Coulombic interaction in Finnish middle school chemistry: a systemic perspective on students' conceptual structure of chemical bonding
Jarkko
Joki
*a,
Jari
Lavonen
b,
Kalle
Juuti
b and
Maija
Aksela
a
aDepartment of Chemistry, University of Helsinki, A.I. Virtasen aukio 1, P.O. Box 55, Helsinki, FI-00014, Finland. E-mail: jarkko.joki@helsinki.fi
bDepartment of Teacher Education, University of Helsinki, PO Box 9 (Siltavuorenpenger 5), Helsinki, FI-00014, Finland
First published on 31st August 2015
Abstract
The aim of this study was to design a novel and holistic way to teach chemical bonding at the middle school level according to research on the teaching and learning of bonding. A further aim was to investigate high achieving middle school students' conceptual structures concerning chemical bonding by using a systemic perspective. Students in one metropolitan area middle school were introduced to this newly designed model and their conceptual structures were studied by a clinical interview (n = 8) at the time when the students were concluding their studies at the middle school. The interview data were analysed by employing a systemic perspective on conceptual structures. Elements of conceptual structures such as concepts, simple models (mnemonic devices), explaining schemas, attributes and hypothesis constructs were identified and coded. Connections between the knowledge elements were also identified. An understanding of these connections helps to illuminate which components are necessary to build an adequate conceptual structure. The study revealed that applying principles relating to Coulombic interaction to understand chemical bonding requires the simultaneous appreciation of several factors: First, electron shells have to be understood in terms of energy levels. Second, the distance between the outer electrons and the nucleus has to be understood on the basis of electron shell construction. On the other hand, the effective nuclear charge also needs to be taken into account. The study introduces two new points of view to chemistry education research (CER): (1) a teaching model of chemical bonding that emphasises electric interaction as the background of most bonding types was developed in the study. This responds to the identified need in CER to test alternative teaching models that avoid the octet framework. (2) In the field of chemistry education research, a systemic approach has not previously been widely used for the examination of conceptual structures. In addition, the systemic perception of the network structure, which consists of these constructions, helps to explain in more detail the relationship between the separate concepts and the constructions and their significance as a whole.
Introduction
Chemical bonding is one of the most central concepts in chemistry. Chemical bonding is used to explain the behaviour of substances or materials in different situations and reactions. The concept of bonding is also used to explain what happens to substances during a chemical reaction. On the other hand, the concept of chemical bonding is highly abstract and difficult to demonstrate since there is no particular macroscopic property that can be directly connected to chemical bonding. (de Jong and Taber, 2014). The difficulties that students experience in learning and understanding chemical bonding have been researched at different levels from secondary education (Harrison and Treagust, 1996; Coll and Treagust, 2001, 2003; Coll and Taylor, 2002; Othman et al., 2008) to university studies (Tsaparlis, 1997). According to reviews conducted by de Jong and Taber (2014), Taber and Coll (2002), Özmen (2004), and Ünal et al. (2006), the problems involved in learning chemical bonding have been widely surveyed. Several problems are connected with the dichotomy model of chemical bond types: ionic and covalent bonding and the octet framework (Taber and Coll, 2002). The octet framework and anthropomorphic language prevent students from constructing meaningful and explanative conceptual structures. The octet framework stems from the quantisation of the energy levels of electrons, but when detached from the quantal framework, it may lead lower and upper secondary school students to think more in terms of magic than science.Several different models have been proposed to reform the teaching of chemical bonding, mostly at the upper secondary level of chemistry teaching. The use of these new models and the benefits of them have not yet been researched in the context of middle school (Taber and Coll, 2002; Levy Nahum et al., 2010; Bergqvist et al., 2013; Dhindsa and Treagust, 2014).
The teaching of the metallic bond is conducted separately from that of ionic and covalent bonding, although the character of the bonding is largely covalent in spite of the delocalised electrons (Allen and Capitani, 1994; Anderson et al., 1994; Gilman, 1999; Levy Nahum et al., 2008; Jensen, 2009).
Teaching of electronegativity in connection with chemical bonding and the use of the differences between electronegativities to suggest the bonding type are not unproblematic (Sproul, 2001; Levy Nahum et al., 2010). Despite large differences in electronegativities, the bond type of the compound can still be characteristically covalent (Woicik et al., 2002).
However, research on the teaching of chemical bonding, as well as suggestions for the use of models and development of instruction, have had only a minor impact on both teaching and textbooks (Bergqvist et al., 2013). Moreover, the use of the models suggested by researchers has not been researched in practice (Taber and Coll, 2002; Levy Nahum et al., 2010; Bergqvist et al., 2013; Dhindsa and Treagust, 2014). Still, it has been stated that faulty ideas related to chemical bonding will hamper students' ability to solve chemistry problems generally and context-based tasks in particular (Broman and Parchmann, 2014). The purpose of this study is to respond to the recent call in the literature to test alternative teaching models for chemical bonding in practice (Ünal et al., 2006; Bergqvist et al., 2013; Dhindsa and Treagust, 2014).
Research on conceptual structures
The concept of “concept” is understood in different ways and has different definitions in the educational research literature (diSessa and Sherin, 1998). Concepts and conceptual structures can be studied at different levels, for example at the level of ontological categories (Chi et al., 1994) or at the level of phenomenological primitives (diSessa and Sherin, 1998; diSessa et al., 2004). Depending on the point of view, conceptual structures can be seen as fragmentary, but developing towards coherence (diSessa et al., 2004) or theory-like structures (Amin et al., 2014). One possible way to reach the synthesis of different points of view is to use a systemic perspective on conceptual structures (Thagard, 1992; Koponen and Huttunen, 2013; Amin et al., 2014). The aim of this study is to investigate middle school students' conceptual structures concerning chemical bonding by using a systemic perspective.Piaget considered that learning is an outcome of the child's inherent curiosity and construction of understanding according to age-dependent development (Piaget, 1988). However, formal teaching is still the most important factor in constructing highly abstract concepts like electrons or chemical bonding. At the beginning, these kinds of concepts are empty and meaningless for students. In the research, these kinds of concepts are thus known as placeholders (Carey, 2011). Formal teaching supports students in constructing the meaning of these placeholders as well as connections between the placeholders and the other concepts during the learning process. However, the construction of meanings for non-observable concepts or models provided by researchers is challenging. The models taught are simplified and reduced from scientific models, and they have been edited to an appropriate age level (Gilbert, 2004). As students do not have preconceptions concerning abstract concepts or models that are alien to everyday life, the first teaching models will construct the basis of the conceptual structure and the foundation for all subsequent learning. Analogies, metaphors and other concepts that have been used in education will have a remarkable effect on how students can construct adequate conceptual structures (Harrison and Treagust, 1996; de Posada, 1999; Talanquer, 2007; Hilton and Nichols, 2011).
Cognitive conflict as an instructional strategy was supposed to be effective for learning concepts. The study has shown rather that some of the folk knowledge and of the alternative conceptual structures are extremely resistant to the attempts to change the conceptual structure through a cognitive conflict (Treagust and Duit, 2008). On the contrary, research has shown that preconceptions and alternative conceptions are very resistant to efforts for change by cognitive conflict. The more scientific concepts appear as competing concepts or parallel alternative concepts, but do not replace alternatives or preconceptions. In addition, research has shown that students can have manifold conceptual structures that compete with each other (Taber, 2000a, 2001a). The students often favour simpler explanation models, even if they have been found to be faulty (Nicoll, 2001). Therefore, the models to be taught should be as accurate as possible from the outset so that there is very little to unlearn during later grades.
Physics education research (PER) has long used the knowledge-in-pieces approach for studying conceptual structures and, recently, the knowledge-in-pieces approach has been recommended as also being fruitful for chemistry education research (de Jong and Taber, 2014; Taber, 2014a). Learning chemical bonding during the 10th grade has recently been studied in order to shed light on fine-grained conceptual structures. Although the diagnostic instrument takes into account canonical knowledge elements, it does not focus on connections between knowledge elements (Yayon et al., 2012). The “big picture” of the conceptual structure concerning chemical bonding, polarities of molecules and structures of matter has recently been studied at the college level (Wang and Barrow, 2013). The study compares students’ networks of conceptual structures after students are divided into high and low conceptual knowledge groups on the basis of three diagnostic instruments. The study found that the lack of understanding of individual concepts was of great importance to the integrity and explanatory power of the whole conceptual structure (Wang and Barrow, 2013).
The present study uses the systemic perspective on conceptual structures, which combines the knowledge-in-pieces and knowledge in a theoretical point of view (Koponen and Huttunen, 2013). From the systemic point of view conceptual structures have been analysed attempting to the fine separation of different kinds of conceptual constructs. In addition, the systemic point of view presents the relationships between the different constructs, which have not been considered in earlier studies (for example Yayon et al., 2012).
A novel way to teach chemical bonding at the middle school level
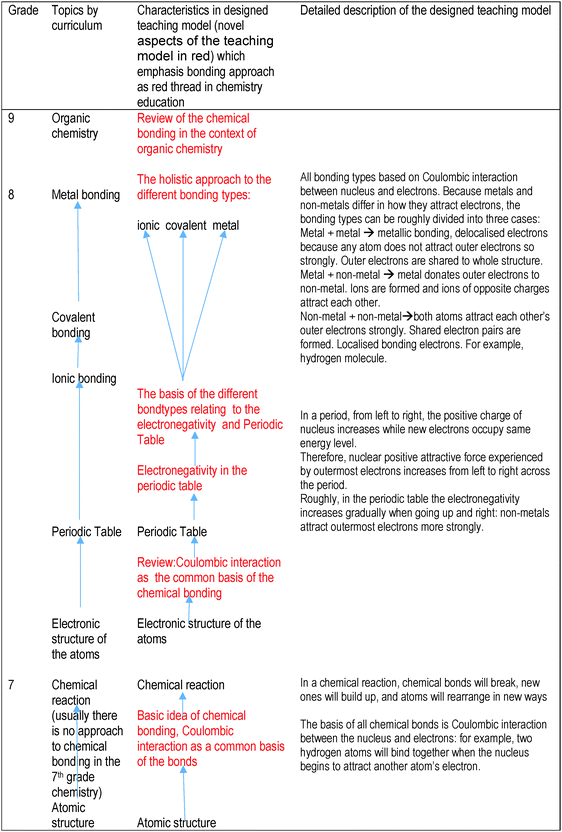
Even though chemical bonding is one of the central concepts of chemistry, there is no direct physical correspondence related to it (Gonthier et al., 2012). However, the concept of chemical bonding and the models used to describe it are central tools in chemistry and are used for perceiving the structure of substances, reactions and the properties of substances. It is particularly challenging to teach chemical bonding in comprehensive schools because the theoretical understanding of chemical bonding is based on quantum mechanical models that are contrary to common sense reasoning. However, quantum mechanics cannot be taught in comprehensive schools so the interactions between the particles of the matter and the teaching of chemical bonding must mainly rely on models of classical physics that have been heavily simplified.The teaching model that has been designed and used in this study does not as such correspond to any model that has been proposed in the research literature because such models have mostly been directed towards the upper secondary school level (upper secondary school/high school) (grades 10–12). The teaching model proposed to be suitable for middle school students (grades 7–9) has been formulated based on a preliminary study (Asunta and Joki, 2003) and has taken shape over ten years. Even though the teaching model has taken shape over several years, some elements stem from recommendations that have been presented in the research literature (Levy Nahum et al., 2007, 2008; Dhindsa and Treagust, 2014). Taber and Coll (2002) recommended avoiding the atom ontology through the use of hypothetic imaginary models (see also Taber, 2012). However, this teaching model approaches the structure and behaviour of substances from the individual atoms point of view, as suggested by Levy Nahum et al. (2007, 2008) in their “from bottom up” teaching model. The hypothetical formation of a chemical bond between two hydrogen atoms is used as the first example of chemical bonding. When the artificiality of this approach is criticised due to the nature of chemistry as a science, it must be remembered that the approach is characteristic of quantum chemistry and for this reason it is not so alien to chemistry in general. Of course, one must emphasise to the students that atoms do not normally appear as individual atoms, but have actually formed bonds and different structures already.
When the model developed in this research is compared to that of Levy Nahum et al. (2007, 2008, 2010), it must be observed that the teaching of intermolecular forces is not included in the chemistry curriculum of Finnish comprehensive schools. The intermolecular forces (the hydrogen bonds and ion–dipole-bonding) may have been implicitly considered in this teaching model in the context of dissolution and conductivity of the ionic compounds in water. The teaching model of Dhindsa and Treagust (2014) is congruent with this model, especially the fact that electronegativity is used as the explanation for different types of bonding. Covalent bonding will be taught at first (look Table 1, implicitly in the 7th class, because coulombic interaction as a common basis of the bonding is presented with two hydrogen atoms without mentioning that there are different bond types and this particular case is covalent) and ionic bonding after that (cf.Dhindsa and Treagust, 2014).
A summary of the designed model and its sequencing across different grades at Finnish lower secondary chemistry education (grades 7–9) is presented in Table 1. Special attention is paid to the fact that the students have used as a peripheral reader a textbook (Ikonen et al., 2009) that represents the octet framework approach.
The purpose of this study is to respond to the stated need in the research literature to test the alternative teaching models for chemical bonding in practice (Dhindsa and Treagust, 2014) and to analyse the conceptual structures and possible problems therein produced by the teaching models. The conceptual structures that are related to chemical bonding have not previously been studied at the lower secondary (middle school) level from a systemic point of view. Another purpose of this study is to produce new information from the conceptual structures and their systemic properties that are related to chemical bonding, when in the teaching an attempt has been made to emphasise Coulombic interaction as the foundation of all chemical bonding types and, on the other hand, the difference in electronegativity caused by the electronic structure of the atoms in the background of different bonding types. The research question that informs the study is: what kind of conceptual structure of chemical bonding does high achieving students acquire when they are taught using the designed teaching model? As the goal of the study is to uncover the conceptual structures created by the new teaching model and the challenges related to them, high-achieving students were chosen for the study to ensure that the examined conceptual structures were as rich as possible and that the image of the conceptual structure produced by the teaching model was as accurate as possible.
Methods
Context of the study
In the Finnish school system, the teaching of chemistry begins at the lower secondary level (grade 7) or in other words in middle school and chemical bonding is taught for the first time during the second year (8th grade). Education is provided by subject teachers at the middle school. Considering the topics of this study, the Finnish lower secondary chemistry curriculum describes these topics briefly and at a very general level. This is because in Finland the curriculum exists in two levels: at a national level Core Curriculum and at the local level more specific and detailed local curriculum. At the core curriculum, objectives are not described to a specific grade (for example grade 7th) but to grade levels (7th to 9th). Therefore, the models designed in this research project are in line with the national framework curriculum. The curriculum (Core Curriculum for basic education, 2004) states: “The instruction relies on an experimental approach in which the starting point is the observation and investigation of substances and phenomena associated with the living nature. The student progresses from that point to the interpretation, explanation, and description of phenomena, and to modelling both the structure of matter and chemical reactions with the symbolic language of chemistry.” Moreover, the curriculum states: “The tasks of chemistry instruction in the seventh through ninth grades is to guide the student in… acquiring knowledge, and in applying that knowledge in different life situations. The students will• learn to describe and model chemical reactions with the aid of reaction equations
• learn to know about the physical and chemical concepts that describe the properties of substances and learn to apply those concepts
• learn concepts and models that describe the chemical bonds and structure of matter and
• learn to apply their knowledge to practical situations and choices.”
The Finnish students are traditionally taught according to the octet framework (Asunta and Joki, 2003). The octet framework is typically found in the Finnish chemistry textbooks used in middle and upper secondary schools. Textbooks also present different bonding types separately or dichotomously (only covalent and ionic bonds). A holistic approach for the common foundation (Coulombic interaction) of the chemical bonding is missing (for example chemistry textbooks used by the students in this study, see Ikonen et al., 2009). The representations in textbooks in Sweden are similar to those in Finnish books (Bergqvist et al., 2013).
New, research-based approaches to teaching chemical bonding must begin in middle school if one wants to avoid the trouble of unlearning the octet framework – if that is even possible (Taber, 2003) – because at least in Finland, the octet framework is introduced by the most used textbooks to the students in middle school chemistry. The recommendations of earlier studies are typically only directed towards upper secondary school teaching (Levy Nahum et al., 2008; Bergqvist et al., 2013; de Jong and Taber, 2014). A teaching model that avoids the octet framework and that has been used from the beginning of middle school has been developed in this study. The objective is to study the kinds of conceptual structures the students have when they graduate from middle school after they have been taught for three years using a novel teaching model.
The new approach (Table 1) to teaching chemical bonding during middle school (from 7th grade to 9th grade) was used for three teaching groups (38 students in total) at a Finnish middle school. The students who participated in this study had studied in the teaching group of the 1st author of this paper throughout the whole middle school period. The school is located in Southern Finland and in an urban area. The students' socio-economic background is mainly higher middle class.
Data collection
When the teaching of chemistry had ended, eight students were invited during the last few weeks of the 2014 spring term to undergo a clinical interview. The students to be interviewed were chosen on the basis of voluntariness and study success.Interview
To obtain a picture of the conceptual structure of each student that is as exact as possible, a clinical semi-structured interview in which the conceptual structure can be analysed with additional focus questions was chosen as the study method.The students' teacher interviewed them individually at the end of a school day in a classroom familiar to them. Each student was asked to reserve 1.5 hours for the interview. The interviews lasted approximately an hour. The interviews were recorded and transcribed. The interviews were held in May 2014, at a time agreed in advance with the students.
The interview (Appendix 1) was divided into four parts: in the first part, the students' motivation regarding the studying of chemistry is questions. However, this warm-up question was not used in the study. After that, questions focusing on the conceptual structures related to the structure of atoms and chemical bonding were asked. In the third part of the interview, how the student uses conceptual structures when explaining the properties and structure of substances (sodium chloride, water, and magnesium ribbon) was studied. In the fourth part, the students' ability to conclude the difference between electronegativities on the basis of the atomic structure and to use electronegativity and the electronic structure of atoms to help in the theoretical explanation of bonding or structures was determined.
The structure of the interview was designed according to the research question and allowed the student to define for him/herself the basic concepts as far as possible while avoiding leading. After that, the concepts that the student introduced were used to explain the structure of substances. Finally, there were diagnostic questions collected from earlier studies (Peterson and Treagust, 1989; Taber, 2000b). With the help of these diagnostic probes, the students' ability to use the electronic structure of the atom and Coulombic interaction in anticipating and explaining differences between electronegativities and bonding structures was clarified.
The term “clinical” refers to the diagnostic character of the interview (Russ et al., 2012). The purpose was to essentially clarify how the student is able to justify his/her views and identify what conceptual structures exist behind the models (diSessa, 2007; Russ et al., 2012).
The students' participation in the interviews was voluntary. The interviewed students had finished studying chemistry in the middle school two months before the interview time due to the periodical schedule of subjects.
The interview was performed in the chemistry classroom, which is familiar to the students, so that the interview environment would be as familiar as possible and natural to the students in connection with the subject. The interviews were conducted in Finnish and the interview material was analysed in Finnish. Only extracts which are presented here were translated by the first author. In the translations all non-necessary words, like “okeay”; “Hmm…”; “as like” are eliminated. Moreover, the slang text was translated to literary language to avoid misunderstanding. However, the meaning of the text from the point of view of chemistry was made similar to original in the translation.
Analysis
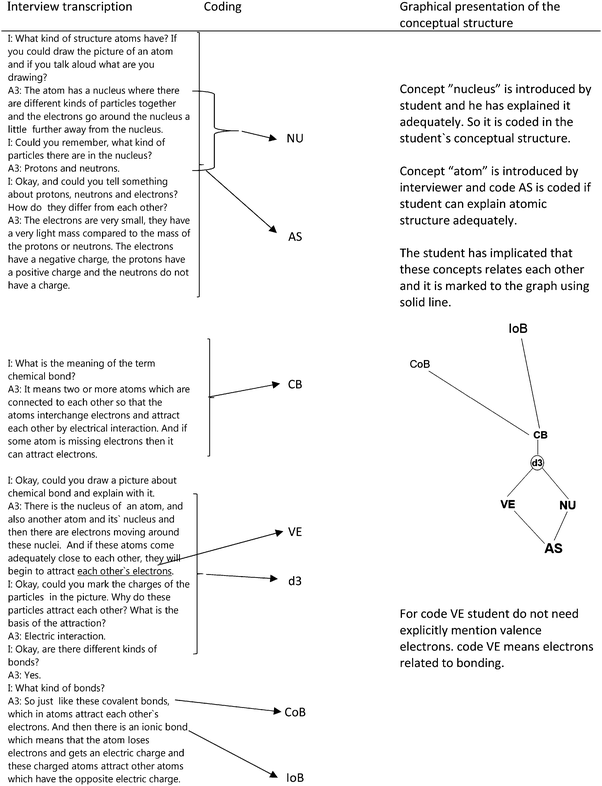
The transcribed interview material was read through several times. The material was analysed according to the notion of inductive content analysis (Elo and Kyngäs, 2008). The systemic analysis model created by Koponen and Huttunen (2013) was applied in the analysis of the conceptual structures in this study. In this model, a conceptual structure is seen as a complex network consisting of different concepts, their attributes, simple models that link the concepts (e.g., mnemonic devices), possible causal constructions behind the models and empirical observations, and the related hypothesis constructions (Koponen and Huttunen, 2013). Unlike in PER in which it is talked about causal schemes we use more cautiously the concept ‘explanatory scheme’ in the context of chemistry.The analysis of the transcribed interviews began by identifying the concepts, simple models, explanatory schemes and hypothesis constructions used by the students. In this context, a chemical bond, which may be either a covalent, ionic or metallic bond, is an example of a concept. Behind a simple model (e.g., a metal and non-metal form an ionic bond), there may be an explanatory scheme that explains the formation of the ionic bond based on the electron configurations and the resulting electronegativity differences of metals and non-metals. On the basis of reading and recognising the concepts, a coding scheme was constructed. The functionality of the coding scheme was tested by coding a few interviews. At the same time, an attempt was made to remove overlapping schemes from the classification. The objective was a clear coding system that, at the same time, would be sufficiently fine-grained. After this, all the interviews were analysed several times, focusing the coding scheme at every iteration. Tables 2–6 introduce the coding scheme and the division of codes. In order to increase the validity of the final coding, the coding grounds and material behind them are presented as openly as possible. For better reliability, all materials were read through several times and the coding scheme for the material was tested at every iteration.
| Central concepts (code) | Concept content |
|---|---|
| AS | Atomic structure |
| N | Non-metal |
| M | Metal |
| CB | Chemical bond |
| CoB | Covalent bond |
| IoB | Ionic bond |
| MeB | Metallic bond |
| VE | Valence electron/bonding electron (implicit appearance) |
| EN | Electronegativity (implicit appearance) |
| PO | Polarity (implicit appearance) |
| NU | Nucleus |
| MS | Molecular structure |
| LS | Lattice structure |
| Simple models | Model content |
|---|---|
| m1 | Metals easily give away their outermost electrons |
| m2 | Non-metals receive electrons to fill their outermost shell |
| m3 | An ionic bond forms between a metal atom and a non-metal atom |
| m4 | A covalent bond forms between two non-metal atoms |
| m5 | A metallic bond forms between two metal atoms |
| Attributes | Description |
|---|---|
| a1 | Valence electron transfer from metal to non-metal |
| a2 | Localised electron pair |
| a3 | Delocalised valence electrons |
| Hypothesis constructs | Description |
|---|---|
| h1 | Compound is crystalline |
| h2 | It is bendy |
| h3 | Highly electrically conductive |
| h4 | High melting and boiling points |
| h5 | Hard but fragile structure |
| Determination constructs/explanatory scheme | Description of the scheme |
|---|---|
| d1 | The full outer shell explanatory principle (Taber, 2002) |
| d2 | Effective attractions of nuclei at the level of the outermost electron shell, which result from the electron configuration of atoms, define how binding electrons are distributed in a bond and what the resulting chemical bond is |
| d3 | A bond is based on the Coulombic interaction between nuclei and binding electrons |
| d4 | Outermost electrons' distance from the nucleus affects the nuclear attraction felt by the electrons |
| d5 | Electric interaction – positive and negative charges aim to cancel each other out (Boo, 1998) |
| d6 | Nuclear charge affects the attraction felt by the outermost electrons |
| d7 | Electrons between the nucleus and the valence electrons shield the valence electrons from the attraction of the nucleus |
| d8 | Another atom draws electrons to itself |
| d9 | A positive or negative charge is too high, which is why the structure is not stable |
| d10 | Electrons repel each other |
| d11 | Nuclear charge is shared out among the residual electrons (Taber, 1998) |
| d12 | Non-charged atoms do not attract each other because there is the same amount of protons and electrons and these charges cancel each other out |
| d13 | Bond is based on different charges of ions |
| d14 | There is more charged protons |
Division grounds of concepts
The concepts (Table 2) were identified either on the basis of a mention in the interview or on the basis of the description. For example, the marking of the concept of the atom structure to the conceptual structure of the student means that the student has described the structure of the atom correctly in the 2nd question.The comments in which the student forms generalising propositions between the concepts without any immediate reasoning, for example “metal and non-metal form the ionic bond”, were classified as simple models (m1–m5), in other words as mnemonic devices (Table 3). The attributes (Table 4) (electron transfer is localised or delocalised) that are related to the bonding electrons are described by (a1–a3) and are connected accordingly in different bonding types.
Explanatory schemes (determination constructs) were explanations of the second level for these mnemonic devices (Table 6). In the explaining/determining schemes, the student drew either on Coulombic interaction or on the octet framework, which functions in a way as a causal principle. The explanatory scheme is not classified as strictly causal in this study like studies in PER, but is an explanatory model that is stronger than the rule of thumb. Some of the explanatory schemes, for example d10 (two objects that are of like charge will repel each other), approach the so-called phenomenological primitives in their simplicity (diSessa et al., 2004). The hypothesis constructions are connected to the macroscopic properties of the material, which find an explanation with the help of the bonding model, at least in the students' understanding.
The systemic point of view of the conceptual structures helps to identify connections between the concepts and the students' use of the concepts, explanatory schemes and mnemonic devices. The graphic presentation (Table 7) of the diagnostic questions (20–25), which are related to the electronic structure and electronegativity, helps separate and identify the determination constructs that the student uses in a given situation. Graphs present whether the student uses many different d-constructs at the same time for a diagnostic question. Sometimes, the student also has to estimate the mutual significance of two different d-constructs for the whole when they would indicate contrary effects. The systemic point of view also offers a general view of the conceptual structure of the student and of the connections between the concepts.
The division of concepts into the concepts, mnemonics, the explanatory schemes, hypothesis constructions and attributes helps to perceive the function of the different particles of the conceptual structure to the whole.
Detailed description of the analysis
The analysis of the interview transcription and the graphical presentation of the students' conceptual structure are presented in detail in Table 8.Ethics
When a teacher examines his own students, one must be aware of the possible distortion of the research material caused by the teacher–student relationship and, on the other hand, keep in mind the ethical factors involved when examining students (Taber, 2014b). The students were informed that agreeing (or not agreeing) to take part in the interviews would not affect their chemistry grade. Furthermore, it was emphasised that any ideas or opinions expressed in the interview would not affect the students' evaluations. For the whole study process, permission to conduct the research was obtained from the education office of the town of Espoo (Licence number: 21/2014, 17.03.2014), which functions as an organiser of the teaching. Furthermore, the underage children's parents/guardians also gave written permission for the children to participate in the study.All research must ethically take into consideration the time that the students donated to the study. Therefore, a cinema ticket was given as compensation for participation in the interview, which lasts for approximately an hour. The material produced by the students was encoded (A1–A8) immediately so that individual students could not be identified.
Results
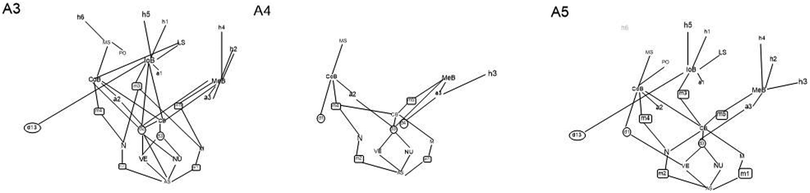
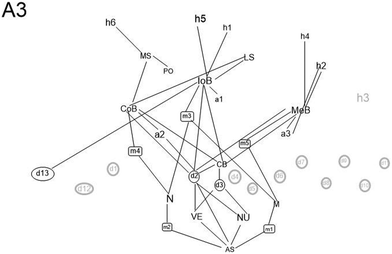
Although the students who had succeeded the most in their chemistry studies were chosen for the study, big differences were identified in the conceptual structures of the examined students. The graphical presentation of the conceptual structures helps to perceive the richness and coherence of the conceptual structure of each student. The more different concept constructions a student has and the more connections there are between them, the richer and more coherent the conceptual structure is. The division of conceptual constructions into mnemonic devices and determination constructs shows whether the student depends on memorising mnemonic devices or on explanatory principles when explaining chemical bonding. Graphs concerning the conceptual structures of three students (A4, A3, and A5) are presented in Fig. 1. The concepts and connections marked in black have been found in the students' interview. Students (A4, A5) lean heavily on mnemonics. This means that students use mnemonics like “non-metal and non-metal forms covalent bonding” but could not explain why is it this way. The first student (A4) neither mentioned an ionic bond in the interview nor knew how to talk about the ionic bond otherwise. The student (A3) conceptual structure is considerably richer and better integrated, and the student has explanatory schemes by which he is able to explain the reason why different kinds of bonding types occur. The graphical presentation of the conceptual structure of student A3 is presented in Fig. 2. The student understood how the different types of chemical bonding are based on the different electronic structures of the atoms (scheme d2). The difference between schemes d2 and d3 is presented in Table 9. Merely understanding that a chemical bond is caused by the Coulombic interaction (d3) is not enough: if a student does not understand the relation of particular ideas such as the structural principle of electron shells, the meaning of an electron's shell related to the energy level, or the effect of the positive charge of the nucleus on the distance from the electrons, it may produce erroneous assumptions (listed in Table 10).| Student has mentioned that chemical bonding is due to the Coulombic interaction (d3), but could not explain why there are different kinds of bond types (missing d2). | Student has mentioned that chemical bonding is due to the Coulombic interaction (d3) and he could explain different bond types using the different kinds of atomic structures (d2). |
| I: Why do the magnesium atoms form bonding among themselves that is different from the bonding type between sodium and a chlorine? | I: All right, yes, so why does it seem that now there will be a different bonding type in table salt than in water? Or why is there a different bonding type in table salt than in magnesium ribbon? |
| A5: I do not know any other reason but magnesium is a metal and when it binds itself then it does it that way… and in sodium chloride there is an ionic bond and they will bind in a different way, but then I do not know in more detail how to explain it | A3: It depends on which atoms the bonding forms between. |
| I: All right. We repeat again now, how will the ionic bond be formed? | I: All right … can you tell me about it little more? |
| A5: In it there is a non-metal and a metal, which it will then make that metal give electrons to the non-metal. | A3: Yes, that kind of metallic bonding forms between perhaps, in other words the metals have the free electrons that are able to move freely in the whole structure. And then the ionic bond will usually form between the metal and the non-metal because the metal has extra electrons which easily leave from there and then the non-metal is able to receive these extra electrons easily, then they will get those electric charges easily and then, in case of two non-metals, so then those electrons are not able to give them in a way so, both begin to attract each other’s electrons, as it forms such a shared electron pair. |
| I: What about if there is merely metals, what happens then? | I: Okay, why do they not donate? Why does it form them into a shared electron pair? |
| A5: Then there will come such shared electrons which then will move freely there | A3: The reason is that neither is able receive that electron. |
| I: Why? | I: Why is one not able to receive? |
| A5: mmm….. as, because, metals cannot receive those electrons directly as such to themselves. | A3: Or that as so either one is unable to donate that electron, or they should donate so many electrons that it will be easier to begin to draw each other’s electron into its half that those own electrons do not need to be given up. |
| I: Why cannot they be received? | I: Why it is so? Why can they not be given? Or where is it based so that they cannot be given? |
| A5: ääh, mmm. I do not know. | A3: It is based on the fact that atoms have these electron shells. Different amounts of electrons fit into different electron shells. The outermost electron shell of these non-metals where all those reactions take place and where all electrons participate in reactions are, is so near to full. So, the electromagnetic force is much stronger so that electrons cannot detach from there or it is not energetically beneficial that they will donate those electrons from there. |
| I: What things are unclear concerning chemical bonding in your opinion? How do you feel, which issue is difficult to understand? | |
| A5: Now it came forth that I do not know, what is the fundamental reason for forming certain bond type between certain substances and why there is different ones and why someone does not form any bond. What is the fundamental reason behind it. | |
| Founded assumptions | Code |
|---|---|
| The student may think that the electric charges always try to cancel each other out | d5 |
| The student may think that the ability of a non-metal atom to draw valence electrons into its half is caused by the protons which are more charged than others. | d14 |
| Having a defective understanding of the electron shells relating to energy levels and neglecting the effective nuclear charge can cause the student to be unable to explain valency relationships without giving causal significance to the octet rule. | d1 |
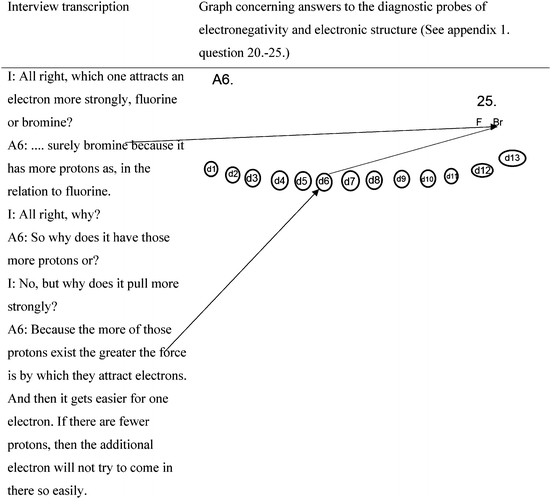
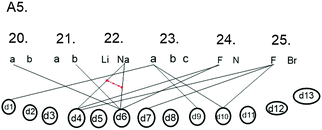
Student A5 connects many macroscopic properties of materials (h1–h5) to different bonding types. Still, the student cannot explain why there are different bonding types (the scheme is missing, d2). However, the student can estimate differences between electronegativity in particular cases (in questions 20–25) (Fig. 3).
Based on atomic electronegativity differences, Coulombic interaction and the configuration principle, some of the students were able to theoretically explain how an argon fluorohydride (Khriachtchev et al., 2000) molecule stays together.
I: All right yes, so now the last question. In the Department of Chemistry of the University of Helsinki, scientists have successfully made this kind of molecule where there is hydrogen, then there will be argon there… you can look from a periodic table and then there is fluorine (drawn the ball model from the molecule to a table) and argon is there. So how you would explain how this molecule holds together? Why do those atoms stay together?
A3: I do not know, I cannot explain.
I: So why do you not know? Why is it problematic in your opinion?
A3: Argon is a stable atom because it has all electron shells full. Therefore, argon should not react at all with anything because it is in a way as, it does not need more electrons in and it does not give away electrons very easily.
I: Yes, but now this one has been found, however, now you should explain why this molecule exists. This is the last question so you can think about it for a little while.
A3: All right… so if fluorine attracts in a way those electrons of argon because one electron is missing from the outermost shell of fluorine, after that there is little room for a one electron which argon atom or the nucleus of the argon atom is able to attract from the hydrogen atom.
However, avoiding the octet framework requires an understanding of the electronic structure of the atoms to be quantised and an understanding of the electron shells relating to energy levels. A student remembered that using the octet rule as a causal explanation was discouraged in teaching, but the reason for this had remained unclear: (question 23):
A2: The nucleus draws that outermost electron to itself, but then it also aims to have its outermost shell full so that it also gives it away easily.
I: Well, why does it want its outermost shell to be full?
A2: Hmm. This is like, I've been told not to ever believe that octet thing, but still, that’s how it sort of goes, but… hmm… I don't know.
The octet rule can appear as a mnemonic device without it having causal significance which appeared in the part of transcription in Table 8 where student A3 explains the basis of different bond types due to the electronic structure of the atoms.
Trends in the electronegativity in the groups and periods taught on the basis of a periodic table help the student to understand different chemical bonding types. In order to succeed in estimating the electronegativity, the student has to take into account the significance of the growing nuclear charge (d6) for the electronegativity when one moves from the left to the right in the period and the significance of the increasing numbers of intervening shells of electrons partially cancelling of the pulling force of the nucleus (d4) moving from the top downward in the group. Forgetting one of these led to a faulty estimate in the 22nd and 25th questions
I: So I now asked if both gave an outermost electron, of course, which one would give it more easily? So on which one will it be more easily removed if one leaves from both?
A2: So no matter with which material it reacts?
I: Yes, no matter, it is not talked about in another part of the reaction…
A2: öööö. hmm. Well, quite difficult questions, I will say, then, lithium.
I: All right, how would you justify it?
A2: It has fewer protons, which would attract that electron and then if somebody would intend to draw that electron so into its half and it would give it more easily.
…
The interview extract below is connected with question 25 (Fig. 4).
I: All right, which one attracts an electron more strongly, fluorine or bromine?
A6: … surely bromine because it has more protons as, in the relation to fluorine.
I: All right, why?
A6: So why does it have those more protons or?
I: No, but why does it pull more strongly?
A6: Because the more those protons exist the greater the force is by which they attract electrons. And then it gets easier for one electron. If there are fewer protons, then the additional electron will not try to come in there so easily.
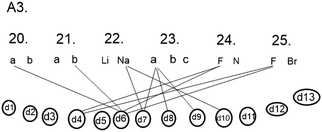
In question number 23, the Na+ ion, Na atom and Na7− ion should be set in order according to stability. All the students chose alternative (a), in which the order was from most stable to most unstable: Na+, Na, and Na7−. In earlier studies, the octet framework has been criticised due to the fact that students presume the ionisation of sodium without any external factors taking place to get the full shell of electrons (Taber, 2000b; Taber and Tan, 2011). However, the question is difficult from the point of view of the student because in the studying situations the student seldom compares the stability of the ions or atoms alone without it being a question of stability of a particle where some other particle is present. However, some of the students (for example, A3, Fig. 5) did indeed spontaneously mention in connection with the interview question that the Na+ the ion will be the most permanent if some other atom draws its electron into its half (d8). It is natural for the student to mention in connection with the students' studying context that an Na+ ion is more permanent than an Na atom because the reaction of the sodium metal with water is a very popular demonstration in chemistry and a good example of the reduction of electronegativity when one moves downwards in the group. In the study of conceptual structures, it has also been shown how the minimal changing of the arrangement of a question or context causes changes in the students' answers (Yates et al., 1988; diSessa et al., 2004; Clark, 2006) so that the reaction of sodium can be compared with the reaction of lithium and thought can be given to why sodium gives its outermost electron more easily than lithium. So, the demonstration can be used to illustrate the effect of the distance between the outermost electron from the nucleus on how easily the electron becomes loose.
In addition to simple models, the student has to perceive the differences in the electronic structures of non-metal and metal atoms, which the bonding types are based on (scheme d2):
I: Is there now this same phenomenon in all these bonding types?
A7: Covalent is tied up and also in the ionic bond, in the metal bonding now not quite so much
I: Why does one not?
A7: Because those electrons are divided in that way, so they can move really freely there.
I: All right, are there still some other reasons that have an effect on the forming of bonding types?
A7: The forming of the bonding types will be effected by the elements and atoms and then the conditions, for example temperature.
I: You defined these bonding types here in that they form according to whether there is metal or non-metal this way? So why?
A7: When metals want to donate their outer electrons, then non-metals will want to receive the electrons.
I: Because of what?
A7: That is because the electron shells get full, so non-metals are missing only a few electrons. It also means that there are all electrons quite close to the nucleus and there are a lot of protons, when the force attracting electrons is quite powerful. Otherwise, in the metals, there may be a few electrons at the outermost shell. So there are also less protons in the nucleus and the attracting force is weaker.
On the other hand, in this case the emphasising of the minor electronegativity of the metals has led the student to think that the metal bonding cannot be entirely explained in terms of the Coulombic interaction:
I: I present the additional question now. Which force is this based on that they stay together?
A7: All bonding is based on the charge of atoms, on electromagnetic charges.
I: To the attractive force?
A7: Yes.
I: Yes, all right now if it is returned here metal… if then, you now think about the magnesium ribbon based on the interview, how would you now explain how magnesium atoms stay together in this one magnesium ribbon? Now I will lead you (laughter) a little.
A7: Hmm…
I: What kind of power could it be based on, that they stay together here?
A7: It could be poorly based on that electromagnetic force because, so on there is neutral charges on those molecules
I: Or on atoms?
A7: On atoms, yes, … in metals not molecules but between the mere metals or the same materials, between the same atoms they are so kind… big so kind… (an unclear word) they…. maybe they somehow attract them… each other.
According to the second student's (A8) view, the outermost electrons are loose from the atoms in the metal bonding. This caused the student to wonder why the loose electrons do not repel each other when they can move freely or why the remaining positive metal ions do not repel each other. So the student did not understand that even though the outermost electrons are delocalised, they will keep the metal atoms together when the nuclei of atoms attract shared electrons.
I: Talking about this magnesium ribbon, how does this bonding model help you to understand the structure of the magnesium ribbon?
A8: hmmm… materials which contain metal bonds are often solid. Perhaps because hmm mmm I don't know why. Their outermost electrons will stay free in the whole structure. And what still exist are the positive ones because there are fewer electrons than protons. I don't know why, but all the positive atoms stay stick together. Maybe it somehow connects with the fact that they can't move much there, these atoms or molecules in the structure.
…
I: How are they attached to each other?
A8: So, with that metallic bond.
I: What it is that metallic bond?
A8: hmmm… I am not quite sure, I remember only the fact that some electrons stay free there. But then I don't know why they do not repel each other.
The Coulombic interaction helps to clearly explain ionic bonding. The theoretical understanding of both metal and covalent bonding (in the metal bonding, the covalent character is mainly in question, in fact) is based on the quantum mechanical examination of electronic structures. So it is symptomatic that the students had difficulty in explaining the formation principle of the metallic and covalent bonding with the help of Coulombic interaction.
A1: So there will be some kind of interaction, but I am not sure, because when I begin to think of this so, for example the oxygen atom has already the protons and electrons in balance, so there is not any electric charges, so why would it want those electrons to come there?
I: Yes.
A1: I do not know, perhaps it is connected to when that shell is full somehow. I do not know actually.
The student had explained a covalent bond earlier, but in connection with the last question (an argon fluorohydride) the student became tangled with his thoughts and started to be surprised at the principle of covalent bonding.
I: Which force is covalent bonding based on?
A5: If they have a shared electron pair and if the another substance which is … if, if, mmm makes them, then not, no, it will not go, it… will… not.
I: So which force is the covalent bonding based on? Why does bond forming happens? Where is it based?
A5: Maybe it is so that, another substance attracts a little more strongly that electron pair, which results negative and positive charge which attracts each other. But then I do not know whether it works because if an oxygen molecule is O2 so, there are just two same atoms. So they are quite similar as the equals so I do not know if then a difference will come even in it, that they would … each other…
I: Why should a difference come in it? So in what way is there a difference?
A5: So that there would be another atom as more negative and another positive.
I: Why should they be in that way?
A5: Well, so that they would stay together somehow.
I: What?
A5: Those atoms.
In the study, it was also noticed that the octet rule is not the only simplifying model that leads students astray. The simple model (m4: a covalent bond forms between two non-metal atoms) caused confusion when the student thought about the reaction between ammonia and hydrogen chloride. However, the student remembered quite rightly that a crystal material is created but could not explain why. The student said that there cannot be an ionic bond involved in it.
Discussion and conclusion
On the basis of this study, a teaching model for chemical bonding based on the Coulombic interaction between the nuclei of atoms and the outermost electrons has produced fairly uniform conceptual structures in typical suburban school students with high grades in chemistry. It has also been noticed in the quasi experiment at the upper secondary school level that the teaching of chemical bonding as a holistic package helps students to better perceive different bonding types and join them to the properties of materials (Karpin et al., 2014).All the interviewees try to use Coulombic interaction (d3) when explaining bonding. Some of the students also had an octet framework (d1). The mnemonic devices (m1–m5) helped the students to perceive the different bonding types. The students connected the macroscopic properties of the material to the bonding types and mainly experienced that the models of chemical bonding helped them to understand the structure of the materials (questions 15 and 16). One student (A5) knew how to connect all the macroscopic properties (h1–h5) to the appropriate bonding types and, in addition, knew how to explain the properties of water on the basis of the polarity of the water molecule caused by the difference of electronegativity between oxygen and hydrogen. However, the student did not understand what the different bonding types are caused by (scheme d2 was missing). In an earlier study concerning the conceptual structure of chemical bonding, manifold conceptions (Taber, 2000a) had been detected. In this study, these manifold explanation models are still seen, albeit at a finer level. On the other hand, thanks to a systemic point of view, which level a student uses in what scheme was also uncovered. For example, student A5 knew how to use the schemes (d4, d6, d9, and d10) concerning electronegativity, Coulombic interaction and electronic structure to answer particular questions, but the student did not know to perceive that the same principles can also be used as a grounding (scheme d2) for the coarse division of different bonding types.
In an earlier study (Taber, 2001a), a transition from the octet framework was followed towards Coulombic interaction and towards the minimum energy principle, and it was said to be a slow process in which the different explanation models compete among themselves and the models have their own ecological niches. In the present study, Coulombic interaction was emphasised in the teaching since the beginning. The octet framework was avoided in the teaching and its nature as a mnemonic device was emphasised. However, the textbook brought out the octet rule. In the present study, it was noticed that the students did not rely to a significant extent on the octet rule as an explanatory scheme. However, some of the students used it for explaining covalent bonding. With one student, the octet framework dominated as an explanatory scheme and displaced Coulombic interaction. With another student, criticizing the octet framework in the teaching had caused confusion and he did not know how the matter should have been understood. Is it the case, however, that the octet rule or any given mnemonic device can become harmful if the student leans too strongly on only the mnemonic devices and does not perceive the determination constructs/explanatory schemes in the background?
There were many challenges to the use of Coulombic interaction in adapting the theoretical understanding of chemical bonding. The students may have thought that the electric charges ultimately always try to cancel each other out (d5). Boo (1998) described the same idea in his study but joined it to the scheme in which discrete molecules that are formed by the ionic bond. In the present study, one student mentioned that molecules formed by the ionic bond but the student in question did not bring out the formation of ions, only the transition of electrons. A similar observation has come forth earlier and has been particularly connected to school teaching (Barker and Millar, 2000). Instead, the student who suggested that the electric charges would cancel each other out presented the proper understanding of the lattice structure of the ion compounds at the beginning of the interview. This may have been caused by the emphasised zero-sum game in connection with the balancing chemical formula of ionic compounds: the sum of the opposite charges of ions must be zero in the formula of a salt and there will be no electric charge on the salt crystal or it may have been a consequence of teaching neutralisation where hydrogen ions and hydroxide ions will produce neutral water.
One can also see as a weakness of the teaching model the emphasis of the minor electronegativity of the metals and delocalization of valence electrons, as a consequence of which the student may remain unsure what force will after all keep the metal atoms together if the nuclei of the atoms do not really attract their valence electrons. Thinking about the Coulombic interaction caused some students to reach a deadlock whereby they were surprised that two uncharged oxygen atoms can attract each other at all if there is only symmetrical charge distribution in the molecule, despite the same students being able to explain covalent bonding at the beginning of the interview.
These examples probably indicate that the covalent bond as a metal bond gets a seeming adhesive tape concept from the Coulombic interaction. However, the Coulombic interaction does not explain that there is the same number of electrons in the particular electron shells despite nuclear charge or why the electrons are found in pairs (de Jong and Taber, 2014). It was also noticed that when gifted students are examined in more detail, the functionality of the explanatory models is in doubt. Recently, the teaching of the basic rules of quantum mechanics has indeed been proposed to be introduced in upper secondary school (high school) chemistry classes and a potential research question has been presented concerning the effect of teaching the basics of the quantum chemistry on the coherence and explanatory power of the conceptual structure of the students (de Jong and Taber, 2014).
The students brought out clearly the delocalisation of valence electrons as a distinction between the metal bonding and covalent bonding. Even though the validity of the concept of the metallic bond has sometimes been problematic, the metal bonding seems a useful conceptual construct at the middle school level on the grounds of delocalisation.
Implications and future research
Being based on the periodic table with the help of the Aufbau principle and the electron shell in terms of energy levels, it is possible to perceive the students' coarse and simplified picture of the different chemical bonding types based on the Coulombic interaction between the nuclei of atoms and the outer electrons. The coarse picture looks fuzzy, however, examined in more detail. Ultimately, one can question whether the concept of chemical bonding is so fuzzy already (Gonthier et al., 2012) that it is impossible to create a clear teaching model from it.However, if one theme that connects bonding types is chosen for the teaching of chemical bonds, it may be justified that Coulombic interaction is more preferable than the octet framework.
In this study, the teaching model has only been tested on gifted students. In the further development and examination of the model, it must be taken into consideration as to how to obtain a model that is sensible and connected students' experiences, but which is also clear and simple enough that it can be used in comprehensive schools (Oh and Oh, 2011). In any case, it is probably clear that in comprehensive schools the introduction of the octet framework should be stopped so that the students do not need to unlearn it at a later stage of their studies.
However, it will not be necessary to totally give up on the full shell principle or the octet rule if basing chemical bonding on Coulombic interaction and the minimum energy principle will first be studied and then later an octet rule or the full shell principle can be used as a mnemonic device in estimating the valencies of atoms. The second side of the understanding of the mnemonic is, of course, the quantised nature of electron shells or energy levels. Does mentioning this support or complicate the learning of the matter (de Jong and Taber, 2014)?
Instead of the dichotomy of bonding types, the trichotomy was emphasised in this teaching model, although the character of bonding types is also presented as a continuum (Levy Nahum et al., 2008). The continuum character is presented in teaching on the basis of the electronegativity of the atoms, which also form a periodic continuum. The students’ understanding of the continuum character came forth, for example, in demonstrating the polarity of the bonding of water molecules on the basis of the differences between electronegativities. On the other hand, the strength of simple models (m3–m5) may have dominated thinking regarding the continuum character of the bonding type when a reaction between ammonia and hydrogen chloride, for example, was considered.
Perhaps it is actually more significant than the teaching order that the different bonding types will be presented during the continuum due to Coulombic interactions and differences in the electronic structures of the atoms. So it is basically a question of understanding the periodic and gradual change of the electronic structures of the atoms. Perceiving the wholeness will be facilitated when all the bonding types are presented at the same time and the variation between them is compared. This point of view has recently received support in the quasi experiment (Karpin et al., 2014) and it is connected to the theoretical framework of variation theory (Bussey et al., 2013).
As the concepts that are related to chemical bonding form a very complex network of schemes, it is difficult to totally avoid mnemonic devices in teaching. From the mnemonic devices, however, faulty ideas will unavoidably be created if they are understood as explanatory schemes with close relations like the natural law. It would be more significant in teaching that meta information is also provided rather than to avoid mnemonics: when there is a mnemonic device helping to categorise (e.g. metal and non-metal usually form ionic bonding) or and when there is a determination construct. The more widely understood problem of teaching chemical bonding is not merely the octet framework, but involves the balance between coarse and categorical mnemonics and explanatory schemes. The student needs mnemonics in order to deal with his new and fairly large conceptual structure, but he also has to perceive the explanatory schemes in the background of mnemonics. Developing the teaching model and adapting it to practice requires the process that has been described as a concept pedagogical transformation in the research literature (Oh and Oh, 2011).
Suggestions for the curriculum
Does the teaching order have significance for avoiding the learning atom/molecule ontology beside the lattice structures as is supposed in the research that recommends the teaching of bonding types should be begun with metal bonding? (Taber, 2001b; Taber, 2012; Bergqvist et al., 2013). In this study, the bonding types were presented to the students as a complete picture but the idea of the bonding was presented with the help of two hydrogen atoms, which form a covalent bond. However, the students mainly knew the lattice structures and knew how to explain that the ionic bond was caused by the electric pulling forces between the ions and not by the transition of electrons, even though the formation of ions and the transition of electrons had been dealt with in connection with the ionic bond. The faulty atom ontology (Taber and Coll, 2002) did not come forth in the interviews, even though the taught idea of chemical bonding used as the first example was the hydrogen molecule, which forms imaginarily from the single hydrogen atoms. However, one student mentioned that molecules also formed by the ionic bond. Even if the teaching of chemical bonding began with a hydrogen molecule, a lattice structure could also be demonstrated during the first year (in the 7th grade) as one structural type of material at the same time as introducing the concepts, atom and molecule. This way, there would be a mental model of the lattice structure and not only discrete atoms or molecules.The leading problem in the teaching of chemical bonding is that there are no macroscopic properties of materials that would be directly connected to strong chemical bonding. The weak chemical bonds are, however, missing from the curriculum of Finnish comprehensive schools and so the introduction to them will take place as late as at the upper secondary school stage. Thus, the connection of the macroscopic properties of the material to the structure of the submicroscopic level remains unavoidably distant and illusory in comprehensive schools. Should the weak bonding be included within the whole chemical bonding topic and should the different bonding types be presented as a continuum based on Coulombic interaction at the comprehensive school level? Schmidt et al. (2009) noted that students still experience difficulty in perceiving the connection of the bonding between the molecules versus the boiling points of substances at the upper secondary school level, instead the students connect a low boiling point to the breaking of the intramolecular bonds.
Limitations
The interview situation and the structure of the interview make this study situation-specific. This refers to a certain kind of conceptual structure in which the student builds on the synergy of his/her earlier conceptual structures and of the particular interview situation. Generalisability of the results is highly questionable. So, these results describe the students' conceptual structure received through studying and the teaching, but also partly the conceptual structure that has been created and modified in the interview situation. The study sample is small and purposely selected, being directed to only those who succeeded in their chemistry studies. This choice was intentional because we wanted to test the weaknesses of the teaching model itself and, on the other hand, to identify what kind of conceptual structure the top students can form at the middle school level.The examination method creates the possibility of seeing the significance of the knowledge pieces of the conceptual structure for the whole conceptual structure. The examination of the whole conceptual structure from a systemic point of view helps to observe what kinds of challenges exist in the conceptual structure produced by the teaching model as well as to identify the problem sections of the teaching model. The challenge of the method is to identify and classify the material in a reasonable way into the separate knowledge element groups. Knowledge elements have to be fine-grained enough and broken into parts but, on the other hand, clear enough and general. The division of the knowledge elements of the conceptual structure into concepts, simple models, determination constructs, attributes and hypothesis constructions helps to perceive the different functions of knowledge elements in the process of forming adequate conceptual structures of chemical bonding (Koponen and Huttunen, 2013). The method was originally developed in PER and has now been adapted for the first time for CER, so the functionality, validity and expediency of the division must, however, still be tested in CER with a more wide range of materials. The new method used in this work to divide the conceptual structures of chemical bonding into concepts, attributes, and simple mnemonics should not be seen as the final solution, but rather as the introduction and first sketch of how the systemic point of view and the division of conceptual structures for different knowledge element groups can be used in the future as a tool to study and develop the conceptual learning of chemistry.
Appendix 1: corpus of the interview
1. What aspects of middle school chemistry have you found particularly interesting?a. What aspects of the chemistry course did you find particularly motivating?
b. What aspects reduced your motivation?
c. What kinds of chemistry-related subjects could increase your motivation?
2. What does “atom” mean?
3. What about “molecule”?
4. Do materials appear as individual atoms? In what?
5. If a material (for example this paper) does not consist of individual atoms, what is it based on?
6. Explain freely and as carefully as possible what “chemical bonding” refers to?
i. Which particles are in question?
ii. Names of particles.
iii. What kinds of systems they will form? Could you name these structures?
The drawing of pictures or models is required. If a student cannot draw the pictures/models, pre-prepared pictures will be shown to them and they will be asked to name the particles.
a. If the student has mentioned the concepts below:
b. Can you say more about the ions? From molecules? From atoms?
7. What different types of bonding are you familiar with?
a. Give an example of every type.
8. Is there a common reason/factor on which all chemical bonding types are based?
9. Are there other issues that can have an effect on the formation of bonds?
10. Why there are different bonding types? What is it based on?
11. What bonding types are involved in the following materials?
a. Water
b. Diamond
c. Sodium chloride
d. Magnesium ribbon
12. What factors affect the bonding type that forms in the particular cases above?
a. Why is there a different bonding type in table salt than in water?
13. What matters related to the theory of chemical bonding are still unclear or difficult to understand?
14. Why?
15. Do the theoretical models of chemical bonding help you to understand the properties and structure of the above-mentioned materials?
16. What properties or features do the models not explain? What do the models that you have learned failed to explain?
17. What makes you motivated for thinking/learning about chemical bonding?
18. Describe some fabulous learning experiences concerning the study of chemical bonding (ionic bonding, covalent bonding, and metallic bonding)?
19. What matters reduce your interest in thinking/learning about chemical bonding?
(Questions 20 and 21; Peterson and Treagust, 1989)
20. Which one of the following best describes the structure of the hydrogen molecule?
(a) H : H (b) H : H
Why?
21. Which one of the following best describes the shared electron pair of hydrogen fluoride?
(a) H : F (b) H : F
Why?
22. Which donates its outermost electron more easily,
(a) lithium or
(b) sodium?
Why?
23. Determine the chemical stability of the following particles:
Na+ ion
Sodium atom
Na7− ion
{These arranged options below are added only for the graphs, students had to determine order without given options
(a) Na+, Na. Na7−
(b) Na, Na+, Na7−
(c) Na+, Na7−, Na
(d) some one other order, what kind of?}
What is an order from most stable to a least stable structure (Taber, 2000b)
24. Which attracts electrons more strongly, nitrogen or fluorine?
a. Why?
25. Which attracts electrons more strongly, fluorine atom or bromine atom?
a. Why?
26. a. HCl is a gas at room temperature. Explain the structure of the molecule. When the gas is introduced into water, the conductance of water will increase. Why? Explain what takes place?
b. When at room temperature, NH3 is reacted with HCl to the same state, whereby two gaseous substances produce a solid material. How do you explain this phenomenon?
27. A HArF molecule has been found both experimentally and computationally. How can the molecule be stable?
References
- Allen L. C. and Capitani J. F., (1994), What is the Metallic Bond? J. Am. Chem. Soc., 116(19), 8810.
- Amin T. G., Smith C. L. and Wiser M., (2014), Student Conceptions and Conceptual Change. Three Overlapping Phases of Research, in Lederman N. G. and Abell S. K. (ed.), Handbook of Research on Science Education, NY: Taylor & Francis, vol. II.
- Anderson W. P., Burdett J. K. and Czech P. T., (1994), What is the Metallic Bond? J. Am. Chem. Soc., 116(19), 8808–8809.
- Asunta T. and Joki J., (2003), Atomirakenteen oppiminen ja siihen liittyviä vaikeuksia. Meisalo, V. (toim) Aineenopettajakoulutuksen vaihtoehdot ja tutkimus 2002. Ainedidaktiikan symposiumi 1.2.2002 (ss. 301–313). Helsingin yliopiston opettajankoulutuslaitoksen julkaisuja 241.
- Barker V. and Millar R., (2000), Students' reasoning about basic chemical thermodynamics and chemical bonding: what changes occur during a context-based post-16 chemistry course? Int. J. Sci. Educ., 22(11), 1171–1200.
- Bergqvist A. C., Drechsler M., de Jong O. and Chang Rundgren S.-N., (2013), Representations of Chemical Bonding models in School Textbooks – Help or Hindrance for Understanding? Chem. Educ. Res. Pract., 14, 589–606.
- Boo H. K., (1998), Students' Understandings of Chemical Bonds and the Energetics of Chemical Reactions, J. Res. Sci. Teach., 35(5), 569–581.
- Broman K. and Parchmann I., (2014), Students' application of chemical concepts when solving chemistry problems in different contexts, Chem. Educ. Res. Pract., 15, 516–529, DOI: 10.1039/c4rp00051j.
- Bussey T. J., Orgill, MK. and Crippen K. J., (2013), Variation theory: a theory of learning and a useful theoretical framework for chemical education research, Chem. Educ. Res. Pract., 14, 9–22.
- Carey S., (2011), Précis of the origin of concepts, Behav. Brain Sci., 34, 113–124.
- Chi M. T. H., Slotta J. D. and de Leeuw N., (1994), From Things to Processes: A Theory of Conceptual Change for Learning Science Concepts, Learn. Instr., 4, 27–43.
- Clark D. B., (2006), Longitudinal conceptual change in students' understanding thermal equilibrium: an examination of the process of conceptual restructuring, Cogn. Instr., 24(4), 467-563.
- Coll R. K. and Taylor N., (2002), Mental Models in Chemistry: Senior Chemistry Students' Mental Models of Chemical Bonding, Chem. Educ.: Res. Pract. Eur., 3(2), 175–184.
- Coll R. K. and Treagust D. F., (2001), Learners' Mental Models of Chemical Bonding, Res. Sci. Educ., 31, 357–382.
- Coll R. K. and Treagust D. F., (2003), Investigation of Secondary School, Undergraduate, and Graduate Learners' Mental Models of Ionic Bonding, J. Res. Sci. Teach., 4(5), 464–486.
- Core Curriculum for basic education, 2004, http://www.oph.fi/download/47672_core_curricula_basic_education_3.pdf, pp. 192–194.
- de Jong O. and Taber K. S., (2014), The Many Faces of High School Chemistry, in Lederman N. G. and Abell S. K. (ed.), Handbook of Research on Science Education, New York: Taylor & Francis, vol. II, pp. 457–480.
- de Posada J. M., (1999), The Presentation of Metallic Bonding in High School Science Textbooks during Three Decades: Science Educational Reforms and Substantive Changes of Tendencies, Sci. Educ., 83, 423–447.
- Dhindsa H. S. and Treagust D. F., (2014), Prospective pedagogy for teaching chemical bonding for smart and sustainable learning, Chem. Educ. Res. Pract., 15, 435–446.
- diSessa A. A., (2007), An Interactional Analysis of Clinical Interviewing, Cogn. Instr., 25(4), 523–565.
- diSessa A. A. and Sherin B. L., (1998), What Changes in conceptual change? Int. J. Sci. Educ., 20(10), 1155–1191.
- diSessa A. A., Gillespie N. M. and Esterly J. B., (2004), Coherence versus fragmentation in the development of the concept of force, Cognit. Sci., 28, 843–900.
- Elo S. and Kyngäs H., (2008), The qualitative content analysis process, J. Adv. Nurs., 62, 107–115.
- Gilbert J. K., (2004), Models and Modelling: Routes to More Authentic Science Education, Int. J. Sci. Math. Educ., 2, 115–130.
- Gilman J. J., (1999), In Defense of the Metallic Bond, J. Chem. Educ., 76(10), 1330–1331.
- Gonthier J. F., Steinmann S. N., Wodrich M. D. and Corminboeuf C., (2012), Quantification of “fuzzy” chemical concepts: a computational perspective, Chem. Soc. Rev., 41, 4671–4687.
- Harrison A. G. and Treagust D. F., (1996), Secondary Students' Mental Models of Atoms and Molecules: Implications for Teaching Chemistry, Sci. Educ., 80(5), 509–534.
- Hilton A. and Nichols K., (2011), Representational classroom practices that contribute to students’ conceptual and representational understanding of chemical bonding, Int. J. Sci. Educ., 33(16), 2215–2246.
- Ikonen M., Tuomisto M., Termonen M. and Perkkalainen P., (2009), ILMIÖ, kemian oppikirja 7-9. Tammi, Helsinki.
- Jensen W. B., (2009), The Origin of the Metallic Bond, J. Chem. Educ., 86(3), 278–279.
- Karpin T., Juuti K. and Lavonen J., (2014), Learning to apply models of materials while explaining their properties, Res. Sci. Technol. Educ., 32(3), 340–351.
- Khriachtchev L., Pettersson M., Runeberg N., Lundell J. and Räsänen M., (2000), A stable argon compound, Nature, 406, 874–876.
- Koponen I. T. and Huttunen L., (2013), Concept Development in Learning Physics: The Case of Electric Current and Voltage Revisited, Sci. Educ., 22, 2227–2254.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Krajcik J., (2007), Developing a New Teaching Approach for the Chemical Bonding Concept Aligned with Current Scientific and Pedagogical Knowledge, Sci. Educ., 91, 579–603.
- Levy Nahum T., Mamlok-Naaman R. and Hofstein A., (2008), A New “Bottom-Up” Framework for Teaching Chemical Bonding, J. Chem. Educ., 85(12), 1680–1685.
- Levy Nahum T., Mamlok-Naaman R., Hofstein A. and Taber K., (2010), Teaching and Learning the Concept of Chemical Bonding, Stud. Sci. Educ., 46(2), 179–207.
- Nicoll G., (2001), A report of undergraduates' bonding misconceptions, Int. J. Sci. Educ., 23(7), 707–730.
- Oh P. S. and Oh S. J., (2011), What teachers of science need to know about models: an overview, Int. J. Sci. Educ., 33(8), 1109–1130.
- Othman J., Treagust D. F. and Chandrasegaran A. L., (2008), An Investigation into the Relationship between Students' Conceptions of the Particulate Nature of Matter and their Understanding of Chemical Bonding, Int. J. Sci. Educ., 30(11), 1531–1550.
- Özmen H., (2004), Some Student Misconceptions in Chemistry: A Literature Review of Chemical Bonding, J. Sci. Educ. Technol., 13(2), 147–159.
- Peterson R. F. and Treagust D. F., (1989), Development and application of a diagnostic instrument to evaluate grade-11 and -12 students' concepts of covalent bonding and structure following a course of instruction, J. Res. Sci. Teach., 26(4), 301–314.
- Piaget J., (1988), Lapsi maailmansa rakentajana, Helsinki: WSOY.
- Russ R. S., Lee V. R. and Sherin B. L., (2012), Framing in cognitive clinical interviews about intuitive science knowledge: dynamic student understandings of the discourse interaction, Sci. Educ., 96(4), 573–599.
- Schmidt H-J., Kaufmann B. and Treagust D. F., (2009), Students' understanding of boiling points and intermolecular forces, Chem. Educ. Res. Pract., 10, 265–272.
- Sproul G., (2001), Electronegativity and Bond Type: Predicting Bond Type, J. Chem. Educ., 78(3), 387–390.
- Taber K. S., (1998), The sharing-out of nuclear attraction: or ‘I Can’t think about Physics in Chemistry’, Int. J. Sci. Educ., 20, 1001–1014.
- Taber K. S., (2000a), Multiple frameworks? Evidence of manifold conceptions in individual cognitive structure, Int. J. Sci. Educ., 22(4), 399–417.
- Taber K. S., (2000b), Case studies and generalizability: grounded theory and research in science education, Int. J. Sci. Educ., 22(5), 469–487.
- Taber K. S., (2001a), Shifting sands: a case study of conceptual development as competition between alternative conceptions, Int. J. Sci. Educ., 23(7), 731–753.
- Taber K. S., (2001b), Building the structural concepts of chemistry: some considerations from educational research, Chem. Educ.: Res. Pract. Eur., 2, 123–158.
- Taber K. S., (2002), Chemical misconceptions – prevention, diagnosis and cure. Volume I: theoretical background, London: Royal Society of Chemistry.
- Taber K. S., (2003), Lost without trace or not brought to mind? – a case study of remembering and forgetting of college science, Chem. Educ. Res. Pract., 4, 249–277.
- Taber K. (ed.), (2012), Teaching Secondary Chemistry. ASE Science Practice. London: Hodder Education.
- Taber K. S., (2014a), The significance of implicit knowledge in teaching and learning chemistry, Chem. Educ. Res. Pract., 15, 447–461.
- Taber K. S., (2014b), Ethical considerations of chemistry education research involving ‘human subjects’, Chem. Educ. Res. Pract., 15, 109–113.
- Taber K. and Coll R. K., (2002), Bonding, in Gilbert J. K., de Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical Education: Towards Research-based Practice, Kluwer Academic Publishers, pp. 213–234.
- Taber K. S. and Tan K. C. D., (2011), The insidious nature of ‘hard core’ alternative conceptions: implications for the constructivist research programme of patterns in high school students' and pre-service teachers' thinking about ionisation energy, Int. J. Sci. Educ., 33(2), 259–297.
- Talanquer V., (2007), Explanations and Teleology in Chemistry Education. Int. J. Sci. Educ., 29(7), 853–870.
- Thagard P., (1992), Conceptual Revolutions, NJ: Princeton University Press.
- Treagust D. F. and Duit R., (2008), Conceptual change: a discussion of theoretical, methodological and practical challenges for science education, Cult. Stud. Sci. Educ., 3, 297–328.
- Tsaparlis G., (1997), Atomic Orbitals, Molecular Orbitals and Related Concepts: Conceptual Difficulties among Chemistry Students, Res. Sci. Educ., 27(2), 271–287.
- Ünal S., Çalik M., Ayas A. and Coll R. K., (2006), A review of chemical bonding studies: Needs, aims, methods of exploring students' conceptions, general knowledge claims and students' alternative conceptions, Res. Sci. Technol. Educ., 24(2), 141–172.
- Wang C-Y. and Barrow L. H., (2013), Exploring conceptual frameworks of models of atomic structures and periodic variations, chemical bonding, and molecular shape and polarity: a comparison of undergraduate general chemistry students with high and low levels of content knowledge, Chem. Educ. Res. Pract., 14, 130–146.
- Woicik J. C., Nelson E. J., Kronik L., Jain M., Chelikowsky J. R., Heskett D., Berman L. E. and Herman G. S., (2002), Hybridization and Bond-Orbital Components in Site-Specific X-Ray Photoelectron Spectra of Rutile TiO2, Phys. Rev. Lett., 89, 1–4.
- Yates J., Bessman M., Dunne M., Jertson D., Sly K. and Wendelboe B., (1988), Are conceptions of motion based on naïve theory or on prototypes? Cognition, 29, 251–275.
- Yayon M., Mamlok-Naaman R. and Fortus D., (2012), Characterizing and representing student's conceptual knowledge of chemical bonding, Chem. Educ. Res. Pract., 13, 248–267.
| This journal is © The Royal Society of Chemistry 2015 |