Open Access Article
Open Access ArticleBrushing up from “anywhere” under sunlight: a universal surface-initiated polymerization from polydopamine-coated surfaces†
Wenbo
Sheng‡
ab,
Bin
Li‡
ac,
Xiaolong
Wang
a,
Bin
Dai
b,
Bo
Yu
*a,
Xin
Jia
*b and
Feng
Zhou
*a
aState Key Laboratory of Solid Lubrication, Lanzhou Institute of Chemical Physics, Chinese Academy of Sciences, 730000, Lanzhou, China. E-mail: yubo@licp.cas.cn; zhouf@licp.cas.cn
bKey Laboratory of Chemical Materials of Xinjiang Uygur Autonomous Region, School of Chemistry and Chemical Engineering, Shihezi University, 832003, Shihezi, China. E-mail: jiaxin@shzu.edu.cn
cUniversity of Chinese Academy of Sciences, 100049, Beijing, China
First published on 15th January 2015
Abstract
We describe a simple yet extremely versatile and generalized surface polymer modification approach based on a surface initiated polymerization from a polydopamine (PDA) layer. PDA deposits on virtually any substrate independent of specific surface chemistries and can act as a photoinitiating layer to initiate the radical polymerization of a variety of (methyl)acrylic/styrene monomers. It does not require any metal/ligand catalyst, additional photoinitiator or dye sensitizer. Another attractive feature of this novel strategy is the ability to spatially control the architectures (pattern, gradient) of the polymer films by altering the areas of light irradiation. It is also adaptable to large area grafting with an ultra-small amount of monomer solution (a thin monomer solution layer).
Introduction
Surface-initiated polymerizations (SIPs) have been widely used for the preparation of well-defined and functional polymer brushes with complex architectures.1–10 Polymer brushes have been explored in a myriad of applications, for example, smart surfaces,11,12 antifouling coatings,13,14 biofunctional surfaces,15,16 and electronics17etc. These applications rely heavily on the ease of the synthetic procedures. The existing approaches for surface grafting, however, depend on the use of surface-specific chemistries, and the examples include the requirement of specific active sites between the modifiers and surfaces (e.g., thiols on gold, silanes on oxides) or the need of multi-step procedures for their implementation. Therefore, the development of new polymerization methods for functionalizing various surfaces by polymerizing a wide range of monomers is critical in this field.Photochemical initiation has several advantages, including a fast initiation rate, low toxicity and biocompatibility, as well as mild reaction conditions, which are very attractive for biologically relevant applications.9,18–20 A wide range of photosensitizers and photoinitiators have been used for surface grafting, e.g. 2,2-azobisisobutyronitrile (AIBN), benzophenone, peroxides, tertiary amines, etc.19,21,22 Furthermore, photochemical initiation is usually fast and allows for lithographic patterning of substrates with a myriad of polymers.23,24 Recently, metal-based photoredox catalysis triggered via light irradiation has further expanded the surface grafting technologies.25–30 However, these reactions usually take place through a radical chain propagation mechanism, and thus require stoichiometric amounts of the transition metal catalyst and initiators. It is highly desirable to develop a more facile approach for generating radical species under mild reaction conditions and avoiding use of transition-metal catalysts and photo initiators which would be expensive or toxic.
Moreover, the development of a simple and generalized strategy for surface modification with multiple classes of materials has proven challenging and, to date, few generalized methods for accomplishing this have been reported. Inspired by the composition of adhesive proteins in mussels,31–33 here we report a generalized and metal/ligand-free photopolymerization strategy which is based on a “universal” polydopamine (PDA) adhesive layer for further surface-initiated photopolymerization under simulated sunlight irradiation. Although dopamine has been widely used for surface modifications, its self-polymerization and the subsequent film deposition are kinetically limited.34–36 In this work, PDA itself acts as a photoactive species that can generate surface bound radicals to trigger the surface-initiated radical polymerization of a variety of vinyl monomers such as methacrylates, acrylates and styrene onto almost any substrate, representing a new advance in sustainable, environmentally friendly radical reactions, which address the above issues and provide a significantly improved performance in the preparation of thin polymer films.
Results and discussion
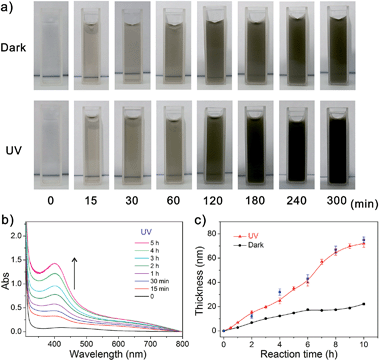
Scheme 1 outlines the procedure for the preparation of surface attached polymers from PDA-based surfaces. This strategy takes advantage of the PDA deposition on almost any substrate, and the PDA layer can generate surface bound radicals under UV light irradiation that can initiate polymerization. The substrates were immersed in an aqueous solution of dopamine (2 mg mL−1, 10 mM Tris–HCl, pH 8.5) and the PDA deposition was triggered via ultraviolet irradiation (36 W lamp), leading to the spontaneous deposition of a thin adherent PDA film. This is practically very important for a well-controlled deposition process which is distinguished from the earlier process with slow kinetics, and PDA was activated with further sunlight irradiation to generate radicals for the polymerization of various monomers. Fig. 1a shows the color changes of dopamine solutions under UV and dark conditions at different reaction times. The UV-irradiated solution turned deep dark after 2 hours, while the color change was slow for the non-irradiated solution. Fig. 1b shows the absorption spectra of a dopamine solution under UV irradiation with the increasing absorbance peak at around 410 nm indicating the growth of PDA with respect to the time. Fig. 1c shows plots of the PDA thickness with respect to the reaction time, analyzed using an ellipsometer. They indicate that the PDA layer thickness was a function of the reaction time and reached up to a value of ca. 80 nm after 10 hours under UV irradiation (red curve). AFM assessment of the PDA films thickness via a scratch test is in good agreement with the data of the ellipsometric thickness (Fig. 1c, blue), and the PDA film (5 h, UV) has a root mean square (RMS) roughness of 2.84 nm (Fig. S6, ESI†). While under dark conditions, the polymerization was very slow and the film had a thickness of only about 20 nm after 10 hours (dark curve). To confirm the mechanism of this photografting process, control polymerizations were conducted on substrates without PDA film or in the absence of light, and both examples demonstrated that the polymerization cannot be triggered without PDA or light, but can be initiated in situ from the PDA surface under simulated “sunlight” irradiation (300 W xenon lamp) at 20 °C (avoiding any thermal reaction during the polymerization). In the absence of PDA, the reaction is retarded. It indicated that the presence of a PDA film is essential for photografting. To confirm the significance of this photochemical process, the kinetics of the same reaction were monitored both with and without light, the polymerization proceeded efficiently under light, but no polymerization was detected under dark conditions. Furthermore, the polymerization did not occur under visible light irradiation when the UV spectrum was filtered out. These experiments indicated that UV light was necessary to bring PDA to its photo-excited state. It is anticipated that this excited PDA* species would generate reactive radicals from PDA through a photosensitized electron transfer process,37–39 which could initiate the polymerization of the monomer through a radical chain propagation mechanism that is in analogy with the traditional radical polymerization process. These features provide compelling evidence that this process is a photo-induced radical polymerization. What is the most advantageous aspect of the photo-induced system, and what is different from the traditional surface photo radical polymerization is that the polymerization is initiated in situ on the PDA surface without adding any metal catalyst, photocatalyst or photosensitizer. Furthermore, it would be better to use sunlight instead of UV light because of the possible negative effects of UV irradiation on polymers, in particular biomaterials.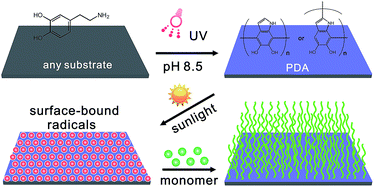 | ||
| Scheme 1 Schematic illustration of sunlight-induced photografting of polymers from a polydopamine layer that is UV deposited on any substrate. | ||
Another distinguished feature of this photopolymerization process is its substrate independence, which allows photografting of polymers on virtually any substrate in the field of materials chemistry. In order to demonstrate its versatility and tolerance to the functional groups and chemical structures of the monomers, we examined the reactivity of different (methyl)acrylate and vinyl monomers, such as neutral N-isopropylacrylamide (NIPAm), styrene, cationic 2-(methacryloyloxy)ethyl trimethylammonium chloride (METAC), anionic 3-sulfopropyl methacrylate potassium salt (SPMA) and methacrylic acid sodium salt (MAA-Na). The PDA coated substrates were submerged in the bulk monomer or monomer–water solutions and irradiated with simulated sunlight, and then, the polymers were spontaneously grafted onto various substrates such as inorganic Al, Fe, Ti, mica and polymeric polydimethylsiloxane (PDMS), PI and PTFE. Attenuated total reflection infrared (ATR-IR) spectroscopy analysis indicated that the above polymers were successfully grafted onto the surfaces (Fig. S5 in ESI†). Contact angle (CA) measurements also confirmed the formation of PDA and the polymers on the surfaces (Fig. 2a and Table S1†), which was demonstrated through the surface polymerization of hydrophilic PSPMA and hydrophobic PS on various substrates coated with thin PDA films. With the PDA layer having medium contact angles, the substrates turned out to be more hydrophilic after the grafting of PSPMA, but more hydrophobic after grafting the PS films. The thickness of the resultant dried polymer films was measured using ellipsometry; the data points are averaged values from separate measurements (Fig. 2b). The AFM image of the PSPMA brushes clearly shows the homogenous growth of PSPMA (with a RMS roughness of 0.25 nm) from the PDA coated gold surface (Fig. S6, ESI†) according to morphology and roughness change. We can prepare functional polymer coatings either in a bulk monomer solution or in aqueous media, enabling the direct polymerization of a broad range of monomers under sunlight without adding any photocatalyst, the way that plants do – using sunlight to construct complex structures. The stability of the PDA/polymer bilayer is important for potential applications, so the sample was immersed in 60 °C water overnight and washed with various solvents (e.g. water, methanol, acetone, etc.), and no measurable changes in the film thickness were found, indicating the good thermal and solvent stability of these surface grafted polymer films.
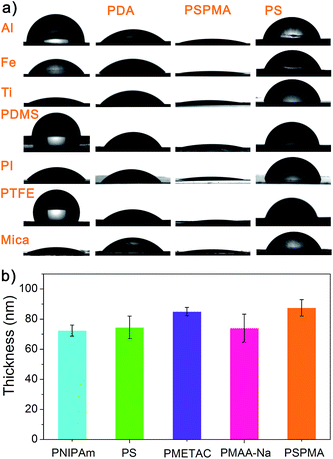 | ||
| Fig. 2 (a) Pictures of water droplets on several unmodified, polydopamine-coated, PSPMA grafted and PS grafted substrates, including inorganic Al, Fe, and Ti, the polymers PDMS, PI, and PTFE, and mica. The contact angle values are shown in Table S1.† (b) Ellipsometric thicknesses of different polymers on a silicon substrate. Reaction conditions: NIPAm (8.8 M, water), 2 h; styrene (bulk), 2 h; METAC (80% in water), 0.5 h; MAA-Na (9.2 M, water), 1.5 h; SPMA (4.0 M, water), 1 h. | ||
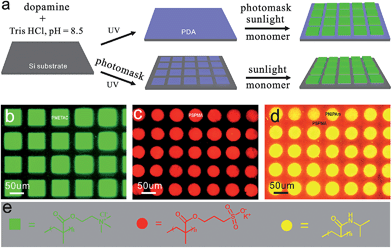
The synthesis of patterned polymer films has been demonstrated using lithography (e.g., photo,23,24,40 electron-beam,41,42 scanning probe,43,44etc.), microcontact printing,45,46 and diffusion controlled strategies.47 An attractive feature of this novel method is the possibility to fabricate complex patterns by readily modulating the light to control the reaction areas on the PDA surface. This single-step procedure allows the formation of patterned polymers directly from the pre-patterned and homogeneous PDA surface using a photomask (Fig. 3a and S3†). We can create patterns through two different ways: first, grafting the polymers from a homogeneously covered PDA substrate by masking the sunlight irradiation; or second, grafting them from pre-patterned PDA surfaces that are formed by masking the UV irradiation (Fig. S4†), which is not easily achieved with current methods due to slow kinetics. A variety of features can be obtained, including squares (PMETAC, 54 μm in length, Fig. 3b) and circles (PSPMA, ca. 50 μm in diameter, Fig. 3c). And most importantly, the non-irradiated areas were kept intact and activated after the first step of polymerization and, therefore, another different polymer can be grafted in a further initiation step. Fig. 3d shows a binary pattern composing two different polymers, i.e., PNIPAm circles (50 μm in diameter) plus backfilled PSPMA. In these cases, polymerization only occurs in the regions where the PDA is exposed to sunlight, resulting in patterned polymers. Thus, this new photografting method enables the preparation of polymeric films with well-defined and spatially confined chemical functionalities.
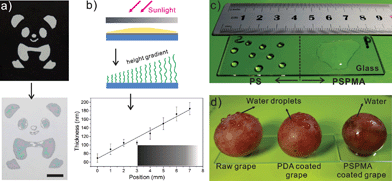
The existing surface grafting polymerization methods often have a low space-time yield, which limits their large scale application. In this work, surface attached polymers can be prepared on large surfaces yet using ultra-small amounts of monomer solution as long as the substrates are completely covered with a thin layer of monomer solution and the simulated sunlight spot is large enough. Fig. 4 shows some of the resultant surfaces on large scales. Instead of using a micromachined photomask, we can make photomasks by printing desired patterns on plastic paper with an office use inkjet printer. Deep black ink can stop the sunlight from passing through the covered area but let it through the blank area. So, with an inversely printed Panda image as the photomask, a polymeric “Panda” can be fabricated directly (Fig. 4a). It is also feasible for creating gradient films using a printed black color gradient as a density filter photomask. This allows the direct formation of gradient films in a single step over large areas. Regions of the substrate that were exposed to more light showed an increase in the kinetic rate of polymer formation, resulting in an increase in polymer thickness (Fig. S7†). Fig. 4b shows how this works and plots the height profile of PMETAC along the substrate. The film’s thickness shows a linear increase from 70 up to 190 nm along a 7 mm distance. Fig. 4c shows a glass slide that was modified with PS and PSPMA, respectively, each side showing either hydrophobic or hydrophilic properties. Fig. 4d shows the implementation of the grafting strategy on a grape, and one can clearly observe the wetting change after each step.
UV-vis analysis provides a preliminary mechanistic insight into this photomediated process. Presumably, in the PDA deposition, UV light increases the chances for the intramolecular cyclization of oxidised quinone acting as a new monomer for the subsequent PDA buildup,48–50 leading to an enhanced reaction rate. For the mechanism of the surface radical polymerization, the interpretation is that in organic chemistry amines are good electron donors which are widely employed for the photochemical single electron transfer (SET) addition of amines to alkenes.37 It is known that the PDA surface is covered by a large number of amino functionalities, which provide the possibility of photopolymerization of vinyl monomers. Upon light irradiation (Scheme S1 in ESI†), surface bound nitrogen-centered radicals are expected to generate photochemically on the PDA surface. Alternatively, such nitrogen-based radicals can undergo proton transfer, producing a protonated amine and a carbon-centered radical that can then couple with the electron-poor monomers, such as (meth)acrylate, which results in the formation of a new chain with a positively charged amine end group. This chain end can either stay protonated or it can become neutral through deprotonation.19,37,51 Nevertheless, there is a possibility that the catechol groups could also initiate polymerization under UV light.52
On the other hand, the polymerization was completely inhibited in the presence of oxygen, an effective radical quencher, further indicating that the surface-attached polymer chains are formed via a free radical polymerization mechanism. Here, the polymerization mechanism becomes even more complicated due to the lack of detailed information about the PDA structure and the corresponding polymerization mechanism,48,53 and the surface polymerization kinetics are highly dependent on parameters such as light, solvent and temperature. Nevertheless, it is clear that this photomediated surface grafting approach is a robust way to prepare well-defined thin polymer films with precisely controlled composition and architectures. Of course, further studies are needed to identify the underlying mechanism.
Experimental
Polydopamine deposition on silicon surfaces
The setup used for the deposition of PDA is shown in Fig. S1.† Oxygen plasma (FEMTO) cleaned silicon wafers were immersed in an aqueous solution of dopamine (2 mg mL−1, in 10 mM Tris buffer, pH 8.5), and the samples were irradiated with a 36 watt fluorescent lamp for a certain time. The same procedure was used for other substrates. Samples were taken periodically for ellipsometry measurements.General procedure for sunlight-induced photografting of polymers
Monomer, solvent and the PDA coated surfaces were placed in a sealed vial and degassed by purging with nitrogen for 30 minutes (Fig. S2†). Photopolymerization was initiated upon exposure to a simulated “sunlight”. After a set reaction time, the substrates were removed from the vial and washed with water and ethanol, yielding the polymer modified surfaces. See ESI† for details.Conclusions
In conclusion, we have developed a new and generalized surface modification approach for fabricating well-defined polymer coatings. Functionalization of the surfaces with self-assembled monolayers (SAMs) of initiators is no longer required and direct polymer grafting on surfaces can be realized in a one-step photo-reaction under “sunlight” irradiation, and shows excellent tolerance to many functional groups, which substantially expands the scope of the monomer and solvent choice. In addition, it can be realized on a wide range of substrates that can be planar, curved, and irregular, virtually any chemical substrate, which is more robust than the existing approaches. Polymers can be easily prepared in a rapid, robust manner through the use of a thin and photosensitive polydopamine film. With this facile technique, a wide range of chemical functionalities can be easily coupled to various substrates. These findings are expected to open a new avenue for surface modifications and are very promising for various applications.Acknowledgements
This work was financially supported by the NSFC (21434009, 21364010, 21125316) and Key Research Program of CAS (KJZD-EW-M01).Notes and references
- R. Barbey, L. Lavanant, D. Paripovic, N. Schüwer, C. Sugnaux, S. Tugulu and H.-A. Klok, Chem. Rev., 2009, 109, 5437 CrossRef CAS PubMed
.
- B. Li, B. Yu, W. T. S. Huck, W. Liu and F. Zhou, J. Am. Chem. Soc., 2013, 135, 1708 CrossRef CAS PubMed
.
- M. Baum and W. J. Brittain, Macromolecules, 2002, 35, 610 CrossRef CAS
.
- C. Li and B. C. Benicewicz, Macromolecules, 2005, 38, 5929 CrossRef CAS
.
- I. M. Rutenberg, O. A. Scherman, R. H. Grubbs, W. Jiang, E. Garfunkel and Z. Bao, J. Am. Chem. Soc., 2004, 126, 4062 CrossRef CAS PubMed
.
- Q. Ye, X. Wang, S. Li and F. Zhou, Macromolecules, 2010, 43, 5554 CrossRef CAS
.
- M. Husseman, E. E. Malmstro, M. McNamara, M. Mate, D. Mecerreyes, D. G. Benoit, J. L. Hedrick, P. Mansky, E. Huang, T. P. Russell and C. J. Hawker, Macromolecules, 1999, 32, 1424 CrossRef CAS
.
- S. Edmondson, V. L. Osborne and W. T. S. Huck, Chem. Soc. Rev., 2004, 33, 14 RSC
.
- D. J. Dyer, Adv. Polym. Sci., 2006, 197, 47 CrossRef CAS
.
- M. Steenackers, S. Q. Lud, M. Niedermeier, P. Bruno, D. M. Gruen, P. Feulner, M. Stutzmann, J. A. Garrido and R. Jordan, J. Am. Chem. Soc., 2007, 129, 15655 CrossRef CAS PubMed
.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101 CrossRef PubMed
.
- F. Zhou and W. T. S. Huck, Phys. Chem. Chem. Phys., 2006, 8, 3815 RSC
.
- S. Jiang and Z. Cao, Adv. Mater., 2010, 22, 920 CrossRef CAS PubMed
.
- H. Ma, J. Hyun, P. Stiller and A. Chilkoti, Adv. Mater., 2004, 16, 338 CrossRef CAS
.
- W. Senaratne, L. Andruzzi and C. K. Ober, Biomacromolecules, 2005, 6, 2427 CrossRef CAS PubMed
.
- B. Li, B. Yu, Q. Ye and F. Zhou, Acc. Chem. Res., 2014 DOI:10.1021/ar500323p
.
- R. Guo, Y. Yu, Z. Xie, X. Liu, X. Zhou, Y. Gao, Z. Liu, F. Zhou, Y. Yang and Z. Zheng, Adv. Mater., 2013, 25, 3343 CrossRef CAS PubMed
.
- D. M. Schultz and T. P. Yoon, Science, 2014, 343, 1239176 CrossRef PubMed
.
-
K. D. Belfield and J. V. Crivello, Photoinitiated Polymerization, ACS Symposium Series 847, American Chemical Society, Washington, DC, 2003 Search PubMed
.
- J. Deng, L. Wang, L. Liu and W. Yang, Prog. Polym. Sci., 2009, 34, 156 CrossRef CAS PubMed
.
- D. J. Dyer, J. Feng, R. Schmidt, V. N. Wong, T. Zhao and Y. Yagci, Macromolecules, 2004, 37, 7072 CrossRef CAS
.
- Y. Yagci, S. Jockusch and N.
J. Turro, Macromolecules, 2010, 43, 6245 CrossRef CAS
.
- J. E. Poelma, B. P. Fors, G. F. Meyers, J. W. Kramer and C. J. Hawker, Angew. Chem., Int. Ed., 2013, 125, 6982 CrossRef
.
- B. Li, B. Yu and F. Zhou, Macromol. Rapid Commun., 2014, 35, 1287 CrossRef CAS PubMed
.
- D. Konkolewicz, K. Schröder, J. Buback, S. Bernhard and K. Matyjaszewski, ACS Macro Lett., 2012, 1, 1219 CrossRef CAS
.
- B. P. Fors and C. J. Hawker, Angew. Chem., Int. Ed., 2012, 51, 8850 CrossRef CAS PubMed
.
- A. Anastasaki, V. Nikolaou, Q. Zhang, J. Burns, S. R. Samanta, C. Waldron, A. J. Haddleton, R. McHale, D. Fox, V. Percec, P. Wilson and D. M. Haddleton, J. Am. Chem. Soc., 2014, 136, 1141 CrossRef CAS PubMed
.
- J. Xu, K. Jung, A. Atme, S. Shanmugam and C. Boyer, J. Am. Chem. Soc., 2014, 136, 5508 CrossRef CAS PubMed
.
- J. Yan, B. Li, F. Zhou and W. Liu, ACS Macro Lett., 2013, 2, 592 CrossRef CAS
.
- S. Dadashi-Silab, M. A. Tasdelen and Y. Yagci, J. Polym. Sci., Part A: Polym. Chem., 2014, 52, 2878 CrossRef CAS
.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426 CrossRef CAS PubMed
.
- J. Sedo, J. Saiz-Poseu, F. Busque and D. Ruiz-Molina, Adv. Mater., 2013, 25, 653 CrossRef CAS PubMed
.
- Q. Ye, F. Zhou and W. Liu, Chem. Soc. Rev., 2011, 40, 4244 RSC
.
- B. Zhu and S. Edmondson, Polymer, 2011, 52, 2141 CrossRef CAS PubMed
.
- M. Kohri, H. Kohma, Y. Shinoda, M. Yamauchi, S. Yagai, T. Kojima, T. Taniguchi and K. Kishikawa, Polym. Chem., 2013, 4, 2696 RSC
.
- Z. Ma, X. Jia, J. Hu, G. Zhang, F. Zhou, Z. Liu and H. Wang, Langmuir, 2013, 29, 5631 CrossRef CAS PubMed
.
-
F. D. Lewis and E. M. Crompton, SET Addition of Amines to Alkene, in CRC Handbook of Organic Photochemistry and Photobiology, ed. W. M. Horspool and F. Lenci, CRC Press, Boca Raton, 2nd edn, 2010 Search PubMed
.
- F. D. Lewis and P. E. Correa, J. Am. Chem. Soc., 1984, 106, 194 CrossRef CAS
.
- Z. Su, P. S. Mariano, D. E. Falvey, U. C. Yoon and S. W. Oh, J. Am. Chem. Soc., 1998, 120, 10676 CrossRef CAS
.
- C. Schuh, S. Santer, O. Prucker and J. Rühe, Adv. Mater., 2009, 21, 4706 CAS
.
- M. Steenackers, R. Jordan, A. Küller and M. Grunze, Adv. Mater., 2009, 21, 2921 CrossRef CAS
.
- S. J. Ahn, M. Kaholek, W.-K. Lee, B. LaMattina, T. H. LaBean and S. Zauscher, Adv. Mater., 2004, 16, 2141 CrossRef CAS
.
- X. Zhou, X. Wang, Y. Shen, Z. Xie and Z. Zheng, Angew. Chem., Int. Ed., 2011, 50, 6506 CrossRef CAS PubMed
.
- M. E. Welch and C. K. Ober, J. Polym. Sci., Part B: Polym. Phys., 2013, 51, 1457 CrossRef
.
- F. Zhou, Z. Zheng, B. Yu, W. Liu and W. T. S. Huck, J. Am. Chem. Soc., 2006, 128, 16253 CrossRef CAS PubMed
.
- B. Li, B. Yu, W. T. S. Huck, F. Zhou and W. Liu, Angew. Chem., Int. Ed., 2012, 51, 5092 CrossRef CAS PubMed
.
- J. Yan, B. Li, B. Yu, W. T. S. Huck, W. Liu and F. Zhou, Angew. Chem., Int. Ed., 2013, 52, 9125 CrossRef CAS PubMed
.
- N. F. Della Vecchia, R. Avolio, M. Alfè, M. E. Errico, A. Napolitano and M. d'Ischia, Adv. Funct. Mater., 2013, 23, 1331 CrossRef CAS
.
- J. Liebscher, R. Mrowczynski, H. A. Scheidt, C. Filip, N. D. Hadade, R. Turcu, A. Bende and S. Beck, Langmuir, 2013, 29, 10539 CrossRef CAS PubMed
.
- W. Xu, X.-M. Zhang and P. S. Mariano, J. Am. Chem. Soc., 1991, 113, 8863 CrossRef CAS
.
- T. G. Ribelli, D. Konkolewicz, S. Bernhard and K. Matyjaszewski, J. Am. Chem. Soc., 2014, 136, 13303 CrossRef CAS PubMed
.
- J. Xia, Y. Xu, J. Lin and B. Hu, Prog. Org. Coat., 2008, 61, 7 CrossRef CAS PubMed
.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: Materials, experimental details and supporting data are reported. See DOI: 10.1039/c4sc03851g |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |