained are shown in Table 1. The results for the HV of (E)-1,3-nonadiene using Cl2Co(dppe) and trimethylaluminum as an additive (Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
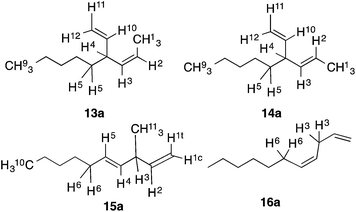
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co = 3) at various temperatures are shown in entries 1–4.74 Thus using 10 mol% of the cobalt complex at −40 °C a clean reaction ensues giving one major (13a) and two minor (14a and 16a) products. These products, arising from 1,4-addition across the 1,3-diene, were identified by comparison of gas chromatographic retention times and spectroscopic data with those of authentic samples, whose structures were rigorously established earlier.57,74 As the temperature is raised, the proportion of the 1,4-linear adduct, 16a, remains constant, but increasing amounts of a new geometric isomer of the major branched product, viz., 14a, is formed (entries 2–4). The reactivity of the substrate and the distribution of products are also dependent on the bite angle of the ligand75,76 employed. The Cl2Co(dppp) complex is slightly better than the corresponding dppe complex in both reactivity and selectivity (compare entries 1 and 5). The most reactive and selective ligand in this series was identified as 1,4-bis-diphenylphosphinobutane (dppb), which yielded the (Z)-1,4-HV product 13a in 96% isomeric purity (entry 9) in less than 0.5 h at 0 °C. Most notably, the (E)-isomer of the 1,4-HV (14a) was not detected by GC (limits of detection <0.5%). The reaction can be done equally effectively using Br2Co(dppb) complex using Me3Al as a promoter (entry 10); but the reaction fails completely when Zn/ZnI2 is used (entry 11), clearly establishing this procedure as distinctly different from the more reducing Hilt protocol (Scheme 1). Yet another remarkable observation, as shown in entries 12 and 13, is the effect of the narrow bite-angle (β = 72) ligand bis-diphenylphosphinomethane (dppm), which yielded a 1,2-HV product 15a as the major product with very little of the 1,4-Z adduct (13a) or the 1,4-linear adduct (16a). The minor component in this reaction was identified as the 1,4-E adduct, 14a. Catalyst derived from dppm is also very reactive, requiring only 3 mol% catalyst to complete the reaction in less than 12 h at −20 °C (entry 13). BISBI (2,2′-(diphenylphospinomethyl)-1,1′-biphenyl), a ligand with a large bite angle (β = 122°) also gives the 1,4-Z-adduct 13a as the major (65%) product, but with up to 34% of the 1,2-adduct 15a. Finally reaction of Cl2Co(Ph3P)2 in the presence of Me3Al gave mostly polymeric materials (entry 15).
Co = 3) at various temperatures are shown in entries 1–4.74 Thus using 10 mol% of the cobalt complex at −40 °C a clean reaction ensues giving one major (13a) and two minor (14a and 16a) products. These products, arising from 1,4-addition across the 1,3-diene, were identified by comparison of gas chromatographic retention times and spectroscopic data with those of authentic samples, whose structures were rigorously established earlier.57,74 As the temperature is raised, the proportion of the 1,4-linear adduct, 16a, remains constant, but increasing amounts of a new geometric isomer of the major branched product, viz., 14a, is formed (entries 2–4). The reactivity of the substrate and the distribution of products are also dependent on the bite angle of the ligand75,76 employed. The Cl2Co(dppp) complex is slightly better than the corresponding dppe complex in both reactivity and selectivity (compare entries 1 and 5). The most reactive and selective ligand in this series was identified as 1,4-bis-diphenylphosphinobutane (dppb), which yielded the (Z)-1,4-HV product 13a in 96% isomeric purity (entry 9) in less than 0.5 h at 0 °C. Most notably, the (E)-isomer of the 1,4-HV (14a) was not detected by GC (limits of detection <0.5%). The reaction can be done equally effectively using Br2Co(dppb) complex using Me3Al as a promoter (entry 10); but the reaction fails completely when Zn/ZnI2 is used (entry 11), clearly establishing this procedure as distinctly different from the more reducing Hilt protocol (Scheme 1). Yet another remarkable observation, as shown in entries 12 and 13, is the effect of the narrow bite-angle (β = 72) ligand bis-diphenylphosphinomethane (dppm), which yielded a 1,2-HV product 15a as the major product with very little of the 1,4-Z adduct (13a) or the 1,4-linear adduct (16a). The minor component in this reaction was identified as the 1,4-E adduct, 14a. Catalyst derived from dppm is also very reactive, requiring only 3 mol% catalyst to complete the reaction in less than 12 h at −20 °C (entry 13). BISBI (2,2′-(diphenylphospinomethyl)-1,1′-biphenyl), a ligand with a large bite angle (β = 122°) also gives the 1,4-Z-adduct 13a as the major (65%) product, but with up to 34% of the 1,2-adduct 15a. Finally reaction of Cl2Co(Ph3P)2 in the presence of Me3Al gave mostly polymeric materials (entry 15).
| Entry | P ∼ P | Bite angle | Cat. (mol%) Al/Co | Temp. (°C), time (h), conversion | Product, yieldb (%) | |||
|---|---|---|---|---|---|---|---|---|
| 13a (1,4-Z) | 14a (1,4-E) | 15a (1,2-E) | 16a (1,4-Linear) | |||||
| a See eqn (7) and ESI for details. b Estimated by GC and NMR. c The rest is starting material. d Entries 5–12 in neat CH2Cl2. e Using Br2Co(dppb). f Use of 20 mol% Zn, 20 mol% ZnI2 at 0 °C-rt for 4 h returns starting material. g 2,2′-(Diphenylphospinomethyl)-1,1′-biphenyl. h Polymerisation. | ||||||||
| 1 | dppe | 85 | 10/3 | −40/14/93c | 78 | 0 | 0 | 15 |
| 2 | dppe | 85 | 10/3 | −20/28/>99 | 64 | 19 | 0 | 12 |
| 3 | dppe | 85 | 10/3 | −12/15/>99 | 66 | 22 | 0 | 11 |
| 4 | dppe | 85 | 10/3 | 0/6/>99 | 73 | 15 | 0 | 12 |
| 5d | dppp | 91 | 10/5 | −40/8/>99 | 85 | 0 | 0 | 15 |
| 6d | dppp | 91 | 10/5 | 13/5/>99 | 76 | 20 | 0 | 4 |
| 7d | dppp | 91 | 10/5 | Rt/1/>99 | 86 | 8 | 0 | 5 |
| 8d | dppb | 98 | 10/5 | −10/8/>99 | 93 | 7 | 0 | 0 |
| 9d | dppb | 98 | 10/5 | 0/0.5/>99 | 96 | 0 | 0 | 4 |
| 10d,e | dppb | 98 | 10/5 | −20/20/>99 | 94 | 0 | 0 | 5 |
| 11d,e,f | dppb | 98 | —f | Rt/4/0 | 0 | 0 | 0 | 0 |
| 12d | dppm | 72 | 10/3 | Rt/2/>99 | 3 | 30 | 67 | 0 |
| 13d | dppm | 72 | 3/5 | −20/12/>99 | 2 | 33 | 64 | 0 |
| 14 | BISBIg | 122 | 100/10 | −12/6/100 | 65 | 0 | 34 | 0 |
| 15 | 2 Ph3P | — | 3/5 | −10/12/—h | 0 | 0 | 0 | 0 |
Identification of the hydrovinylation products
For an understanding of the mechanism of the reaction and for any further applications of the products, rigorous identification of all of the isomers of the hydrovinylation is critical. Fortunately, the remarkable ligand effects seen in these reactions enable preparation of several of the products in nearly pure state, which make their identification, and, the identification of minor products sometimes formed along with these compounds, fairly straight forward. We have also established conditions for gas chromatographic separation of all compounds including those of the enantiomers on appropriate columns.74 All estimates of isomeric ratios derived from proton NMR data have been corroborated by GC analysis.The products 13a, 14a, 15a and 16a derived from hydrovinylation of (E)-1,3-nonadiene (eqn (7)) are typical. The assignments of signals described below have been confirmed by COSY, NOESY and appropriate decoupling experiments (see Fig. 2). The major product seen in most hydrovinylation reaction, 13a, is characterized by, among other signals, the vinyl–CH3–hydrogens (H1) which appear at δ 1.622 (dd, 6.5 Hz, 2.0 Hz) and the bis-allylic hydrogen (H4) at δ 3.011 (m, NOE with H1). The 13C signal for the methyl carbon linked to this cis-double bond appears at δ 14.07 ppm. In 14a, the corresponding trans-1,4-HV adduct, this methyl carbon appears at δ 17.96, in keeping with the generally observed trend of lower field for trans alkene–methyls.77a This is also reflected in the 1H NMR signals of the CH3 groups of the cis- and trans compounds. The trans CH3 signals are slightly lower field compared to the cis-CH3 signals. In the present case the 1H NMR the methyl signal in the trans-compound 14a appears at δ 1.669 (dd, 6.0 Hz, 0.5 Hz) and of the cis-compound (13a) at δ 1.622.77b The bis allylic hydrogen H4 in 14a appears at δ 2.619 (tdd, 7.5 Hz, 7.5 Hz, 7.5 Hz). This signal, corresponding to the bis-allylic hydrogens, is distinctly different for all 4 isomers and is highly diagnostic. The 1,2-HV-adduct 15a is characterized by an up-field methyl doublet at δ 1.075 (d, J = 6.5 Hz, H11) and a bis-allylic hydrogen (H3) signal at δ 2.807 (ddq, 6.0 Hz, 6.0 Hz, 6.0, Hz). The (E)-geometry of the alkene is obvious from the large J4,5 value in 15a (15.5 Hz). Closely related compounds have been described in the literature.78,79 The linear adduct 16a is characterized by bis-allylic H's (H3) at δ 2.778 (t, 2H) and allylic H's (H6) at δ 2.019 (q, 2H). The GC retention time of this isomer (16) in every case is significantly longer than the other three isomers on a methylsilicone column. In addition, 16a is the only compound among the adducts that is achiral, and thus showing no resolution on the chiral stationary phase (CSP) GC columns. On a typical 30 m polymethylsiloxane column at 80 °C the following retention times are observed for the various adducts: 13a: 21.169 min.; 14a: 21.93 min.; 15a: 22.77 min.; at 75 °C: 13a: 28.22 min, 16a: 40.77 min. CSP GC (Cyclodex B) 60 °C 13a: RT = 26.07 min. (S), 27.25 (R); 16a: 44.79 min. The assignment of the absolute configuration is based on the observation that the product from Fe-catalysed asymmetric hydrovinylation of 1,3-pentadiene3 gave the laevorotatory product (S) as the major one and the same product was obtained in the asymmetric HV of 1,3-pentadiene using Cl2Co[(R,R) (−)-DIOP]. Accordingly, the levorotatory enantiomer was assumed to have the (S)-configurations. Configurations of all other 1,4-adducts were assigned by analogy (Fig. 2).
Promoters
Even though methyl–aluminum reagents such as methylaluminoxane have been widely used for the activation of cobalt–halide complexes in polymerisation reactions,19,20,80 the use of trimethylaluminum in our reactions is novel and noteworthy, since it has been reported that in cobalt-catalysed hydrovinylation of styrene this additive is ineffective.55 Our protocol is also distinctly different from the hydroalkenylation of 1,3-dienes reported by Hilt in which a bromide complex Br2Co(P ∼ P) [(P ∼ P) = dppm, dppe, dppp] is used along with Zn and ZnI2 as additives (Scheme 1) to get high yields of the adducts (8). While we find that Br2Co(dppb) complex works just as well as the corresponding chloride complex in our protocol using Me3Al (eqn (8)), this bromide complex does not catalyse the HV reaction in the presence of Zn and ZnI2 (eqn (9), Hilt conditions, also entry 17, Table 2).81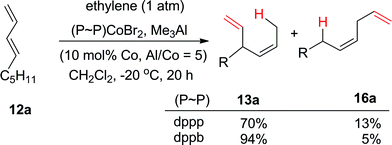 | (8) |
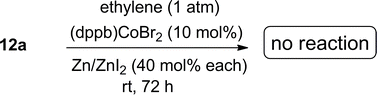 | (9) |
| Entry | P ∼ P | Additive (mol%) | Temp (°C), time (h) | Conversion [products] |
|---|---|---|---|---|
a See eqn (7) and ESI for details. All using Cl2Co(P ∼ P) unless indicated otherwise. Entries 13–20 using (E)-C8H17–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH–CH CH–CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2.
b No volatile products (polymers?).
c 2% Each 2 other isomers.
d
Ref. 81 (in hydroalkenylation of isoprene). CH2.
b No volatile products (polymers?).
c 2% Each 2 other isomers.
d
Ref. 81 (in hydroalkenylation of isoprene).
|
||||
| 1 | dppp | No additive | Rt (2) | 0 |
| 2 | dppp | Me3B (100) | Rt (2) | 0 |
| 3 | dppp | Et3B (100) | Rt (2) | 0 |
| 4 | dppp | Ph3B (100) | Rt (2) | 0 |
| 5 | dppp | i-BuAlH (100) | Rt (2) | 0 |
| 6 | dppp | LiEt3BH (100) | Rt (2) | 0 |
| 7 | dppb | Zn/ZnI2 (5, 5) | Rt (16) | 0 |
| 8 | dppe | PhMgBr (400) | Rt (7) | 0 |
| 9 | dppe | Mn (100) | Rt (14 h) | 0 |
| 10 | dppe | InI3 (100) | −10 – rt (10) | 0 |
| 11 | dppe | Et2AlOEt (100) | Rt (2) | <2% |
| 12 | dppm | MeMgBr, AgOTf (100, 100) | 0 – rt (4) | 0 |
| 13 | dppb | Et2AlCl (50) | −10 (2) | 0b |
| 14 | dppb | EtAlCl2 (50) | −10 (2) | 0b |
| 15 | dppp | Zn/ZnI2 (20, 20) | 0 – rt (5) | 0 |
| 16 | dppe | Zn/ZnI2 (20, 20) | 0 – rt (5) | 72 [56% 1,4-Z (13a); 11% 1,4-Z-lin (16a)]c |
| 17 | Br2Co (dppb) | Zn/ZnI2 (20, 20) | 0 – rt (4) | 0 |
| 18d | Br2Co (dppb) | Zn/ZnI2 (10, 10) | Rt (16) | 0 |
| 19 | Br2Co (dppe) | Zn/ZnI2 (20, 20) | 0 – rt (4) | 100 [85% 1,4-Z (13a); 15% 1,4-Z-lin (16a)] |
| 20 | Br2Co (dppp) | Zn/ZnI2 (20, 20) | 0 – rt (4) | 100 [79% 1,4-Z (13a); 21% 1,4-Z-lin (16a)] |
In light of the observations documented in the previous paragraph, it is of interest to note that several of the commonly used promoters (hydride reagents, alkylating agents, Lewis acids) also do not promote the hydrovinylation reactions in the presence of Cl2Co(P ∼ P) complexes (Table 2, entries 1–15). Combination of Zn/ZnI2 does affect the reaction giving moderate yields of 13a and 16a if Cl2Co(dppe) is used instead of Cl2Co(dppp) (entries 15 versus 16, Table 2). The corresponding complexes with CoBr2 catalyze the reaction, but with significant proportion of the linear 1,4-adduct 16 (entries 19 and 20). This stark difference between various bis-diphenylphosphine ligands (dppm, dppe vs. dppb) has been noted earlier by Hilt in his hydroalkenylation studies (entry 18, Table 2).81
Ligands
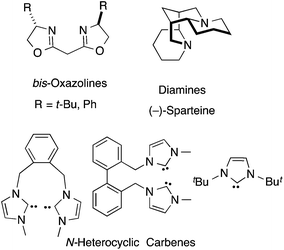
Having identified a chelating bis-phosphine and Me3Al as a viable ligand/promoter combination for an efficient cobalt-catalysed HV, we also examined three other popular classes of ligands, bis-oxazolines, chelating bis-amines and N-heterocyclic carbenes (Fig. 3) for this reaction. Cobalt(II)-complexes of these ligands failed to yield any HV products in reactions of (E)-1,3-nonadiene under conditions similar to what has been prescribed for the bis-phosphines in eqn (7).Scope of 1,3-diene substrates
The full scope of the new protocol for the Co-catalysed hydrovinylation reaction was examined with the help of the substrates shown in Table 3. The general optimized procedure used for the reactions is shown in eqn (10). In this procedure, a round-bottomed flask with a side-arm is charged with the X2Co(P ∼ P) complex (5–10 mol%) in degassed methylene chloride at 0 °C under argon. When methylaluminoxane (MAO) is used as the promoter, it is charged to the reaction flask right after the addition of the cobalt-complex. If Me3Al is used, a solution of Me3Al in toluene is added to the Co-complex and the reaction vessel is carefully evacuated and refilled with ethylene from a balloon, which is kept in place until the reaction is quenched. It is subsequently cooled to the prescribed temperature and the substrate is added and the mixture stirred for the periods shown in the table. Progress of the reaction maybe followed by gas chromatography, where baseline separation of the various possible adducts is observed. Excess methanol is added to quench the reaction and the products are extracted with pentane/ether. The conversions generally are quantitative under conditions shown in the table and, the yield, as judged by estimated weight of the product and its purity (GC and NMR) is >95% in most cases. The lower yields of some of the products result from the volatility of the hydrocarbon products. Attempts to remove last traces of the solvent (especially on small scales) result in some loss of materials. Details of the characterization of the isomeric products obtained from prototypical substrates (eqn (7)) and the diagnostic peaks in the 1H NMR that characterize the various isomers are documented in the ESI.†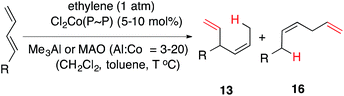 | (10) |
| Entry | Diene (12) R in 12eqn (10) | Cl2Co(P ∼ P) (P ∼ P), mol% | Al/Co | Conditions temp. (°C)/time (h) | Yield | |
|---|---|---|---|---|---|---|
| 13 | 16 | |||||
a See eqn (10) for procedure.
b Solvent CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) toluene 4 toluene 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
c MAO as ‘Al–Me’ source.
d 1,2-branched product 15g (99%).
e 1,2-branched product 15l, 97%.
f Product 15l 62%.
g Co 1.
c MAO as ‘Al–Me’ source.
d 1,2-branched product 15g (99%).
e 1,2-branched product 15l, 97%.
f Product 15l 62%.
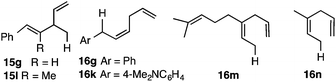
g Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Al 1 Al 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Z)-2-methyl-6-(3-propenyl)octa-2,6-diene (16m). See Fig. 4 for structures of 15g, 15l, 16g, 16k, 16m and 16n. 3 (Z)-2-methyl-6-(3-propenyl)octa-2,6-diene (16m). See Fig. 4 for structures of 15g, 15l, 16g, 16k, 16m and 16n.
|
||||||
| 1 | C5H11 (12a) | dppb (10) | 5 | 0/0.5 | >95 | |
| 2 | C6H13 (12b) | dppb (5) | 3 | −10/6b | >95 | |
| 3 | C7H15 (12c) | dppb (5) | 3 | −10/6b | >95 | |
| 4 | C8H17 (12d) | dppb (5) | 3 | −10/6b | >95 | |
| 5 | C8H17 (12d) (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z 54 Z 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46) 46) |
dppb (10) | 20 | −10/8c | 82 | |
| 6 | Cyclohexyl (12e) (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z 45 Z 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 55) 55) |
dppb (10) | 20 | −10/8c | 90 | |
| 7 | CH3 (12f) | dppb (5) | 3 | −10/6b | >95 | |
| 8 | Ph (12g) | dppp (10) | 3 | −20/4 | 0 | >99 (86) |
| 9 | dppb (10) | 3 | −10/6 | 0 | >99 (87) | |
| 10 | dppm (10) | 3 | −20/7 | 0 | 0d | |
| 11 | Ph (12g) (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z 40 Z 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60) 60) |
dppb (10) | 20 | −10/8c | 0 | >99 |
| 12 | CH2CO2Et (12h) | dppp (10) | 10 | 5/11 | 0 | 0 |
| 13 | dppb (10) | 10 | 5/11 | 0 | 0 | |
| 14 | dppm (10) | 10 | 0/15 | 84 | ||
| 15 | CH2CH2OBn (12i) | dppb (10) | 20 | −15/14c | >97 | <2 |
| 16 | CH2CH2Ph (12j) (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z 53 Z 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47) 47) |
dppb (10) | 20 | −10/8c | 84 | |
| 17 | 4-Me2N–C6H4 (12k) | dppb (10) | 20 | 0/13c | 0 | 79 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Other dienes | ||||||
| 18 |
|
dppm (5) | 3 | −10/6b | 0 | 0e |
| 19 | dppp | 3 | 10/6 | 0 | 0f | |
| 20 | β-myrcene (12m) | dppb (10) | 3 | 0/6 | 0 | >95g |
| 21 | dppp (10) | 3 | Rt/1 | 0 | 83g | |
| 22 | Isoprene (12n) | dppp (10) | 10 | −20/4 | 0 | >99 |
As shown in eqn (10), the cobalt-catalysed hydrovinylation of 1,3-dienes is most useful to prepare two types of adducts, 1,4-adducts 13 and 16 depending on the nature of the substituent R and the chelating bis-phosphine that is used. In general, alkyl- and substituted alkyl-1,3-dienes give excellent yields of the 1,4-(Z)-adduct 13 (entries 1–7) with the Cl2Co(dppb) complex with either Me3Al or MAO as the activating agent. The ester bearing substrate 12h (entries 12–14) is uniquely different in its reactivity in that neither the commonly used dppp complex (entry 12) nor the dppb complex (entry 13) is able to effect the hydrovinylation of this substrate. Yet a cobalt complex with a bis-phosphine ligand with a narrow bite-angle, dppm gives an excellent yield of the product 13h (entry 14). Recall that dppm-complex with the substrate with a simple alkyl substituent (12a) gave a 1,4 E-adduct 14a and a 1,2 E-adduct 15a (Table 1, entries 12 and 13).
1,3-Dienes with aromatic ring in conjugation with the diene belong to a class of substrates that behaves dramatically differently (12g: entries 8–11, 12k: entry 17). These substrates give linear 1,4-adducts 16g and 16k (Fig. 4) with a (Z)-geometry in the HV reactions catalysed by Cl2Co(dppb) and Cl2Co(dppp) complexes (entries 8, 9, 17). The Cl2Co(dppm) complex, in sharp contrast, gives exclusively a 1,2-(E)-adduct, 15g (entry 10, Fig. 4). Most notably, there is no contamination from the isomeric impurities in either of these reactions. Likewise (E)-2-methyl-1-phenyl-1,3-butadiene (12l) gives only a 1,2-HV product (15l, Fig. 4) with both the dppm and dppp complexes (entries 18 and 19).
3-Substituted 1,3-butadienes, β-myrcene (12m) and isoprene (2-methyl-1,3-butadiene, 12n) gave very high yields of 1,4-linear adducts 16m and 16n (Fig. 4) in which the hydrogen adds to the less substituted double bond (entries 20–22).
 | (11) |
Iron(III)-catalysed codimerization of ethylene and (E)-nona-1,3-diene
Iron(III)-catalysed codimerisation of ethylene and 1,3-butadiene (60 kg cm−2, 30–80 °C) in the presence of alkylaluminum reagents is among the earliest reported examples of these reactions.1,2 Other related reactions involving Fe-catalysis include codimerization of 1,3-pentadiene and ethylene,3 dimerization of α-olefins,83 and 1,4-addition of α-olefins to dienes.84 In a brief exploration to establish a direct comparison of reactivity of Cl3Fe(dppe) with Cl2Co(dppe) revealed that the former is much less reactive, with no particular advantages in terms of selectivity of products obtained, even though 1,4-Z-adduct 13a is the major product in both cases. One of the moderately successful experiments is depicted in eqn (12).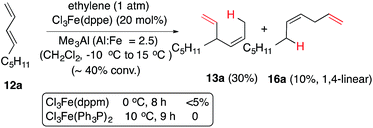 | (12) |
Asymmetric hydrovinylation of 1,3-dienes
| Entry | Ligandc | Conversion | Products (%ratio/%ee)b | |||
|---|---|---|---|---|---|---|
| 13a | 14a | 15a | 16a | |||
| a See eqn (13) for typical procedure (R = C5H11). b Determined by CSP GC. c See Fig. 5 for structures of ligands. d At −45 °C/8 h. e Rest starting material. | ||||||
| 1 | L1 | 99 | 41/78(S) | 4/— | 55/16 | 0 |
| 2 | L2 | 99 | 18/44 | 15/— | 62/1 | 2 |
| 3 | L3 | 99 | 90/8 | 0 | 0 | 9 |
| 4 | L4 | 66 | 54/1 | 0 | 0 | 12 |
| 5 | L5 | 40d | 7/— | — | 31/8 | |
| 6 | L6 | 0d | — | — | — | — |
| 7 | L7 | 99 | 96/97(R) | 0 | 0 | <1 |
| 8 | L8 | 99 | 95/95(S) | 0 | 0 | <1 |
| 9 | L9 | 99 | 86/64(S) | 11/— | 2/— | 0 |
| 10 | L10 | 0 | — | — | — | — |
| 11 | L11 | 0 | — | — | — | — |
| 12 | L12 | 88 | 66/8 | 0 | 0 | 7 |
| 13 | L13 | 95 | 63/14 | 0 | 11/31 | 4 |
| 14 | L14 | 80 | 39/95(S) | 0 | 0 | 40 |
| 15 | L15 | ∼6e | 6/— | 0 | 0 | 0 |
| 16 | L16 | 99 | 0 | 28/20 | 59/12 | 0 |
| 17 | L17 | 87d | 79/10 | 0 | 0 | 8 |
| 18 | L18 | 95 | 95/87(S) | 0 | 0 | 4 |
| 19 | L19 | 0 | — | — | — | — |
| 20 | L20 | 0 | — | — | — | — |
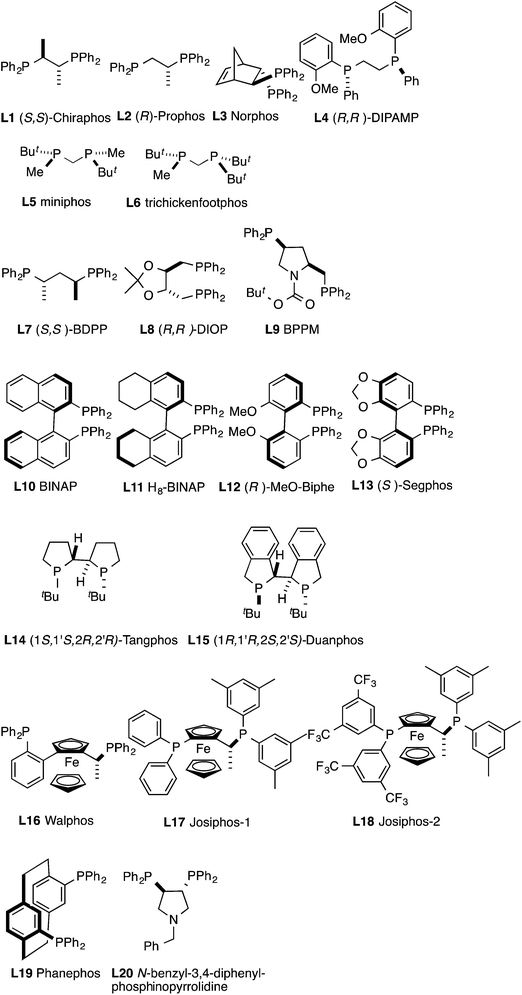
The reactivities of the chelating chiral ligands roughly parallel those of the corresponding achiral ligands with similar bite angles. In general, bis-diarylphosphino-ligands with bite angles comparable to dppe, dppp and dppb are the most reactive (Table 4, entries 1–3 and 7–9, 17, 18; ligands L1, L2, L3, L7, L8, L9, L17, L18). As expected, ligands with narrow bite-angles, (S,S)-chiraphos (L1) and (R)-prophos (L2) give, as the major product the 1,2-HV product 15a, along with minor amounts of 13a, the 1,4-(Z)-adduct (entries 1 and 2). The enantioselectivities for these products are low, with the ee for 13a almost always higher than that of the any other adducts in these reactions. Two other phosphines containing 2-carbon chains in the backbone, L3 and L4, give 13a as the major product with very low enantioselectivities (entries 3 and 4). Two chiral analogs of dppm, miniphos L5 and trichickenfootphos L6 are electron-rich phosphines,85 which are poor ligands for this reaction (entries 5 and 6).
 | (13) |
The dppp analog (S,S)-BDPP (L7) and dppb analog (R,R)-DIOP (L8) gave the best yields (99%) and highest enantioselectivities (97% and 95% respectively) for the hydrovinylation of 1,3-nonadiene (entries 7 and 8). In these cases less than 1% of the isomeric linear product (16a) is formed as the only contaminant. A constrained analog of dppb, L9, derived from proline also gives 13a as the major product, albeit in relatively low ee (entry 9).
2,2′-Diphenylphosphino-1,1-biaryls are among the most widely studied ligands in asymmetric catalysis, and in the present case only the biaryl ligands that gave any products are (R)-MeO-Biphe (L12) and (S)-Segphos (L13). Only low ee's (entries 12 and 13) were observed in these cases. Co(II)-complexes of BINAP (L10) and H8-BINAP (L11) failed to affect the reaction (entries 10 and 11).
Cobalt(II) complexes of electron-rich phospholanes L14 and L15 are poor catalysts, with the latter giving <6% conversion (entries 14 and 15). It is not unreasonable to assume that in the cases of ligands L6 (entry 6) and L15 (entry 15) the reactions might be retarded by both electronic and steric properties of these ligands. Yet, Tangphos (L14), an electron-rich bis-phospholane is among the best ligands (95% ee) for the formation of the 1,4-Z-HV product 13a, even though it gave up to 40% of the linear achiral product 16a (entry 14).
The complex derived from ferrocene-based ligand Walphos (L16) is the only ligand among the many we tested that gave none of the most commonly observed major product13a (entry 16). This complex gave the (E)-1,4 and (E)-1,2- adducts 14a and 15a as the major products, both in only modest selectivities (entry 16). Two other related ligands Josiphos-1 (L17) and Josiphos-2 (L18) are among the best ligands, the latter giving up to 87% ee for the branched product 13a, with <4% of the linear adduct 16a as a contaminant (entry 18). A remarkable electronic effect was observed in going from complex of L17, a ligand with diphenylphosphino-substituent, to one with a more electron-withdrawing di-(bis-1,3-CF3-phenylphosphino)-ligand L18. The enhancement of reactivity (yield: improvement from 87% to >95%) and selectivity (from 10% ee to 87% ee) observed in this ligand change is quite uncommon, and is reminiscent of similar effects seen in Ni-catalysed asymmetric hydrocyanation of vinylarenes.86 Electronic tuning of ligands might offer an attractive option to enhance enantioselectivity in the asymmetric hydrovinylation of 1,3-dienes. In addition the complex from ligand Josiphos 2 (L18) effects these reactions at 0.01 equivalent loading level (See Table 5, entries 4, 7, 9).
| Entry | Diene (12) R in 12eqn (13) | (P ∼ P) in Cl2Co(P ∼ P) | Al/Co | Conditions temp. (°C)/time (h) | Yield (%) | 13 (%ee)b | 16 (%) |
|---|---|---|---|---|---|---|---|
a See eqn (13) for procedure.
b Determined by CSP GC. Configurations are tentative and are based on the known product of HV of 1-methylbuta-1,3-diene (see Ref. 3). See ESI for details.
c 1 mol% Co.
d Reaction stopped after most of the (E)-isomer was converted, recovered starting material (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 1 Z = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17).
e Reaction stopped after most of the (E)-isomer is converted, recovered starting material (E 17).
e Reaction stopped after most of the (E)-isomer is converted, recovered starting material (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 1 Z = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49).
f Estimated by GC, volatile products.
g 1,2 –Adduct, 15g (43% yield; 52% ee), rest linear 16g.
h 5 mol%.
i Rest starting material.
j Using MAO. 49).
f Estimated by GC, volatile products.
g 1,2 –Adduct, 15g (43% yield; 52% ee), rest linear 16g.
h 5 mol%.
i Rest starting material.
j Using MAO.
|
|||||||
| 1 | C5H11 (12a-E) | L8 (R,R)-DIOP | 3 | −45/6 | >95 | 95.0(S) | |
| 2 | L7 (S,S)-BDPP | 3 | −45/6 | 97.1 | 97.1(R) | ||
| 3 | L14 Tangphos | 3 | −10/8 | 39 | 95.0(S) | 40 | |
| 4 | L18 Josiphos 2c | 3 | −20/14 | >95 | 87.0(S) | <4 | |
| 5 | C6H13 (12b-E) | L8 (R,R)-DIOP | 3 | −45/6 | >95 | 95.3(S) | |
| 6 | C7H15 (12c-E) | L8 (R,R)-DIOP | 3 | −45/6 | >98 | 95.4(S) | |
| 7 | L18 Josiphos 2c | 3 | −20/14 | >95 | 87.0(S) | <3 | |
| 8 | C8H17 (12d-E) | L8 (R,R)-DIOP | 3 | −45/6 | 95 | 96.1(S) | |
| 9 | L18 Josiphos 2c | 3 | −20/14 | 88 | 86.0(S) | <3 | |
| 10 | C8H17 (12d) (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 54 Z = 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 46) 46) |
L8 (S,S)-DIOP | 5 | −45/1 | 53d | 74.0(R) | 5 |
| 11 | Cyclohexyl (12e) (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 47 Z = 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53) 53) |
L8 (S,S)-DIOP | 3 | −10/8 | 49e | 84.0(R) | 5 |
| 12 | CH3 (12f-E) | L8 (R,R)-DIOP | 3 | −45/6 | >95f | 90.1(S) | |
| 13 | Ph (12g-E) | L8 (S,S)-DIOP | 3 | 0/5 | 46 | —g | 55 |
| 14 | CH2CO2Et (12h-E) | L7 (S,S)-BDPP | 10 | 0/15 | 84 | 92(R) | x |
| 15 | L18 Josiphos 2h | 3 | 10/8 | 0 | 0 | 0 | |
| 16 | CH2CH2OBn (12i-E) | L8 (R,R)-DIOP | 3 | −20/6 | 40i | 99.0(S) | 0 |
| 17 | L8 (S,S)-DIOPj | 3 | −10/6 | 99 | 94.0(R) | 6 | |
| 18 | L7 (S,S)-BDPPj | 3 | −10/9 | >99 | 92.0(R) | 0 | |
Finally two other ligands, Phanephos (L19) and the bis-phosphinopyrrolidine L20 are not competent for this reaction under the standard conditions (entries 19 and 20).
Scope of substrates in the asymmetric hydrovinylation of 1,3-dienes
A close examination of the results presented in Table 4 suggests that under optimized conditions the (Z)-1,4-HV product 13a can be prepared in synthetically useful yield and enantioselectivity by asymmetric hydrovinylation of (E)-1,3-dienes. The best ligands for this process have been identified as BDPP (L7), DIOP (L8) and Josiphos 2 (L18) and they appear to have much broader applications. A variety of simple and functionalized 1,3-dienes undergo the reaction giving excellent yields and enantioselectivities and the results are documented in Table 5. For simple 1,3-dienes such as 12a–g (entries 1–13) DIOP and BDPP gave the best results. In most cases, under optimized conditions, nearly quantitative yield of the product (13) is obtained in >95% ee with very little if any of contamination by an isomeric product, which is usually the linear adduct 16. Complexes of Josiphos 2 (L18, Fig. 5) are much more active as compared to BDPP (L7, Fig. 5) and DIOP (L8, Fig. 5), even though the ee's are slightly lower (1 mol% catalyst under otherwise identical conditions entry 6 vs. 7 and 8 vs. 9). Only in the case of Tangphos ligand L14 the linear product 16 is formed in significant amount (entry 3). As expected from studies described earlier, this product is also the major one in the cases of 1,3-dienes in conjugation with an aromatic nucleus, e.g., 12g (entry 13). In this case, up to 55% of linear adduct 16g is formed with the DIOP complexes. The major chiral product is the 1,2 E-adduct 15g, which is formed in a modest ee of 52%.The reaction is compatible with an ester group (12h) on the diene as shown in entries 14 and 15. Unexpectedly, cobalt complex of the Josiphos 2 ligand (L18) failed to affect the reaction of this substrate even with higher catalyst loading (entry 15). The diene containing a benzyl ether (12i) undergoes the reaction giving excellent yield and selectivity in the presence of CoCl2 complexes of DIOP and BDPP (entries 16–18). The DIOP complex, especially at low temperature is excellent for this reaction (>99% ee).
During these studies we also noticed significant difference in the rates of reactions of (E)- and (Z)-1,3-dienes.57c While this difference is hardly perceptible with the achiral Cl2Co(dppb) complex (entries 5, 6, 11 and 16 in Table 3), with the Cl2Co(DIOP) the (E)-isomers react significantly faster, leaving behind essentially unreacted (Z)-isomer near the end of the reaction (entries 10, 11, Table 5). In the case of substrate 12e (entry 11, E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 47
Z = 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 53), the unreacted starting material left behind at the end of the HV reaction (−10 °C, 8 h) using the complex Cl2Co(DIOP) is essentially pure (Z)-isomer (E
53), the unreacted starting material left behind at the end of the HV reaction (−10 °C, 8 h) using the complex Cl2Co(DIOP) is essentially pure (Z)-isomer (E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z = 1
Z = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49). Similar behavior is also seen with substrate 12d (entry 10). Note that while the isomerically pure (E)-12d leads to 96.1 %ee (entry 8), the E/Z mixture gives only 74% ee (entry 10), suggesting that the E- and Z-isomers of a given diene lead to different proportion of the enantiomers in the asymmetric HV.
49). Similar behavior is also seen with substrate 12d (entry 10). Note that while the isomerically pure (E)-12d leads to 96.1 %ee (entry 8), the E/Z mixture gives only 74% ee (entry 10), suggesting that the E- and Z-isomers of a given diene lead to different proportion of the enantiomers in the asymmetric HV.
Discussion
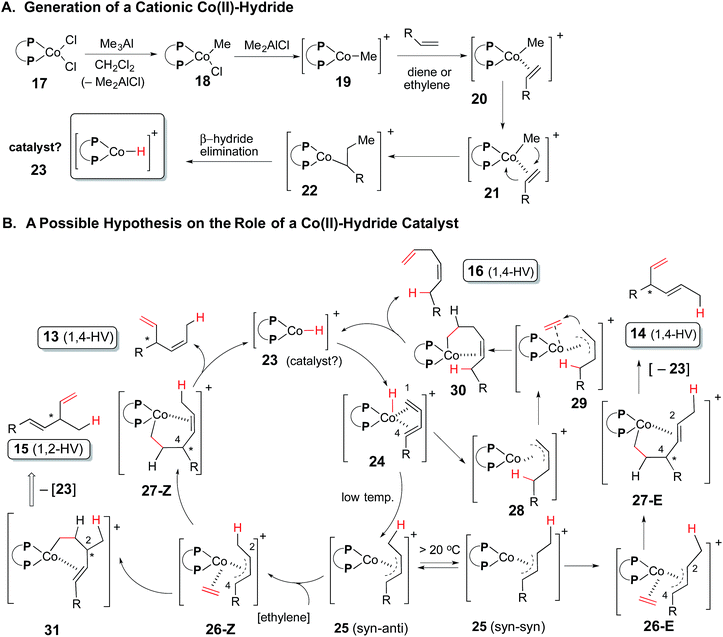
There are several key features that differentiates this reaction from the more well-studied (P)Ni(allyl)Br-catalysed hydrovinylation of 1,3-dienes87 and the hydroalkenylation reaction of 1,3-dienes catalysed by Br2Co(P ∼ P)/Zn/ZnI2.88 Whereas the nickel-catalysed reaction is completely inhibited by chelating ligands,67,89 the cobalt-catalysed reaction works well with a wide range of chelating ligands, and, by appropriate tuning of these ligands it is possible to obtain synthetically useful yields and selectivities of specific isomers of these hydrovinylation products. For example, cobalt complexes of chelating bis-phosphine ligands with narrow bite angles such as dppm give predominantly the 1,2-adduct (15, eqn (7)) whereas complexes of almost all other ligands lead to 1,4-HV leading to the major product 13 in which the initial hydride addition takes place at the terminal carbon and the vinyl group at the C4-carbon. The major contamination in these reactions results from a 1,4-addition with the hydride adding to the C4-carbon and the vinyl group to C1, leading to linear adducts (16). When the reaction is carried out at low temperature, the configuration of the internal double bond is almost exclusively (Z). When the diene is conjugated with an aromatic nucleus this linear product is formed almost exclusively.The remarkable selectivity seen in the HV of 1,3-dienes catalysed by cobalt complexes of dppp- and dppb-analogs (Table 3) stands in stark contrast to the corresponding Ni-catalysed reaction, which leads to an intractable mixture of products. We ascribe the poor results in the Ni-catalysed HV to the inability of this metal (small, limited coordination possibilities) to control the conformational mobility (s-cis/s-trans) of the 1,3-dienes. Based on several anecdotal observations in the literature5,20,56,71,80 and analogy to our own previous work on the mechanism of Ni-catalysed hydrovinylation of vinylarenes,68 we propose a mechanism for this reaction which is shown in Scheme 3. In this mechanism we hypothesize that a cationic cobalt hydride 23 is the true catalyst in the reaction (panel A). This species is formed by a sequence of reactions which start with the reaction of Cl2Co(P ∼ P) (17) with Me3Al to form 18. Abstraction of a halide by the in situ generated Lewis acid (Me2AlCl) would give the coordinately unsaturated complex 19, which facilitates an alkene (ethylene or the 1,3-diene) insertion into the Co–Me bond to generate 22. A β-hydride elimination from this species would produce the putative catalyst 23.
The catalyst 23 upon coordination of a diene would form 24 (panel B). We believe it is this η4-complex that is responsible for the high selectivity of this reaction. Isoelectronic metal complexes containing two chelating phosphines and a hydride are known in the literature71 and this is not an unreasonable intermediate for this process. Such a structure would limit the conformation of the coordinated diene to the s-cis arrangement, a possibility that does not exist in any corresponding Ni(II)-species. Two modes of addition of hydride to the intermediate 24 are possible. Addition of hydride to the terminal position (C1) would lead to an η3-allyl complex 25 (syn–anti), which after incorporation of ethylene at C4 leads to 27, and from there, to 13 with regeneration of the catalyst 23 by β-hydride elimination. Instead, if the ethylene addition takes space at C2 of 26, 31 will result, which after β-hydride elimination of 23 would lead to the 1,2-HV product 15. In the second mode, hydride addition takes place at C4 of 24 to give 28, which upon addition of ethylene at C1 would lead to the 1,4-linear adduct, 16.
We expect the initially formed adduct 25 (syn–anti) to be configurationally stable at low temperature and this would account for the Z-geometry seen in the major product 13 and the E-geometry seen in 15. However, if the syn–anti isomer of 25 undergoes isomerization to the more stable 25(syn–syn), for example at higher temperature, the 1,4-adduct 14 with an E-configuration of the double bond will result.66,90,91 This indeed has been observed (compare entries 1–3, or entries 5, 6 in Table 1). Further support for the intermediacy of the η4-complex also comes from the enhanced reactivity of the E-isomer in a mixture of (Z)- and (E)-terminal 1,3-dienes (entries 10 and 11 in Table 5). For steric reasons, the formation of the η4-complex should be significantly favored for the (E)-isomer of the diene, which can adapt an s-cis conformation much more easily as compared to the corresponding (Z)-diene. This differential reactivity is especially striking in the case of a highly discriminating ligand such as DIOP.
The differences between the hydrovinylation reactions catalysed by Cl2Co(P ∼ P)/Me3Al and the corresponding reactions carried out under the reducing conditions carried out by Hilt et al. [Br2Co(P ∼ P)/Zn/ZnI2]46,88 are also noteworthy. The former reaction proceeds in nearly quantitative yields with a broad selection of chelating bis-phosphines from dppm (bite angle 72) to BISBI (bite angle 122) [Table 1]. The reaction under reducing conditions (Zn/ZnI2) is limited to cobalt complexes of dppm, dppe and dppp.81 We have confirmed these ligand effects in additions of ethylene to 1,3-nonadiene (See Table 2, entries 17, 19, 20). Further, while the more easily reduced complex Br2Co(dppp) is catalytically competent upon treatment with Zn/ZnI2, the corresponding Cl2Co(dppp) returned no products under identical conditions (entry 15 vs. entry 20 in Table 2). It is reasonable to assume that under the highly reducing conditions, the reactions probably involve Co(I)-intermediates, where as under our reaction conditions, viz., using [Cl2Co(P ∼ P)/Me3Al], a cationic cobalt hydride is the active catalyst. All attempts to obtain discernable NMR spectra of any of the putative intermediates under our conditions have been unsuccessful, suggesting involvement of paramagnetic intermediates. On the other hand, (dppe)2CoH, an isolable diamagnetic complex (1H 16 ppm, 31P 69 ppm), described in literature69,70 is competent to effect the hydrovinylation of (E)-1,3-dodecadiene (12d), albeit in relatively low yield (10 mol%, CH2Cl2, 0 °C to rt, 7 h, 43% conversion to 13d and 16d). This experiment provides indirect support to the idea of a Co(I) hydride in some of the HV reactions. Other observations in the literature, for example, Et2AlCl is a better activator than Et3Al for HV reaction of 1,3-butadiene in the presence of isolated (dppe)2CoH,92 is not also inconsistent with the viability of a cobalt hydride in these reactions. Further studies to clarify the mechanisms of these reactions and to expand their scope will be reported in due course.
Conclusions
In the presence of bidentate 1,n-bis-diphenylphos-phinoalkane-CoCl2 complexes {Cl2Co[P ∼ P]} and Me3Al or methylaluminoxane, acyclic (E)-1,3-dienes react with ethylene (1 atmosphere) to give excellent yields of hydrovinylation products. The regioselectivity (1,4- or 1,2-addition) and the configuration (E- or Z- internal alkene) of the product depend on the nature of the ligand and temperature at which the reaction is carried out. Cobalt(II)-complexes of 1,1-dipheylphosphinomethane and similar ligands with narrow bite angles give mostly 1,2-addition, retaining the E-geometry of the original diene. Complexes of most other ligands at low temperature (−40 °C) give almost exclusively the branched product, (Z)-3-alkylhexa-1,4-diene, which arises from a 1,4-hydrovinylation reaction. A minor product is the linear adduct, 6-alkyl-hexa-1,4-diene, also arising from a 1,4-addition of ethylene. As the temperature is increased, a higher proportion of the major 1,4-adduct appears as the (E)-isomer. The unexpectedly high selectivity seen in the Co-catalysed reaction as compared to the corresponding Ni-catalysed reaction can be explained by invoking an η4-[(diene)[P ∼ P]CoH]+-complex and its subsequent reactions. The enhanced reactivity of terminal E-1,3-dienes over the corresponding Z-dienes can also be explained on the basis of the ease of formation of the η4-complex in the former case. The complete lack of reactivity of the X2Co(dppb) (X = Cl, Br) complexes in the presence of Zn/ZnI2 sets the Me3Al-mediated reaction apart from the previously reported hydroalkenylation of dienes. Electron-rich phospholanes, bis-oxazolines and N-heterocyclic carbenes appear to be poor ligands for the Co(II)-catalysed hydrovinylation of 1,3-dienes. An extensive survey of chiral ligands reveals that complexes of DIOP, BDPP and Josiphos ligands are quite effective for these reactions even at −45 °C and enantioselectivities in the range of 90–99% ee can be realized for a variety of 1,3-dienes.Acknowledgements
Financial assistance for this research provided by US National Science Foundation CHE-1362095 and National Institutes of Health (General Medical Sciences R01 GM075107).References and Notes
- G. Hata, J. Am. Chem. Soc., 1964, 86, 3903 CrossRef CAS.
- M. Iwamoto and S. Yuguchi, J. Org. Chem., 1966, 31, 4290 CrossRef CAS.
- J. Ehlers, W. A. König, S. Lutz, G. Wenz and H. tom Dieck, Angew. Chem., Int. Ed. Engl., 1988, 27, 1556 CrossRef PubMed.
- D. Wittenberg, Angew. Chem., Int. Ed. Engl., 1964, 3, 153 Search PubMed.
- M. Iwamoto and S. Yuguchi, Bull. Chem. Soc. Jpn., 1968, 41, 150 CrossRef CAS.
- L. Pu, A. Yamamoto and S. Ikeda, J. Am. Chem. Soc., 1968, 90, 7170 CrossRef CAS.
- T. Kagawa, Y. Inoue and H. Hashimoto, Bull. Chem. Soc. Jpn., 1970, 43, 1250 CrossRef CAS.
- A. Yamamoto, S. Kitazume, L. S. Pu and S. Ikeda, J. Am. Chem. Soc., 1971, 93, 371 CrossRef CAS.
- G. Wilke, B. Bogdanović, P. Hardt, P. Heimbach, W. Keim, M. Kroner, W. Oberkirch, K. Tanaka, E. Steinrucke, D. Walter and H. Zimmermann, Angew. Chem., Int. Ed., 1966, 5, 151 CrossRef CAS PubMed.
- R. G. Miller, T. J. Kealy and A. L. Barney, J. Am. Chem. Soc., 1967, 89, 3756 CrossRef CAS.
- K. Maruya, T. Mizoroki and A. Ozaki, Bull. Chem. Soc. Jpn., 1970, 43, 3630 CrossRef CAS.
- B. Bogdanović, B. Henc, B. Meister, H. Pauling and G. Wilke, Angew. Chem., Int. Ed., 1972, 11, 1023 CrossRef PubMed Among metal-catalysed asymmetric carbon-carbon bond-forming reactions, only Nozaki's Cu(II)-catalysed cyclopropanation of styrene with ethyl diazoacetate predates this discovery. See: H. Nozaki, S. Moriuti, H. Takaya and R. Noyori, Tetrahedron Lett., 1966, 7, 5239 CrossRef.
- P. Denis, A. Jean, J. F. Crcizy, A. Mortreux and F. Petit, J. Am. Chem. Soc., 1990, 112, 1292 CrossRef CAS.
- (a) T. Alderson, E. L. Jenner and R. V. Lindsey Jr, J. Am. Chem. Soc., 1965, 87, 5638 CrossRef CAS; (b) R. Cramer, J. Am. Chem. Soc., 1967, 89, 1633 CrossRef CAS.
- (a) G. J. P. Britovsek, K. J. Cavell and W. Keim, J. Mol. Catal. A: Chem., 1996, 110, 77 CrossRef CAS; (b) R. Bayersdörfer, B. Ganter, U. Englert, W. Keim and D. Vogt, J. Organomet. Chem., 1998, 552, 187 CrossRef; (c) N. J. Hovestad, E. B. Eggeling, H. J. Heidbüchel, J. T. B. H. Jastrzebski, U. Kragl, W. Keim, D. Vogt and G. van Koten, Angew. Chem., Int. Ed., 1999, 38, 1655 CrossRef CAS; (d) U. Englert, R. Haerter, D. Vasen, A. Salzer, E. B. Eggeling and D. Vogt, Organometallics, 1999, 18, 4390 CrossRef CAS; (e) E. B. Eggeling, N. J. Hovestad, T. B. H. Jastrzebski, D. Vogt and G. van Kotten, J. Org. Chem., 2000, 65, 8857 CrossRef CAS PubMed; (f) W.-J. Shi, J.-H. Xie and Q.-L. Zhou, Tetrahedron: Asymmetry, 2005, 16, 705 CrossRef CAS PubMed; (g) I. Ayora, R. M. Ceder, M. Espinel, G. Muller, M. Rocamora and M. Serrano, Organometallics, 2011, 30, 115 CrossRef CASFor a rare example of a platinum-catalysed hydrovinylation, see: M. E. Cucciolito, A. D'Amora and A. Vitagliano, Organometallics, 2005, 24, 3359 CrossRef CAS.
- H. Umezaki, Y. Fujiwara, K. Sawara and S. Teranishi, Bull. Chem. Soc. Jpn., 1973, 46, 2230 CrossRef CAS.
- For reviews, see, (a) H. Muller, D. Wittenberg, H. Seibt and E. Scharf, Angew. Chem., Int. Ed. Engl., 1965, 4, 327 CrossRef PubMed; (b) A. C. L. Su, Codimerization of ethylene and butadiene, Adv. Organomet. Chem., 1979, 17, 269 CrossRef CAS; (c) Y. Chauvin and H. Olivier, Dimerization and Codimerization, in Applied Homogeneous Catalysis with Organometallic Compounds, ed. B. Cornils and W. A. Herrmann, VCH, New York, 1996, Vol. 1, pp. 258–268 Search PubMed; (d) P. W. Jolly and G. Wilke, Hydrovinylation, in Applied Homogeneous Catalysis with Organometallic Compounds, ed. B. Cornils and W. A. Herrmann, VCH, New York, 1996, Vol. 2, pp. 1024–1048 Search PubMed; (e) T. V. RajanBabu, Chem. Rev., 2003, 103, 2845 CrossRef CAS PubMed; (f) G. Hilt, Eur. J. Org. Chem., 2012, 4441 CrossRef CAS PubMed; (g) T. V. RajanBabu, G. A. Cox, H. J. Lim, N. Nomura; R. K. Sharma; C. R. Smith and A. Zhang, Hydrovinylation Reactions in Organic Synthesis, in Comprehensive Organic Synthesis, ed. G. A. Molander and P. Knochel, Elsevier, Oxford, 2nd edn, 2014, Vol. 5, pp. 1582–1620 Search PubMed.
- L. K. Johnson, C. M. Killian and M. Brookhart, J. Am. Chem. Soc., 1995, 117, 6414 CrossRef CAS.
- B. L. Small, M. Brookhart and A. M. A. Bennett, J. Am. Chem. Soc., 1998, 120, 4049 CrossRef CAS.
- G. J. P. Britovsek, M. Bruce, V. C. Gibson, B. S. Kimberley, P. J. Maddox, S. Mastroianni, S. J. McTavish, C. Redshaw, G. A. Solan, S. Strömberg, A. J. P. White and D. J. Williams, J. Am. Chem. Soc., 1999, 121, 8728 CrossRef CAS.
- D. Takeuchi, R. Matsuura, S. Park and K. Osakada, J. Am. Chem. Soc., 2007, 129, 7002 CrossRef CAS PubMed.
- C. S. Yi, D. W. Lee and Y. Z. Chen, Organometallics, 1999, 18, 2043 CrossRef CAS.
- Z. He, C. S. Yi and W. A. Donaldson, Org. Lett., 2003, 5, 1567 CrossRef CAS PubMed.
- T. V. RajanBabu, N. Nomura, J. Jin, M. Nandi, H. Park and X. Sun, J. Org. Chem., 2003, 68, 8431 CrossRef CAS PubMed.
- Z. He, C. S. Yi and W. A. Donaldson, Synlett, 2004, 1312 CAS.
- R. P. Sanchez and B. T. Connell, Organometallics, 2008, 27, 2902 CrossRef CAS.
- Q.-S. Wang, J.-H. Xie, W. Li, S.-F. Zhu, L.-X. Wang and Q.-L. Zhou, Org. Lett., 2011, 13, 3388 CrossRef CAS PubMed.
- G. Jiang and B. List, Chem. Commun., 2011, 47, 10022 RSC.
- Y. Hiroi, N. Komine, S. Komiya and M. Hirano, Org. Lett., 2013, 15, 2486 CrossRef CAS PubMed.
- N. Nomura, J. Jin, H. Park and T. V. RajanBabu, J. Am. Chem. Soc., 1998, 120, 459 CrossRef CAS.
- (a) G. Franció, F. Faraone and W. Leitner, J. Am. Chem. Soc., 2002, 124, 736 CrossRef PubMed; (b) N. Lassauque, G. Francio and W. Leitner, Adv. Synth. Catal., 2009, 351, 3133 CrossRef CAS PubMed; (c) N. Lassauque, G. Francio and W. Leitner, Eur. J. Org. Chem., 2009, 3199 CrossRef CAS PubMed.
- A. Zhang and T. V. RajanBabu, Org. Lett., 2004, 6, 1515 CrossRef CAS PubMed.
- C. R. Smith and T. V. RajanBabu, Org. Lett., 2008, 10, 1657 CrossRef CAS PubMed.
- A. Zhang and T. V. RajanBabu, J. Am. Chem. Soc., 2006, 128, 5620 CrossRef CAS PubMed.
- W.-J. Shi, Q. Zhang, J.-H. Xie, S.-F. Zhu, G.-H. Hou and Q.-L. Zhou, J. Am. Chem. Soc., 2006, 128, 2780 CrossRef CAS PubMed.
- C. R. Smith, H. J. Lim, A. B. Zhang and T. V. RajanBabu, Synthesis, 2009, 2089 CAS.
- A. Zhang and T. V. RajanBabu, J. Am. Chem. Soc., 2006, 128, 54 CrossRef CAS PubMed.
- B. Saha, C. R. Smith and T. V. RajanBabu, J. Am. Chem. Soc., 2008, 130, 9000 CrossRef CAS PubMed.
- D. J. Mans, G. A. Cox and T. V. RajanBabu, J. Am. Chem. Soc., 2011, 133, 5776 CrossRef CAS PubMed.
- R. Kumareswaran, N. Nandi and T. V. RajanBabu, Org. Lett., 2003, 5, 4345 CrossRef CAS PubMed.
- W. Liu and T. V. RajanBabu, J. Org. Chem., 2010, 75, 7636 CrossRef CAS PubMed.
- (a) C. Y. Ho and L. S. He, Angew. Chem., Int. Ed., 2010, 49, 9182 CrossRef CAS PubMed; (b) C. Y. Ho, C.W. Chan and L. He, Angew. Chem., Int. Ed., 2015, 54, 4512 CrossRef CAS PubMed.
- For a review of reductive coupling of alkynes, allenes and alkenes catalysed by cobalt and nickel complexes, see: M. Jeganmohan and C.-H. Cheng, Chem.–Eur. J., 2008, 14, 10876 CrossRef CAS PubMed.
- A recent review of applications of cobalt chemistry: H. Pellissier and H. Clavier, Chem. Rev., 2014, 114, 2775 CrossRef CAS PubMed.
- M. Iwamoto, K. Tani, H. Igaki and S. Yuguchi, J. Org. Chem., 1967, 32, 4148 CrossRef CAS.
- G. Hilt, F.-X. du Mesnil and S. Lüers, Angew. Chem., Int. Ed., 2001, 40, 387 CrossRef CAS.
- G. Hilt and S. Lüers, Synthesis, 2002, 609 CrossRef CAS.
- M. Arndt, M. Dindaroglu, H.-G. Schmalz and G. Hilt, Org. Lett., 2011, 13, 6236 CrossRef CAS PubMed.
- G. Hilt, M. Danz and J. Treutwein, Org. Lett., 2009, 11, 3322 CrossRef CAS PubMed.
- M. Arndt, A. Reinhold and G. Hilt, J. Org. Chem., 2010, 75, 5203 CrossRef CAS PubMed.
- M. A. Bohn, A. Schmidt, G. Hilt, M. Dindaroglu and H.-G. Schmalz, Angew. Chem., Int. Ed., 2011, 50, 9689 CrossRef CAS PubMed.
- G. Hilt and S. Roesner, Synthesis, 2011, 662 CrossRef CAS PubMed.
- M. Arndt, M. Dindaroglu, H.-G. Schmalz and G. Hilt, Synthesis, 2012, 44, 3534 CrossRef CAS PubMed.
- M. M. P. Grutters, C. Müller and D. Vogt, J. Am. Chem. Soc., 2006, 128, 7414 CrossRef CAS PubMed.
- (a) M. M. P. Grutters, J. I. van der Vlugt, Y. X. Pei, A. M. Mills, M. Lutz, A. L. Spek, C. Muller, C. Moberg and D. Vogt, Adv. Synth. Catal., 2009, 351, 2199 CrossRef CAS PubMed; (b) G. Kiefer, H. Vrubel, R. Scopelliti and K. Severin, Eur. J. Inorg. Chem., 2013, 4916 CAS.
- C. Bianchini, G. Giambastiani, A. Meli and A. Toti, Organometallics, 2007, 26, 1303 CrossRef CAS.
- (a) R. K. Sharma and T. V. RajanBabu, J. Am. Chem. Soc., 2010, 132, 3295 CrossRef CAS PubMed; (b) J. P. Page and T. V. RajanBabu, J. Am. Chem. Soc., 2012, 134, 6556 CrossRef CAS PubMed; (c) Y. N. Timsina, S. Biswas and T. V. RajanBabu, J. Am. Chem. Soc., 2014, 136, 6215 CrossRef CAS PubMed.
- C. R. Smith, Metal-Catalysed Reactions of Ethene: Asymmetric Hydrovinylation Reaction and Palladacycle-Mediated Low-Pressure Vinylation of Aryl Halides, Ph. D. Thesis, The Ohio State University, 2010.
- V. H. Rawal and S. Kozmin, 1,3-Dienes, in Science of Synthesis, B. M. Trost, Thieme, Stuttgart, 2009, Vol. 46, pp. 1–739 Search PubMed.
- K. Mikami and K. Aikawa, Asymmetric Ene Reactions and Cycloadditions, in Catalytic Asymmetric Synthesis, ed. I. Ojima, Wiley, Hoboken, 2010, pp. 683–737 Search PubMed.
- M. P. Doyle, Asymmetric Addition and Insertion Reactions of Catalytically Generated Metal Carbenes, in Catalytic Asymmetric Synthesis, I. Ojima, Wiley-VCH, New York, 2000, pp. 191–228 Search PubMed.
- H. M. L. Davies and X. Dai, Total Synthesis of Natural Products Using the Combined C-H Activation/Cope Rearrangement as the Key Step, in Strategies and Tactics in Organic Synthesis, ed. M. Harmata, Elsevier, Greenwich, 2008, Vol. 7, pp. 383–407 Search PubMed.
- T. Horiuchi, T. Ohta, E. Shirakawa, K. Nozaki and H. Takaya, Tetrahedron, 1997, 53, 7795 CrossRef CAS.
- A. L. Watkins and C. R. Landis, Org. Lett., 2011, 13, 164 CrossRef CAS PubMed.
- M. Iwamoto and S. Yuguchi, Bull. Chem. Soc. Jpn., 1966, 39, 2001 CrossRef CAS.
- C. A. Tolman, J. Am. Chem. Soc., 1970, 92, 6777 CrossRef CAS.
- M. Nandi, J. Jin and T. V. RajanBabu, J. Am. Chem. Soc., 1999, 121, 9899 CrossRef CAS.
- J. Joseph, T. V. RajanBabu and E. D. Jemmis, Organometallics, 2009, 28, 3552 CrossRef CAS PubMed.
- F. Zingales, F. Canzini and A. Chiesa, Inorg. Chem., 1963, 2, 1303 CrossRef CAS.
- A. Sacco and R. Ugo, J. Chem. Soc., 1964, 3274 RSC.
- R. Ciancanelli, B. C. Noll, D. L. DuBois and M. R. DuBois, J. Am. Chem. Soc., 2002, 124, 2984 CrossRef CAS PubMed.
- W. D. Horrocks, G. R. Van Hecke and D. D. Hall, Inorg. Chem., 1967, 6, 694 CrossRef CAS.
- M. R. Friedfeld, M. Shevlin, J. M. Hoyt, S. W. Krska, M. T. Tudge and P. J. Chirik, Science, 2013, 342, 1076 CrossRef CAS PubMed.
- See ESI† for details of experimental procedures, analytical and spectroscopic data for all new compounds.
- P. W. N. M. van Leeuwen, P. C. J. Kamer, J. N. H. Reek and P. Dierkes, Chem. Rev., 2000, 100, 2741 CrossRef CAS PubMed.
- M. N. Birkholz, Z. Freixa and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2009, 38, 1099 RSC.
- (a) E. Breitmaier and W. Voelter, Carbon-13 NMR Spectroscopy, VCH, Weinheim, 1987, p. 116 Search PubMed; (b) G. Hata and D. Aoki, J. Org. Chem., 1967, 32, 3754 CrossRef CAS; (c) R. B. Bates and D. M. Gales, J. Am. Chem. Soc., 1960, 82, 5749 CrossRef CAS.
- Y. Yamamoto, S. Yamamoto, H. Yatagai and K. Maruyama, J. Am. Chem. Soc., 1980, 102, 2318 CrossRef CAS.
- H. Yatagai, J. Org. Chem., 1980, 45, 1640 CrossRef CAS.
- K. P. Tellmann, V. C. Gibson, A. J. P. White and D. J. Williams, Organometallics, 2005, 24, 280 CrossRef CAS.
- G. Hilt and J. Treutwein, Chem. Commun., 2009, 1395 RSC.
- J. Jin and T. V. RajanBabu, Tetrahedron, 2000, 56, 2145 CrossRef CAS.
- B. L. Small and A. J. Marcucci, Organometallics, 2001, 20, 5738 CrossRef CAS.
- B. Moreau, J. Y. Wu and T. Ritter, Org. Lett., 2009, 11, 337 CrossRef CAS PubMed.
- T. Imamoto, K. Tamura, T. Ogura, Y. Ikematsu, D. Mayama and M. Sugiya, Tetrahedron: Asymmetry, 2010, 21, 1522 CrossRef CAS PubMed.
- (a) A. L. Casalnuovo, T. V. RajanBabu, T. A. Ayers and T. H. Warren, J. Am. Chem. Soc., 1994, 116, 9869 CrossRef CAS; (b) T. V. RajanBabu and A. L. Casalnuovo, Pure Appl. Chem., 1994, 66, 1535 CrossRef CAS.
- T. V. RajanBabu and C. R. Smith, Enantioselective Hydrovinylation of Alkenes, in Comprehensive Chirality, E. M. Carreira and H. Yamamoto, Elsevier, London, 2012, Vol. 5, pp. 355–398 Search PubMed.
- G. Hilt, Eur. J. Org. Chem., 2012, 4441 CrossRef CAS PubMed.
- T. V. RajanBabu, N. Nomura, J. Jin, B. Radetich, H. Park and M. Nandi, Chem.–Eur. J., 1999, 5, 1963 CrossRef CAS.
- G. Consiglio and R. M. Waymouth, Chem. Rev., 1989, 89, 257 CrossRef CAS.
- B. M. Trost and C. Lee, Asymmetric Allylic Alkylation Reactions, in Catalytic Asymmteric Synthesis, ed. I. Ojima, Wiley-VCH, New York, 2000, pp. 593–649 Search PubMed.
- M. Iwamoto and S. Yuguchi, Chem. Commun., 1968, 28 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5sc00929d |
| ‡ Current address. Department of Chemistry, IIT Jodhpur, Old Residency Road, Jodhpur-342011, Rajasthan, India (E-mail: rks@iitj.ac.in) |
| This journal is © The Royal Society of Chemistry 2015 |