Open Access Article
Open Access ArticleOrthoester exchange: a tripodal tool for dynamic covalent and systems chemistry†
René-Chris
Brachvogel
and
Max
von Delius
*
Friedrich-Alexander-University Erlangen-Nürnberg (FAU), Department of Chemistry and Pharmacy, Henkestrasse 42, 91054 Erlangen, Germany. E-mail: max.vondelius@fau.de
First published on 3rd December 2014
Abstract
Reversible covalent reactions have become an important tool in supramolecular chemistry and materials science. Here we introduce the acid-catalyzed exchange of O,O,O-orthoesters to the toolbox of dynamic covalent chemistry. We demonstrate that orthoesters readily exchange with a wide range of alcohols under mild conditions and we disclose the first report of an orthoester metathesis reaction. We also show that dynamic orthoester systems give rise to pronounced metal template effects, which can best be understood by agonistic relationships in a three-dimensional network analysis. Due to the tripodal architecture of orthoesters, the exchange process described herein could find unique applications in dynamic polymers, porous materials and host–guest architectures.
Introduction
Dynamic covalent chemistry (DCC)1 has developed into a powerful tool for probing non-covalent interactions,2 identifying ligands for medicinally relevant biotargets,3 and for synthesizing interesting molecules,4 porous materials5 and polymers.6 Increasingly, dynamic covalent libraries (DCLs) are also investigated from the perspective of the emerging field of systems chemistry.7Central to DCC are reversible covalent exchange reactions that allow molecular building blocks to interact under thermodynamic control.8 Currently, the most popular exchange reactions in this area are imine, hydrazone, boronate and disulfide exchange.1,2b Motivated by the prospect of accessing new molecular architectures and creating DCLs under unprecedented conditions, there is unabated interest in developing new additions to the toolbox of DCC. Recent developments include reversible triazolinedione ‘click’ chemistry,9 dynamic alkoxyamines,10 dynamic selenium–pyridine aggregates,11 exchange of thiols and amines with certain Michael acceptors,12 dynamic urea linkages,13 reversible native chemical ligation,14 dynamic enamines15 and diselenide exchange as a variant of disulfide exchange.16
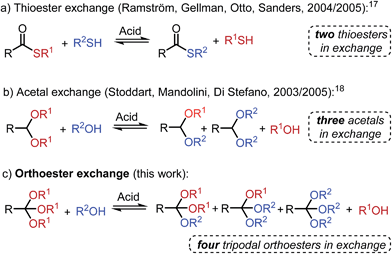
Herein, we introduce a new tool for DCC: the acid-catalyzed exchange reaction between simple O,O,O-orthoesters and alcohols, a process that is most closely related to the existing DCC tools of (thiol-) thioester17 and (alcohol-) acetal18 exchange (Scheme 1). Orthoester exchange is unique among all other dynamic covalent reactions, because it intrinsically features a tripodal architecture, which we believe will be of benefit in a range of applications, from molecular sensing19 to three-dimensional framework materials.5a In contrast to all established exchange processes (Scheme 1), the tripodal geometry of orthoesters also implies that a higher degree of molecular complexity can be generated from a given number of starting materials, which is a highly desirable feature in the field of systems chemistry (vide infra).
Results and discussion
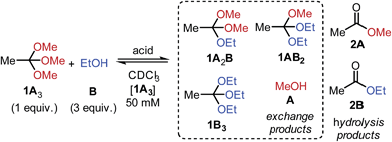
Inspired by Tan's recent studies on ‘scaffolding ligands’ (N,O,P- or N,N,O-orthoesters),20 we wondered whether the exchange of simple O,O,O-orthoesters with alcohols could be developed into a reliable tool for DCC. Although the reaction of orthoesters with alcohols has been used previously as a synthetic strategy to transform one orthoester into another,21 we realized that traditional reaction protocols – generally featuring neat reagents, large reaction scale, reaction at reflux and purification by distillation – are too remote from the mild conditions typically required for the creation of DCLs.We therefore aimed at identifying conditions under which orthoester exchange would reliably occur at room temperature and in dilute solution. To this end, we treated methyl orthoacetate (1A3) and ethanol (B) with a diverse range of acid catalysts and monitored the reaction outcome by 1H NMR spectroscopy (Table 1).
| Entry | Acid | t |
1A3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1A2B 1A2B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1AB2 1AB2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1B3b 1B3b |
Σ1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Σ2b Σ2b |
|
|---|---|---|---|---|---|
| a Reaction conditions: 1A3 (37.5 μmol), B (112.5 μmol), acid, CDCl3 (total volume: 750 μL), RT. Reactions were conducted in screw-capped NMR tubes. CDCl3 and EtOH were dried over molecular sieves (MS) prior to use. PhCOOH: benzoic acid; TFA: trifluoroacetic acid; MsOH: methanesulfonic acid; TfOH: trifluoromethanesulfonic acid. b Product ratios were determined by integration of 1H NMR spectra (estimated error ±5%). | |||||
| 1 | PhCOOH | 1% | 24 h | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 2 | TFA | 1% | 1 h | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 42 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 26 26 |
>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 3 | TFA | 0.1% | 1 h | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 4 | MsOH | 1% | 1 h | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 5 | TfOH | 1% | 1 h | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28 28 |
98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 6 | AlCl3 | 1% | 1 h | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 44 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
>98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
After an equilibration time of one to 24 hours, all tested acid catalysts gave rise to nearly identical product distributions (entries 1 to 6). Hydrolysis to esters 2A and 2B was not observed to appreciable extent, as long as moisture was excluded from the reaction mixture (vide infra). An investigation of solvents acetonitrile, DMSO and benzene revealed that in all cases the same statistical product distributions were obtained, accompanied by only marginal hydrolysis to esters 2 (see ESI† for 1H NMR spectra).
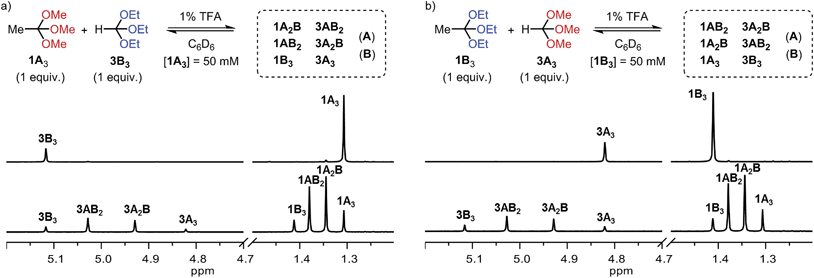
Encouraged by these findings, we tested whether two pristine orthoesters (1A3 and 3B3) would exchange alcohol substituents under the influence of TFA. As demonstrated by the 1H NMR spectra shown in Fig. 1a, this metathesis reaction, which to the best of our knowledge has no literature precedence, proceeded cleanly to a statistical product distribution within only one hour. Equilibration times on this order were found throughout our investigation, establishing orthoester exchange as a relatively fast dynamic covalent reaction. Further experiments on orthoester metathesis revealed that an identical product ratio was reached when starting from orthoesters 1B3 and 3A3 (Fig. 1b), confirming that the reaction outcome indeed corresponds to the system's thermodynamic minimum. Moreover, we found that a high concentration of orthoesters and the presence of around one mol% of water in the solvent dramatically increased the rate of equilibration. Both observations suggest that the formal metathesis reaction is in fact mediated by small quantities of free alcohols A and B (see ESI† for 1H NMR spectra), which are initially generated via hydrolysis.22 A particularly interesting trait of this orthoester metathesis reaction is that two simple starting materials suffice to generate a dynamic library comprising eight products (Fig. 1). Other dynamic covalent reactions require either larger numbers of starting materials or the possibility of forming cyclic products for generating such a level of complexity.
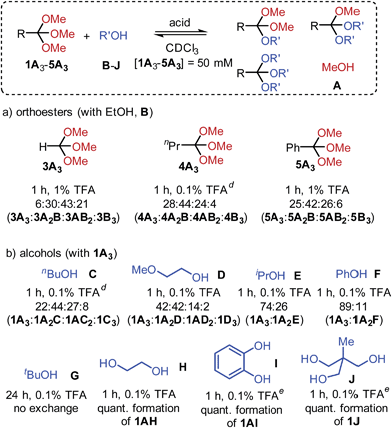
An investigation into the scope of orthoester exchange in solvents CDCl3 and C6D6 revealed that commercial orthoesters trimethyl orthoacetate (1A3), orthoformate (3A3), orthobutyrate (4A3) and orthobenzoate (5A3) differ noticeably in their reactivity. While 1A3 and 4A3 exchange rapidly with EtOH using 0.1% TFA, 3A3 and 5A3 require a larger amount of acid to reach equilibrium within the standard equilibration time of one hour (see Scheme 2a).23
When different alcohols were allowed to exchange with orthoester 1A3, it was not the kinetics of exchange, but the final product distribution that was affected by the nature of the substrate. For example, primary alcohols C and D (Scheme 2) led to similar distributions, whereas 2-propanol (E) and phenol (F) only led to the formation of orthoesters featuring one secondary alcohol (1A2E and 1A2F, respectively).24 This observation is likely due to significant steric crowding in the products that were not observed, a hypothesis that is supported by our finding that the reaction with tert-butanol (G) did not lead to detectable quantities of exchange products.21c,25 These results indicate that orthoester exchange could serve as a remarkably sensitive tool for probing subtle steric effects under thermodynamic control. With diols ethylene glycol and catechol (H, I) and with triol J we observed equilibrium distributions shifted entirely towards products 1AH, 1AI and 1J. Notably, this type of chelate effect has been exploited previously for synthetic purposes such as the face-selective protection of carbohydrates.21c,26
The main limitation of dynamic orthoester exchange is arguably its incompatibility with aqueous (co-)solvent. Similar to acetals, orthoesters hydrolyze to the corresponding esters (e.g.2A) in the presence of acid catalyst and excess water.27 However, after the acid catalyst is quenched, we have found that orthoesters can be surprisingly stable, e.g. they can be treated with bicarbonate solution (see ESI†), subjected to analysis by reverse-phase HPLC-MS or GC-FID (see ESI†), and orthoesters incorporating a chelating diol or triol (e.g.H–J) are generally most robust.
For the synthesis of materials such as covalent organic frameworks and polymers, but also in DCLs, a dynamic covalent reaction should allow shifting the equilibrium towards certain exchange products. Using the model reaction of orthoesters 1A3 or 1E3 with 2-methoxyethanol (D), we have identified strategies for achieving the amplification of three different predominant exchange products (Fig. 2). First, the equilibrium could be shifted towards product 1D3 by addition of molecular sieves (4 Å), which act as a thermodynamic sink for methanol (Fig. 2b). Second, an unusually large quantity of twofold-exchange product 1ED2 was formed when ‘high-energy’ starting material 1E3 (steric repulsion between isopropyl residues)19 was used (Fig. 2c). Third, and perhaps most intriguingly, addition of metal template sodium tetrakis[3,5-bis(trifluoromethyl)phenyl]borate (NaBArF) gave rise to a significant amplification of yet another exchange product: 1E2D (Fig. 2d).
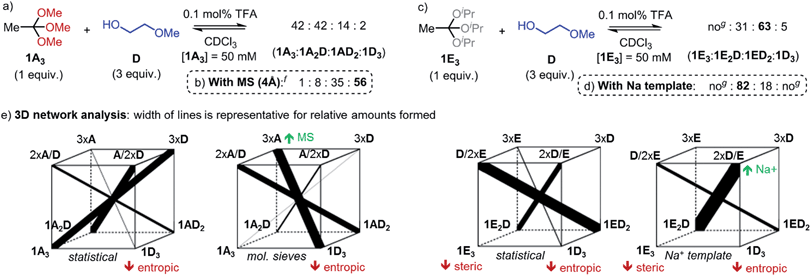 | ||
| Fig. 2 (a–d) Exchange experiments illustrating different strategies for influencing the equilibrium distributions in dynamic orthoester systems. (e) Graphical analysis of the four observed reaction outcomes (‘3D constitutionally dynamic networks’).28 A red arrow indicates a destabilizing effect on the corresponding species, while a green arrow indicates a stabilizing effect. Reaction conditions: 1A3 or 1E3 (37.5 μmol), D (112.5 μmol), TFA (0.1 mol%), CDCl3 (total volume: 750 μL), RT, NaBArF (experiment (d) only, 37.5 μmol). Reactions were conducted in screw-capped NMR tubes, product ratios were determined by integration of 1H NMR spectra (estimated error ±5%). CDCl3 was dried over MS prior to use. f1% TFA was added every 24 h. gno: not observed. | ||
While one could be tempted to explain this observation by the formation of a particularly stable coordination complex between 1E2D and Na+, we realized from a comparison with the non-templated reaction that the amplification of 1E2D must have a more complex origin. In our view, the key to understanding the outcome of the above experiments lies in considering the agonistic and antagonistic relationships between the four possible orthoester products and the free alcohols in solution. A useful graphical approach for exploring such complex relationships was recently proposed by Lehn.28 The analysis presented in Fig. 2e and discussed below is only the second report of a ‘3D constitutionally dynamic network’ put into practice,29 which highlights the potential of orthoester exchange as a tool for fundamental studies on complex dynamic systems.
The four cubes shown in Fig. 2e correspond to the four experiments presented in Fig. 2a–d. Each bottom corner represents one of the four possible orthoester products, while the top corners represent unbound alcohols in solution in such a way that the compounds on opposing corners of the cube are engaged in an agonistic relationship28–30 (i.e., if one of the two compounds is stabilized or destabilized, the other one is amplified or deamplified as a direct result). In experiment (d) for example, the key to understanding the somewhat counterintuitive reaction outcome is to realize that unbound alcohol D is most effective at stabilizing the Na+ template through chelate binding. Hence, the agonist pair of 3 × D and 1E3 could be the ideal candidate for stabilizing the naked sodium ion, however, the amplification of this pair of compounds is precluded by the high ground state energy of agonist 1E3. The second best solution for stabilizing Na+ would be the agonist pair of alcohols 2 × D/1 × E and orthoester 1E2D, which is indeed found as major product, despite orthoester 1E2D being evidently more strained than compound 1ED2 (see Fig. 2c and Scheme 2b for comparison). Crucially, this type of analysis provides an accessible rational explanation for the system's behaviour in all four experiments shown in Fig. 2, including the counter-intuitive reaction outcome upon metal addition (Fig. 2d).31
Conclusions
We have demonstrated that the exchange of O,O,O-orthoesters with simple alcohols proceeds cleanly under relatively mild conditions. Our experiments show that the reaction is suitable for the generation of DCLs and that the equilibrium distribution can be shifted towards different products by removal of methanol, by using a sterically crowded orthoester starting material or by addition of a metal template. Within the diverse toolbox of DCC, the dynamic orthoester motif stands out due to its tripodal geometry, which enables the rapid self-assembly of molecules with three fold symmetry from up to four components in only one step. Thanks to their three ‘docking sites’ for alcohols, orthoesters provide access to dynamic systems of high complexity in one single step. The high potential of orthoester exchange for applications in the field of systems chemistry was demonstrated in this work by the observation of an unexpected template effect and its rationalization by agonistic relationships in a 3D dynamic network. Our findings are relevant to all current applications of DCC, in which the use of aqueous (co-)solvent is not required during the exchange process. Further studies on the exploitation of template effects en route to more complex orthoester architectures are underway in our laboratory.Acknowledgements
This work was supported by the Fonds der Chemischen Industrie (Liebig fellowship for M.v.D.; FCI doctoral fellowship for R.-C.B.) and the Deutsche Forschungsgemeinschaft (Emmy-Noether grant DE1830/2-1). We thank Andreas Zech and Michael Bothe for experimental work carried out as part of undergraduate projects.Notes and references
-
(a) P. T. Corbett, J. Leclaire, L. Vial, K. R. West, J.-L. Wietor, J. K. M. Sanders and S. Otto, Chem. Rev., 2006, 106, 3652 CrossRef CAS PubMed
; (b) Y. Jin, C. Yu, R. J. Denman and W. Zhang, Chem. Soc. Rev., 2013, 42, 6634 RSC
.
-
(a) S. Otto, R. L. E. Furlan and J. K. M. Sanders, Science, 2002, 297, 590 CrossRef CAS PubMed
; (b) A. Herrmann, Chem. Soc. Rev., 2014, 43, 1899 RSC
.
-
(a) O. Ramström and J.-M. Lehn, Nat. Rev. Drug Discovery, 2002, 1, 26 CrossRef PubMed
; (b) M. Y. M. Abdelrahim, M. Tanc, J.-Y. Winum, C. T. Supuran and M. Barboiu, Chem. Commun., 2014, 50, 8043 RSC
; (c) M. Mondal, N. Radeva, H. Köster, A. Park, C. Potamitis, M. Zervou, G. Klebe and A. K. H. Hirsch, Angew. Chem., Int. Ed., 2014, 53, 3259 CrossRef CAS PubMed
.
-
(a) K. S. Chichak, S. J. Cantrill, A. R. Pease, S.-H. Chiu, G. W. V. Cave, J. L. Atwood and J. F. Stoddart, Science, 2004, 304, 1308 CrossRef CAS PubMed
; (b) J.-F. Ayme, J. E. Beves, D. A. Leigh, R. T. McBurney, K. Rissanen and D. Schultz, Nat. Chem., 2012, 4, 15 CrossRef CAS PubMed
; (c) N. Ponnuswamy, F. B. L. Cougnon, J. M. Clough, G. D. Pantoş and J. K. M. Sanders, Science, 2012, 338, 783 CrossRef CAS PubMed
.
-
(a) S.-Y. Ding and W. Wang, Chem. Soc. Rev., 2013, 42, 548 RSC
; (b) G. Sforazzini, E. Orentas, A. Bolag, N. Sakai and S. Matile, J. Am. Chem. Soc., 2013, 135, 12082 CrossRef CAS PubMed
; (c) Y. Jin, Q. Wang, P. Taynton and W. Zhang, Acc. Chem. Res., 2014, 47, 1575 CrossRef CAS PubMed
.
-
(a) J.-M. Lehn, Prog. Polym. Sci., 2005, 30, 814 CrossRef CAS PubMed
; (b) E. Moulin, G. Cormos and N. Giuseppone, Chem. Soc. Rev., 2012, 41, 1031 RSC
; (c) Z. Wei, J. H. Yang, J. Zhou, F. Xu, M. Zrinyi, P. H. Dussault, Y. Osada and Y. M. Chen, Chem. Soc. Rev., 2014, 43, 8114 RSC
.
-
(a) M. Kindermann, I. Stahl, M. Reimold, W. M. Pankau and G. von Kiedrowski, Angew. Chem., Int. Ed., 2005, 44, 6750 CrossRef CAS PubMed
; (b) R. F. Ludlow and S. Otto, Chem. Soc. Rev., 2008, 37, 101 RSC
; (c) J. M. A. Carnall, C. A. Waudby, A. M. Belenguer, M. C. A. Stuart, J. J.-P. Peyralans and S. Otto, Science, 2010, 327, 1502 CrossRef CAS PubMed
; (d) Q. Ji, R. C. Lirag and O. S. Miljanic, Chem. Soc. Rev., 2014, 43, 1873 RSC
; (e) A. Jiménez, R. A. Bilbeisi, T. K. Ronson, S. Zarra, C. Woodhead and J. R. Nitschke, Angew. Chem., Int. Ed., 2014, 53, 4556 CrossRef PubMed
.
-
(a) J.-M. Lehn, Chem.–Eur. J., 1999, 5, 2455 CrossRef CAS
; (b) J.-M. Lehn, Chem. Soc. Rev., 2007, 36, 151 RSC
.
- S. Billiet, K. De Bruycker, F. Driessen, H. Goossens, V. Van Speybroeck, J. M. Winne and F. E. Du Prez, Nat. Chem., 2014, 6, 815 CrossRef CAS PubMed
.
- H. Otsuka, Polym. J., 2013, 45, 879 CrossRef CAS
.
- Y. Yi, H. Xu, L. Wang, W. Cao and X. Zhang, Chem.–Eur. J., 2013, 19, 9506 CrossRef CAS PubMed
.
-
(a) G. Joshi and E. V. Anslyn, Org. Lett., 2012, 14, 4714 CrossRef CAS PubMed
; (b) A. G. Campaña, D. A. Leigh and U. Lewandowska, J. Am. Chem. Soc., 2013, 135, 8639 CrossRef PubMed
; (c) Y. Zhong, Y. Xu and E. V. Anslyn, Eur. J. Org. Chem., 2013, 2013, 5017 CrossRef CAS
.
- H. Ying, Y. Zhang and J. Cheng, Nat. Commun., 2014, 5, 3218 Search PubMed
.
- Y. Ruff, V. Garavini and N. Giuseppone, J. Am. Chem. Soc., 2014, 136, 6333 CrossRef CAS PubMed
.
- A. Sanchez-Sanchez, D. A. Fulton and J. A. Pomposo, Chem. Commun., 2014, 50, 1871 RSC
.
-
(a) S. Ji, W. Cao, Y. Yu and H. Xu, Angew. Chem., Int. Ed., 2014, 53, 6781 CrossRef CAS PubMed
; (b) B. Rasmussen, A. Sorensen, H. Gotfredsen and M. Pittelkow, Chem. Commun., 2014, 50, 3716 RSC
.
-
(a) R. Larsson, Z. Pei and O. Ramström, Angew. Chem., Int. Ed., 2004, 43, 3716 CrossRef CAS PubMed
; (b) M. G. Woll and S. H. Gellman, J. Am. Chem. Soc., 2004, 126, 11172 CrossRef CAS PubMed
; (c) J. Leclaire, L. Vial, S. Otto and J. K. M. Sanders, Chem. Commun., 2005, 1959 RSC
; (d) Y. Ura, J. M. Beierle, L. J. Leman, L. E. Orgel and M. R. Ghadiri, Science, 2009, 325, 73 CrossRef CAS PubMed
; (e) S. Ghosh, L. A. Ingerman, A. G. Frye, S. J. Lee, M. R. Gagné and M. L. Waters, Org. Lett., 2010, 12, 1860 CrossRef CAS PubMed
; (f) F. Mansfeld, H. Au-Yeung, J. Sanders and S. Otto, J. Syst. Chem., 2010, 1, 12 CrossRef CAS
; (g) S. A. Berg and B. J. Ravoo, Soft Matter, 2014, 10, 69 RSC
.
-
(a) B. Fuchs, A. Nelson, A. Star, J. F. Stoddart and S. Vidal, Angew. Chem., Int. Ed., 2003, 42, 4220 CrossRef CAS PubMed
; (b) R. Cacciapaglia, S. Di Stefano and L. Mandolini, J. Am. Chem. Soc., 2005, 127, 13666 CrossRef CAS PubMed
; (c) R. Cacciapaglia, S. Di Stefano and L. Mandolini, Chem.–Eur. J., 2006, 12, 8566 CrossRef CAS PubMed
; (d) D. Berkovich-Berger and N. Gabriel Lemcoff, Chem. Commun., 2008, 1686 RSC
; (e) R. Cacciapaglia, S. Di Stefano, L. Mandolini, P. Mencarelli and F. Ugozzoli, Eur. J. Org. Chem., 2008, 2008, 186 CrossRef
; (f) S. Di Stefano, J. Phys. Org. Chem., 2010, 23, 797 CrossRef CAS
; (g) J. A. Berrocal, R. Cacciapaglia and S. Di Stefano, Org. Biomol. Chem., 2011, 9, 8190 RSC
; (h) J. A. Berrocal, R. Cacciapaglia, S. Di Stefano and L. Mandolini, New J. Chem., 2012, 36, 40 RSC
; (i) A. Ruggi, R. Cacciapaglia, S. Di Stefano, E. Bodo and F. Ugozzoli, Tetrahedron, 2013, 69, 2767 CrossRef CAS PubMed
.
-
(a) A. Buryak and K. Severin, Angew. Chem., Int. Ed., 2005, 44, 7935 CrossRef CAS PubMed
; (b) L. You, J. S. Berman and E. V. Anslyn, Nat. Chem., 2011, 3, 943 CrossRef CAS PubMed
; (c) J. R. Askim, M. Mahmoudi and K. S. Suslick, Chem. Soc. Rev., 2013, 42, 8649 RSC
; (d) J. F. Teichert, D. Mazunin and J. W. Bode, J. Am. Chem. Soc., 2013, 135, 11314 CrossRef CAS PubMed
.
-
(a) K. L. Tan, ACS Catal., 2011, 1, 877 CrossRef CAS
; (b) T. E. Lightburn, M. T. Dombrowski and K. L. Tan, J. Am. Chem. Soc., 2008, 130, 9210 CrossRef CAS PubMed
.
-
(a) E. R. Alexander and H. M. Busch, J. Am. Chem. Soc., 1952, 74, 554 CrossRef CAS
; (b) R. M. Roberts, T. D. Higgins and P. R. Noyes, J. Am. Chem. Soc., 1955, 77, 3801 CrossRef CAS
; (c) R. H. DeWolfe, Synthesis, 1974, 153 CrossRef CAS
.
- M. Ciaccia, S. Pilati, R. Cacciapaglia, L. Mandolini and S. Di Stefano, Org. Biomol. Chem., 2014, 12, 3282 CAS
.
- These differences in reaction rate can be explained by the different substituents' electronic effect on the intermediate oxonium ions (e.g., Me: electron-donating, Ph: electron-withdrawing).
- These observations are consistent with the (subtle) differences in steric demand between methyl, ethyl, isopropyl, phenyl and tert-butyl groups (A-values 1.70, 1.75 2.15, 3, >4), see for example:
Stereochemistry of Organic Compounds, ed. E. L. Eliel, S. H. Wilen and L. N. Mander, Wiley, New York, 1994 Search PubMed
.
- R. P. Narain and R. C. Mehrotra, Proc. Natl. Acad. Sci., India, Sect. A, 1963, 33, 45 CAS
.
-
(a) F. Kong, Carbohydr. Res., 2007, 342, 345 CrossRef CAS PubMed
; (b) A. M. Vibhute, A. Vidyasagar, S. Sarala and K. M. Sureshan, Chem. Commun., 2012, 48, 2448 RSC
.
- L. Li, Y. Xu, I. Milligan, L. Fu, E. A. Franckowiak and W. Du, Angew. Chem., Int. Ed., 2013, 52, 13699 CrossRef CAS PubMed
.
- J.-M. Lehn, Angew. Chem., Int. Ed., 2013, 52, 2836 CrossRef CAS PubMed
.
- N. Hafezi and J.-M. Lehn, J. Am. Chem. Soc., 2012, 134, 12861 CrossRef CAS PubMed
.
-
(a) G. Vantomme, S. Jiang and J.-M. Lehn, J. Am. Chem. Soc., 2014, 136, 9509 CrossRef CAS PubMed
; (b) S. Ulrich and J.-M. Lehn, Chem.–Eur. J., 2009, 15, 5640 CrossRef CAS PubMed
.
- We carried out 1H NMR binding studies (titrations) between NaBArF and compounds 1D3 and D in order to provide experimental support for our interpretation of the system's complex behaviour. However, the metal salt proved to be not soluble over a suitably wide concentration range (in CDCl3), preventing us from obtaining full binding isotherms. At a qualitative level, our results indicate that 1D3 is only slightly better suited to stabilize Na+ than is 3 × D, which is in line with our analysis (3D dynamic constitutional network).
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and spectroscopic data. See DOI: 10.1039/c4sc03528c |
| This journal is © The Royal Society of Chemistry 2015 |