Open Access Article
Open Access ArticleA prochelator peptide designed to use heterometallic cooperativity to enhance metal ion affinity†
Bruno
Alies
,
Jacob D.
Wiener
and
Katherine J.
Franz
*
Department of Chemistry, Duke University, Durham, North Carolina 27708, USA. E-mail: katherine.franz@duke.edu
First published on 5th May 2015
Abstract
A peptide has been designed so that its chelating affinity for one type of metal ion regulates its affinity for a second, different type of metal ion. The prochelator peptide (PCP), which is a fusion of motifs evocative of calcium loops and zinc fingers, forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn
2 Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) peptide complex at pH 7.4 that increases its affinity for Zn2+ ∼3-fold in the presence of Tb3+ (log
peptide complex at pH 7.4 that increases its affinity for Zn2+ ∼3-fold in the presence of Tb3+ (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 from 13.8 to 14.3), while the 1
β2 from 13.8 to 14.3), while the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 luminescent complex with Tb3+ is brighter, longer lived, and 20-fold tighter in the presence of Zn2+ (log
1 luminescent complex with Tb3+ is brighter, longer lived, and 20-fold tighter in the presence of Zn2+ (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K from 6.2 to 7.5). This unique example of cooperative, heterometallic allostery in a biologically compatible construct suggests the possibility of designing conditionally active metal-binding agents that could respond to dynamic changes in cellular metal status.
K from 6.2 to 7.5). This unique example of cooperative, heterometallic allostery in a biologically compatible construct suggests the possibility of designing conditionally active metal-binding agents that could respond to dynamic changes in cellular metal status.
Introduction
The intersections of metal homeostasis pathways have important ramifications for cellular function and dysfunction.1 Crosstalk among Na+, K+, and Ca2+ regulatory pathways, for example, orchestrates changes in the relative concentrations of these s-block ions that form the basis of neuronal activity. Recognition is now focusing increasingly on how Ca2+ fluxes associated with cell signaling may induce dynamic mobiliza-tion and redistribution of d-block ions like Zn2+ and Cu+.2–5 Reciprocally, dynamic changes of Zn2+ may impinge on Ca2+ homeostasis.6,7 Tools to explore this crosstalk are needed to better understand the complexity of interconnected metal pathways and ultimately remedy dysfunctional aspects.As part of our efforts to design conditionally active prochelators that change their metal chelating ability in response to specific stimuli,8,9 we envisioned biologically compatible molecules that could increase their metal binding affinity for one metal in response to dynamic changes in the concentration of a second metal. This kind of allosteric regulation of metal chelation has precedent in biology. For example, the antibacterial properties of the mammalian immunodefense protein calprotectin are related to its ability to bind Mn2+ and Zn2+,10 the affinity for which increases in the presence of Ca2+.11–13 Other members of the S100 family of calcium-binding proteins contain Zn2+ binding sites that positively influence Ca2+ binding,14 while others show negative allostery, suggesting that Zn2+ in these cases might inhibit these proteins' responses to Ca2+ signals.15–17 An interesting example of differential binding based on oxidation state is the bacterial metal resistance protein CopK, which displays cooperative binding of Cu+ and Cu2+.18
The concept of allosteric regulation in biology has inspired numerous efforts to develop artificial receptor systems, including ones where one or both of the effector and substrate species is a metal ion.19–21 Many of these systems do not operate in water and are not compatible with biological applications. The field of de novo peptide and protein design offers elegant examples of bio-inspired cooperativity, for example an artificial metallohydrolase that uses a Hg2+ binding site to stabilize a catalytic Zn2+ site,22 and the use of metal templating to mediate cooperative assembly along protein surfaces to generate sophisticated multi-subunit protein arrays.23 Recently, a β-peptide bundle was shown to exhibit positive allosteric cooperativity in water for binding two Cd2+ ions at distinct sites.24 In these cases, cooperative metal binding serves to stabilize the overall construct for structural or catalytic purposes. In contrast, the goal for metal-activated prochelators is to influence the environment around the construct by altering metal concentrations in a synergistic way.
Results and discussion
Here we present the first ProChelator Peptide (PCP) that contains distinct binding sites for two different metal ions that bind cooperatively, meaning that coordination of one increases the affinity for the other. We specifically wanted to incorporate synergistic zinc and calcium binding sites, and chose to use Tb3+ (Tb) as a surrogate for Ca2+ due to its similar coordination preferences and useful luminescence.25 A starting point for our design was a lanthanide binding tag (LBT) developed by Imperiali et al. via iterative redesign of classic calcium-binding loops.26–28 The crystal structure of the optimized Tb–LBT complex revealed that a hydrophobic patch generated by flanking tyrosine and leucine residues helps to stabilize the loop and improve the Kd for Tb from 8 μM for the original construct to 57 nM for the LBT shown in Fig. 1.28 We hypothesized that replacing the flanking hydrophobic residues with histidines and cysteines could generate constructs that use Zn2+ (Zn) as a stabilizing factor via formation of a Cys2His2 motif reminiscent of classic zinc fingers. The sequences of the resulting PCP and several control peptides are shown in comparison to the template LBT in Fig. 1.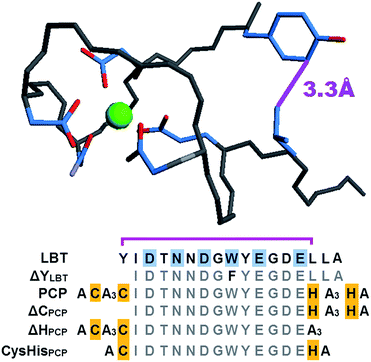 | ||
| Fig. 1 Top: structure of the parent Tb–LBT complex (PDB 1TJB). For clarity, only the Tb-binding ligands and flanking Tyr1 and Leu13 residues are shown, with their proximity emphasized by the purple line. Bottom: amino acid sequences of LBT, the ProChelator Peptide (PCP), and several controls. Putative Zn-binding residues are highlighted in yellow, with known Tb-binding residues of the LBT highlighted in cyan. Monodentate carboxylates from Asp1 and Asp5, bidentate carboxylates from Glu9 and Glu12, carboxamide O from Asn3, and backbone carbonyl O of Trp7 provide an 8-coordinate binding site for Tb3+. | ||
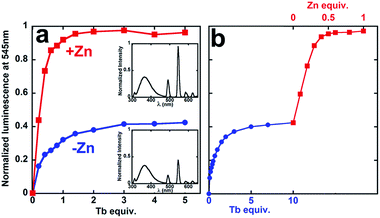
To investigate possible synergistic binding of Tb and Zn to PCP, the peptide was titrated with Tb in the absence and presence of Zn (Fig. 2). Both the intensity and the lifetime of Tb luminescence are sensitive to coordination environment. Aqueous Tb3+ is only weakly luminescent, but close proximity of a chromophore like tryptophan sensitizes intense Tb emission, which is also enhanced by displacement of inner-sphere H2O molecules that quench Tb emission. In the absence of Zn, the sensitized Tb emission for PCP plateaus after 1 equivalent of Tb has been added, suggesting 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Tb
1 Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCP stoichiometry (Fig. 2). If Zn is present either at the beginning (Fig. 2a, red squares) or end of the Tb titration (Fig. 2b, red squares), the intensity increases 150%. This boost in Tb emission maximizes at 0.5 equivalent of Zn, suggesting a 1
PCP stoichiometry (Fig. 2). If Zn is present either at the beginning (Fig. 2a, red squares) or end of the Tb titration (Fig. 2b, red squares), the intensity increases 150%. This boost in Tb emission maximizes at 0.5 equivalent of Zn, suggesting a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn
2 Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCP ratio. The luminescence lifetime also increases dramatically, gaining +1.1 ms in the presence of Zn (Table 1 and Fig. S1†). These combined results indicate that Zn has a clear effect on the coordination environment of Tb. Furthermore, the observation that the order of metal addition does not impact the Zn-induced Tb emission indicates a system in dynamic equilibrium.
PCP ratio. The luminescence lifetime also increases dramatically, gaining +1.1 ms in the presence of Zn (Table 1 and Fig. S1†). These combined results indicate that Zn has a clear effect on the coordination environment of Tb. Furthermore, the observation that the order of metal addition does not impact the Zn-induced Tb emission indicates a system in dynamic equilibrium.
| Peptides | Zn association constanta (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2) & pZnb β2) & pZnb |
Tb association constantc,d (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K) & pTbb K) & pTbb |
Lifetime (±0.1 ms) | |||
|---|---|---|---|---|---|---|
| (−) Tb | (+) Tb | (−) Zn | (+) Zn | (−) Zn | (+) Zn | |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 (pZn) β2 (pZn) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 (pZn) β2 (pZn) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K (pTb) K (pTb) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K (pTb) K (pTb) |
|||
| a Zn affinity measured using PAR competition. b pM values calculated for [peptide] = 2 μM, [M1] = 0.5 μM and [M2] either zero or saturating. c Determined by direct titration. d Determined by competition with ΔYLBT or LBT. e Does not compete with PAR. See ESI for further details. | ||||||
| LBT | ≪10e | ≪10e | 7.8 ± 0.2c (8.3) | 7.8 ± 0.2c (8.3) | 2.5 | 2.5 |
| ΔYLBT | ≪10e | ≪10e | 6.9 ± 0.1d (7.4) | 6.8 ± 0.1d (7.3) | 2.4 | 2.4 |
| PCP | 13.8 ± 0.2 (8.1) | 14.3 ± 0.2 (8.6) | 6.2 ± 0.2d (6.9) | 7.5 ± 0.2d (8.1) | 1.3 | 2.4 |
| ΔCPCP | 10.6 ± 0.4 (6.3) | 10.6 ± 0.4 (6.3) | 5.5 ± 0.1c (6.5) | 5.4 ± 0.1c (6.5) | 1.0 | 1.0 |
| ΔHPCP | 11.9 ± 0.2 (6.7) | 11.9 ± 0.2 (6.7) | 6.7 ± 0.1c (7.2) | 6.4 ± 0.1c (7.0) | 1.0 | 1.0 |
| CysHisPCP | 11.5 ± 0.3 (6.5) | 11.5 ± 0.3 (6.5) | 6.2 ± 0.1d (6.9) | 5.9 ± 0.1d (6.7) | 1.2 | 1.5 |
Formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Tb
1 Tb![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCP complex was expected and consistent with previous studies using similar sequences.26–28 While our original design imagined a 1
PCP complex was expected and consistent with previous studies using similar sequences.26–28 While our original design imagined a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 tetrahedral Zn bind-ing site created by flanking Cys2 and His2 motifs of a single PCP, the data in Fig. 2 indicate a 1
1 tetrahedral Zn bind-ing site created by flanking Cys2 and His2 motifs of a single PCP, the data in Fig. 2 indicate a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Zn
2 Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCP stoichiometry.
PCP stoichiometry.
In order to test the importance of these flanking residues on Zn-dependent Tb luminescence, we synthesized control peptides that lack both N-terminal cysteines (ΔCPCP), both C-terminal histidines (ΔHPCP) or only have one Cys and one His on each end (CysHisPCP). Substituting polar cysteines and histidines with alanines resulted in peptides with diminished solubility, whereas truncating the ends better replicated the solution properties of the parent peptide. The affinities for Zn and Tb were the same for truncated CysHisPCP compared to a longer Ala-substituted counterpart A4CysHisPCP (ESI Fig. S3 and S5†), validating the truncation strategy. In contrast to PCP, Zn did not change the Tb luminescence lifetime of LBT, ΔCPCP, or ΔHPCP, and caused only a very minor 0.3 ± 0.1 ms increase in the case of CysHisPCP. The three Cys and His truncated controls all showed a minor but consistent decrease in Tb emission intensity as a function of Zn, perhaps a result of direct competition with Tb for the carboxylate ligands in these cases. The control peptides have a lower affinity for Zn compared to PCP, in line with having fewer ligands available for Zn binding (Table 1). Even though the Tb binding site remains the same, Tb affinity varies slightly across this series, suggesting a contribution from the second coordination sphere, as seen for other metal–peptide complexes.29 These combined observations from the control peptides support a model of Zn binding that requires at least one C-terminal histidine and one N-terminal cysteine from each peptide to form a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex. This 1
2 complex. This 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 stoichiometry and cysteine ligation are further supported by the appearance of a characteristic Zn–thiolate charge transfer at 230 nm that plateaus at 0.5 equiv. of added Zn (see Fig. S8†).30,31 The absence of cooperative Zn and Tb binding by the control peptides highlights the uniqueness of the amino acid arrangement in PCP that fosters Zn-dependent changes in the Tb coordination, likely a consequence of a peptide conformational change.
2 stoichiometry and cysteine ligation are further supported by the appearance of a characteristic Zn–thiolate charge transfer at 230 nm that plateaus at 0.5 equiv. of added Zn (see Fig. S8†).30,31 The absence of cooperative Zn and Tb binding by the control peptides highlights the uniqueness of the amino acid arrangement in PCP that fosters Zn-dependent changes in the Tb coordination, likely a consequence of a peptide conformational change.
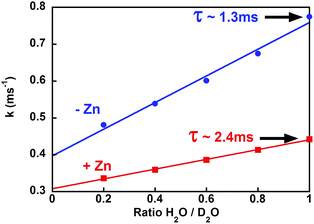
To further investigate this structural change, we estimated the number of coordinated water molecules (q) on Tb by measuring the Tb lifetime at different ratios of H2O/D2O (Fig. 3 and S4†).32 For Tb–PCP, a q value of 1.5 decreases to 0.5 when Zn is present, a reduction that is illustrated by a smaller variation of luminescence decay rate in the presence of Zn than in its absence (Fig. 3 red vs. blue, respectively). These data indicate that Zn binding to the PCP–Tb complex induces a conformational change that better shields Tb from water and even occludes an H2O ligand from the inner coordination sphere. Concurrent with the increase in lifetime is an increase in emission intensity upon Zn binding (see Fig. S1† PCP), which suggests that the conformational change triggered by Zn more favourably aligns the Trp for energy transfer to Tb, perhaps by improving the peptide's Tb affinity.
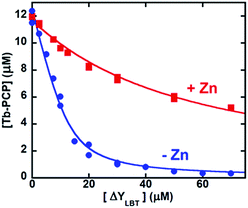
Measuring the binding affinity of PCP for Tb required a competitive ligand with specificity for Tb3+ over Zn2+. Because the template LBT itself has negligible affinity for Zn (Table 1), we made a derivative, ΔYLBT, that lacks the first tyrosine and replaces the tryptophan with phenylalanine. Deletion of Tyr1 lowers Tb affinity,28 and replacement of the Trp quiets the Tb emission (a remaining Tyr retains some luminescence). Fig. 4 displays a Tb competition experiment between PCP and ΔYLBT. In the absence of Zn (Fig. 4, blue circles), the concentration of ΔYLBT needed to displace half of the Tb from PCP is about 10 μM, whereas in the presence of Zn it is 50 μM. The conditional association constants of PCP for Tb are obtained from fitting these titration curves (Table 1). Expressed as dissociation constants, the Kd for Tb drops from 630 nM to 30 nM upon addition of Zn. Overall, this ∼20-fold amplification in Tb affinity as a result of Zn is of the same magnitude as the effect of Ca on Zn or Mn binding to calprotectin,11,12 a protein with over 200 amino acids and multiple Ca-binding sites.13,33
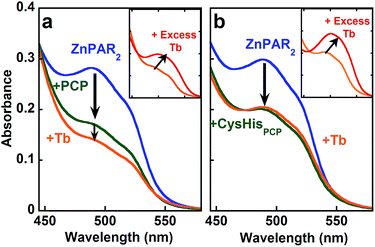
In order to explore the full cooperativity between Tb and Zn, we measured the affinity of PCP binding to Zn as a function of Tb by using the metal chelator 4-(2-pyridylazo)resorcinol (PAR), which forms colored complexes with various metal ions. The Zn(PAR)2 complex exhibits a band with a λmax at 490 nm (Fig. 5a, solid blue line). The addition of PCP (solid green line) causes a decrease in intensity at 490 nm, suggesting removal of Zn from ZnPAR2. Subsequent addition of Tb (solid orange line) leads to a further decrease at 490 nm, which suggests that the binding of Tb to PCP increases its affinity for Zn. This behavior is unique for PCP and is not observed for CysHisPCP (Fig. 5b) or other control peptides (Fig. S5†). If an excess of Tb is added (Fig. 5 inset, solid red line), another colored complex grows in with λmax of 510 nm, which is consistent with formation of a Tb–PAR complex. Because the Zn and Tb–PAR complexes absorb at different wavelengths, the concentrations of the various species in solution can be calculated and the conditional affinity of PCP for Zn determined in the presence and absence of Tb (Table 1). A 3-fold increase in PCP's Zn affinity occurs in the presence of Tb, whereas the Zn affinity of the control peptides is unchanged. Although modest, this change in affinity is significant since metal concentrations in living system are regulated within a narrow range.
Combined, the data presented here show that PCP has the ability to bind both Tb and Zn in a cooperative manner. Direct comparison of PCP Tb and Zn affinity constants (Table 1) is misleading because of their differing stoichiometries (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vs. 1
1 vs. 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), therefore we calculated pM values (−log[M]free) to assess how much free (uncomplexed) metal would be present under a defined set of conditions. The pM values in Table 1 were calculated for 0.5 μM M1 and 2 μM apo-peptide or M2-loaded peptide, with a wider range of M1 concentrations shown in Fig. 6. For PCP, a pZn of 8.1 increases to 8.6 in the presence of Tb, while its pTb goes from 6.9 to 8.1 for the apo and Zn-loaded forms, respectively. In many cases, allostery is only observable for the stronger binding substrate in the presence of a weaker binding effector,20 but in this case the similarity in Zn and Tb affinities enables observation of the influence of metal binding in both directions, meaning Zn and Tb are both substrate and effector for each other.
2), therefore we calculated pM values (−log[M]free) to assess how much free (uncomplexed) metal would be present under a defined set of conditions. The pM values in Table 1 were calculated for 0.5 μM M1 and 2 μM apo-peptide or M2-loaded peptide, with a wider range of M1 concentrations shown in Fig. 6. For PCP, a pZn of 8.1 increases to 8.6 in the presence of Tb, while its pTb goes from 6.9 to 8.1 for the apo and Zn-loaded forms, respectively. In many cases, allostery is only observable for the stronger binding substrate in the presence of a weaker binding effector,20 but in this case the similarity in Zn and Tb affinities enables observation of the influence of metal binding in both directions, meaning Zn and Tb are both substrate and effector for each other.
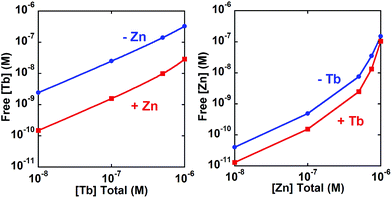 | ||
| Fig. 6 Calculated concentration (logarithmic scale) of unbound Tb (left panel) or Zn (right panel) in the presence or absence of the second metal. Free metal calculated at 2 μM PCP. | ||
Conclusions
The present work establishes that a simple peptide built from native amino acids and designed to harbor two metal binding sites can self assemble into a heterometallic species in which the chelation of one metal ion enhances the affinity for another, and vice versa (Scheme 1). This concept has been exemplified with Tb3+ and Zn2+ as the allosteric partners, but the readily accessible peptide template could be reconfigured for targeting other metals. Such constructs may be interesting for studying heterometallic allostery in aqueous environments, and potentially for controlling free metal ion concentrations in complex environments like cells and organisms, where dynamic alterations in various metal concentrations influence function.Acknowledgements
We thank the NSF (CHE-1152054) and Duke University for financial support, and Alex Owens for early data collection.Notes and references
- D. S. Folk, F. Kielar and K. J. Franz, in Comprehensive Inorganic Chemistry II, ed. J. Reedijk and K. Poeppelmeier, Elsevier, Amsterdam, 2013, pp. 207–240 Search PubMed.
- W. Maret, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 12325–12327 CrossRef CAS PubMed.
- Y. Qin, P. J. Dittmer, J. G. Park, K. B. Jansen and A. E. Palmer, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 7351–7356 CrossRef CAS PubMed.
- S. C. Dodani, D. W. Domaille, C. I. Nam, E. W. Miller, L. A. Finney, S. Vogt and C. J. Chang, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 5980–5985 CrossRef CAS PubMed.
- A. Grubman, S. A. James, J. James, C. Duncan, I. Volitakis, J. L. Hickey, P. J. Crouch, P. S. Donnelly, K. M. Kanninen, J. R. Liddell, S. L. Cotman, M. D. de Jonge and A. R. White, Chem. Sci., 2014, 5, 2503–2516 RSC.
- J. Liu, J. E. Kohler, A. L. Blass, J. A. Moncaster, A. Mocofanescu, M. A. Marcus, E. A. Blakely, K. A. Bjornstad, C. Amarasiriwardena, N. Casey, L. E. Goldstein and D. I. Soybel, PLoS One, 2011, 6, e19638 CAS.
- D. Guo, Y. Du, Q. Wu, W. Jiang and H. Bi, Arch. Biochem. Biophys., 2014, 560, 44–51 CrossRef CAS PubMed.
- L. K. Charkoudian, D. M. Pham and K. J. Franz, J. Am. Chem. Soc., 2006, 128, 12424–12425 CrossRef CAS PubMed.
- D. S. Folk and K. J. Franz, J. Am. Chem. Soc., 2010, 132, 4994–4995 CrossRef CAS PubMed.
- S. M. Damo, T. E. Kehl-Fie, N. Sugitani, M. E. Holt, S. Rathi, W. J. Murphy, Y. Zhang, C. Betz, L. Hench, G. Fritz, E. P. Skaar and W. J. Chazin, Proc. Natl. Acad. Sci. USA, 2013, 110, 3841–3846 CrossRef CAS PubMed.
- J. A. Hayden, M. B. Brophy, L. S. Cunden and E. M. Nolan, J. Am. Chem. Soc., 2013, 135, 775–787 CrossRef CAS PubMed.
- M. B. Brophy, J. A. Hayden and E. M. Nolan, J. Am. Chem. Soc., 2012, 134, 18089–18100 CrossRef CAS PubMed.
- D. M. Gagnon, M. B. Brophy, S. E. J. Bowman, T. A. Stich, C. L. Drennan, R. D. Britt and E. M. Nolan, J. Am. Chem. Soc., 2015, 137, 3004–3016 CrossRef CAS PubMed.
- J. Baudier, N. Glasser and D. Gerard, J. Biol. Chem., 1986, 261, 8192–8203 CAS.
- M. Koch, S. Bhattacharya, T. Kehl, M. Gimona, M. Vašák, W. Chazin, C. W. Heizmann, P. M. H. Kroneck and G. Fritz, Biochim. Biophys. Acta, 2007, 1773, 457–470 CrossRef CAS PubMed.
- M. C. Bauer, H. Nilsson, E. Thulin, B. Frohm, J. Malm and S. Linse, Protein Sci., 2008, 17, 760–767 CrossRef CAS PubMed.
- H. M. Botelho, M. Koch, G. Fritz and C. M. Gomes, FEBS J., 2009, 276, 1776–1786 CrossRef CAS PubMed.
- L. X. Chong, M.-R. Ash, M. J. Maher, M. G. Hinds, Z. Xiao and A. G. Wedd, J. Am. Chem. Soc., 2009, 131, 3549–3564 CrossRef CAS PubMed.
- M. Elhabiri and A.-M. Albrecht-Gary, Coord. Chem. Rev., 2008, 252, 1079–1092 CrossRef CAS PubMed.
- C. Kremer and A. Lützen, Chem.–Eur. J., 2013, 19, 6162–6196 CrossRef CAS PubMed.
- L. Kovbasyuk and R. Krämer, Chem. Rev., 2004, 104, 3161–3188 CrossRef CAS PubMed.
- M. L. Zastrow, A. F. A. Peacock, J. A. Stuckey and V. L. Pecoraro, Nat. Chem., 2011, 4, 118–123 CrossRef PubMed.
- J. D. Brodin, X. I. Ambroggio, C. Tang, K. N. Parent, T. S. Baker and F. A. Tezcan, Nat. Chem., 2012, 4, 375–382 CrossRef CAS PubMed.
- J. P. Miller, M. S. Melicher and A. Schepartz, J. Am. Chem. Soc., 2014, 136, 14726–14729 CrossRef CAS PubMed.
- J.-C. G. Bunzli and C. Piguet, Chem. Soc. Rev., 2005, 34, 1048–1077 RSC.
- M. Nitz, K. J. Franz, R. L. Maglathlin and B. Imperiali, ChemBioChem, 2003, 4, 272–276 CrossRef CAS PubMed.
- K. J. Franz, M. Nitz and B. Imperiali, ChemBioChem, 2003, 4, 265–271 CrossRef CAS PubMed.
- M. Nitz, M. Sherawat, K. J. Franz, E. Peisach, K. N. Allen and B. Imperiali, Angew. Chem., Int. Ed., 2004, 43, 3682–3685 CrossRef CAS PubMed.
- B. Alies, C. Bijani, S. Sayen, E. Guillon, P. Faller and C. Hureau, Inorg. Chem., 2012, 51, 12988–13000 CrossRef CAS PubMed.
- M. Vasak and J. H. R. Kagi, Met. Ions Biol. Syst., 1983, 15, 213–273 CAS.
- L.-J. Jiang, M. Vašák, B. L. Vallee and W. Maret, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 2503–2508 CrossRef CAS.
- W. D. Horrocks and D. R. Sudnick, J. Am. Chem. Soc., 1979, 101, 334–340 CrossRef CAS.
- I. P. Korndörfer, F. Brueckner and A. Skerra, J. Mol. Biol., 2007, 370, 887–898 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and Fig. S1–S9 showing plots of lifetime, absorbance, titration, HPLC and mass spectra. See DOI: 10.1039/c5sc00602c |
| This journal is © The Royal Society of Chemistry 2015 |