Fostering spatial skill acquisition by general chemistry students
Deborah
Carlisle
*a,
Julian
Tyson
b and
Martina
Nieswandt
c
aDepartment of Education, University of Massachusetts, Amherst, Massachusetts 01003, USA. E-mail: dcarlisle@educ.umass.edu
bDepartment of Chemistry, University of Massachusetts, Amherst, Massachusetts 01003, USA
cDepartment of Teacher Education and Curriculum Studies, University of Massachusetts, Amherst, Massachusetts 01003, USA
First published on 10th March 2015
Abstract
The study of chemistry requires the understanding and use of spatial relationships, which can be challenging for many students. Prior research has shown that there is a need to develop students' spatial reasoning skills. To that end, this study implemented guided activities designed to strengthen students' spatial skills, with the aim of improving their understanding of spatial relationships that exist within and between molecules. Undergraduate STEM majors, taking the second semester of a two-semester general chemistry course, engaged in these activities. This study followed a quasi-experimental design, in which the experimental (n = 209) and the control group (n = 212) were administered a pre-test. At the completion of the semester, both groups participated in a post-test designed to measure spatial skill acquisition. A one-way ANOVA confirmed that student performance differed significantly between the three interventions and the control group. Students who completed three activities scored higher than those who completed only two, suggesting continuity is an important feature of spatial training. In particular, our results show improvement in three skill areas: symmetry plane identification, visualization of molecules and translation between 2D and 3D representations. The results of this study show that spatial skills can be successfully enhanced through the use of relevant guided activities designed to improve student understanding of external representations. Our findings show that the use of activities which require students to sketch molecular models from different perspectives, locate a plane of symmetry on a 3D model and on a 2D sketch of the same molecule, as well as position physical models to match 2D sketches containing dash/wedge cues is an effective approach to the teaching and learning of spatial skills in general chemistry.
Introduction
It has been widely recognized that spatial ability is an important contributor to the successful learning of scientific principles in chemistry. The teaching and learning of chemistry is orchestrated through a variety of molecular representations, reactions, and theoretical concepts that require students to reason with spatial information. Students of chemistry must be able to visualize three-dimensional molecules from a two-dimensional sketch and from a chemical formula. They must also be capable of mentally manipulating three-dimensional molecular shapes to deduce an individual molecule's functional behavior, and to consider molecular interactions within a collection of molecules. For example, an understanding of molecules in three-dimensional space is critical for foundational concepts such as valence shell electron pair repulsion (VSEPR) theory, polarity, and inter-particulate attractions. Further spatial knowledge is required as novice students continue on to more advanced chemistry coursework where they learn R and S configurations of organic compounds, stereo-selective mechanisms, crystal structures and group theory applications. Yet the details of spatial properties are often not explicitly taught to chemistry students (Ramadas, 2009; Sorby, 2009; Harle and Towns, 2011). Previous studies have pointed out that without instruction, students interpretation of spatial properties are based on their own assumptions, leading to misconceptions or incomplete understanding (Wu and Shah, 2004; Gilbert, 2005; Ramadas, 2009). Additionally, spatial information is often implied, because it is embedded in abstract content that is complex, yet oversimplified for a variety of reasons; one being the need to move through material “quickly,” and another being the simple fact that spatial skills are not adequately recognized as an area that requires teaching. All too often there is an assumption students will “pick up” the necessary spatial information by observing or visualizing during lessons (Ramadas, 2009; Hegarty, 2012).Given general chemistry's central role in the undergraduate STEM curriculum it is important to consider the implications of spatial training more broadly, recognizing it's significance within all STEM disciplines as well as to the selection of a STEM career (Ferguson et al., 2008; Ramadas, 2009; Sorby, 2009; Wai et al., 2009; NSB, 2010; PCAST, 2010). Research has shown that spatial training may be enough to significantly “boost” achievement in STEM disciplines by helping students persist in early challenging course work (Uttal and Cohen, 2012). Therefore, spatial training experienced in general chemistry courses will assist students regardless of whether they pursue chemistry at the more advanced levels.
The purpose of this study was to examine the outcomes of engaging students taking the second semester of a two-semester general chemistry course in activities designed to strengthen their spatial reasoning skills. Two research questions were addressed: (1) How effective was the spatial intervention in supporting the acquisition of students spatial reasoning skills? (2) What can be learned from this study regarding curriculum development for spatial skill acquisition in general chemistry courses? The premise of this research is that the acquisition and development of spatial skills may benefit from the use of guided activities, designed to promote the acquisition of skills appropriate for the understanding of spatial relationships within and between molecular structures.
The elusive nature of spatial ability
As described by Carroll, spatial abilities “have to do with individuals' abilities in searching the visual field, apprehending the forms, shapes and positions of objects as visually perceived, forming mental representations of those forms, shapes and positions, and manipulating such representations mentally” (Carroll, 1993). Considerable effort has been made to study and identify the factors involved in spatial abilities (Lohman, 1979; Carroll, 1993; Wai et al., 2009). In a review of the spatial ability literature and its connection to chemistry Harle and Towns (2011) cite two foundational meta-analytic studies carried out by Lohman and Carroll, in which major and minor factors of spatial ability were identified. Here we cite the three major factors identified by Lohman, and later supported by Carroll:(1) Spatial relations – requires the mental rotation of an object within a plane (2-D), or out of a plane (3-D)
(2) Spatial orientation – ability to imagine how an object would look from a different perspective by changing one's egocentric reference.
(3) Spatial visualization – while the most general factor is defined by complex tasks that may require movement or displacement of parts of a spatial figure relative to other parts, combining of different figures, and or multiple transformations (Yilmaz, 2009). This aspect is considered more complex than relation or orientation tasks.
The fact that spatial ability is a comprehensive construct defined by a collection of cognitive factors, and the fact that these same factors are used by cognitive scientists to explain different components of spatial ability, has lead to some confusion when determining which specific factors are important (Yilmaz, 2009; Harle and Towns, 2011). Spatial ability tests are designed to measure how individuals deal with material that is presented in space in one, two, or three dimensions, or how individuals orient themselves in space (Lohman, 1979). Some examples of spatial ability tests include: Guay's Purdue Spatial Visualizations Test (PSVT), Bodner and Guay's Purdue Visualization of Rotations Test (PVROT) (1997), and Perspective Taking/Spatial Orientations Test (Hegarty and Waller, 2004). The PSVT and PVROT are frequently used in chemistry to measure the factors listed above. For example, the mental rotation of a molecule may be necessary to consider the bond angles between the atoms and this skill is related to the spatial relation factor. These factors are also used to discern similarities and differences between molecular structures as when assessing isomers, and symmetry relationships.
It is important to note that identifying what types, or understanding what types of spatial reasoning individuals employ is confounded by the fact that spatial tests do not cleanly measure all identified factors (Bodner and Guay, 1997; Harle and Towns, 2011), because, specific features of spatial ability may overlap in cognitive processes making it challenging to determine specific strategies used by students (Harle and Towns, 2011; Stieff et al., 2012). Regardless of the elusive nature and complexity of spatial reasoning, there is much that can be done to improve student learning, simply by building upon the recognized facets. Several studies have shown that spatial skills can be improved with practice and are retained to have long-term effects on student learning (Ferguson et al., 2008; Terlecki et al., 2008; Sorby, 2009). Such studies have specific implications for the teaching of chemistry (Mohler, 2008; Terlecki et al., 2008; Harle and Towns, 2011).
Visuospatial skills and chemistry
Visuospatial skills are used frequently in everyday life when we seek to reason with visual representations. This component of cognitive functioning allows us to interpret visual information, which includes where objects are located in space. For example, visuospatial skills allow us to recognize a square or a cube, to judge distances between objects, and to find our way across a familiar campus. These skills also include recognizing shades of color, light and dark areas, and identifying spatial features from the shape of a face to intersecting angles. Chemistry by its very nature involves reasoning with visual information. Chemistry instruction incorporates the use of molecular models, both virtual and hand-held, that incorporate many different visuospatial features. For example, three-dimensional computer images rely on shaded colors, light and dark regions, and for-shortened lines to make a molecular structure appear three-dimensional on a two-dimensional screen. Hand-held models show how atoms are physically connected, allowing for better 3D perception visually, which may also be augmented by tactile manipulations offering a kinesthetic feature as well. The language of chemistry also relies on a variety of structural representations and chemical formulae. All of these different external representations provide the basis for learning about molecules and their interactions. Tasks involving visuospatial reasoning are required for students to understand conceptual information that is communicated through visual modes of instruction. Thus, visuospatial abilities play a key role in the teaching and learning of chemistry (Wu and Shah, 2004; Gilbert, 2005; Ramadas, 2009).Novices enrolled in general chemistry often lack awareness of the spatial relationships within and between molecules, consequently these relationships are not able to be developed to the extent required for meaningful understanding (Wu and Shah, 2004; Kozma and Russell, 2005; Ramadas, 2009). Therefore students may not acquire the necessary background for more advanced coursework. Further support for these ideas was found in an earlier pilot study with a group of chemistry majors (Carlisle, 2013). While it is recognized that some high ability students have an innate ability to process spatial information, the majority of students require practice and guidance in order to acquire these skills (Wu and Shah, 2004; Mohler, 2008; Wai et al., 2009; Uttal and Cohen, 2012). It is also important to note that visuospatial skills are required for a larger set of skills known as representational competence proposed by Kozma and Russell (2005), where these skills are integrated with chemistry content knowledge for the purpose of generating, interpreting and evaluating representations.
Toward building skills
The skills that influence spatial reasoning ability in chemistry such as visualization, diagrammatic/representational skills, and the ability to translate between different types of representations have all been the subject of educational research (Coleman and Gotch, 1998; Schwartz and Heiser, 2005; Kozma and Russell, 2005; Stieff, 2010; Taagepera et al., 2011; Hegarty, 2012). In their review, Harle and Towns (2011) put forth specific suggestions for chemistry instruction:• Explicitly articulate 3D cues
• Provide ongoing instruction on molecular representation
• Continuously demonstrate visuospatial analytic techniques
• Provide visualization resources for students to practice spatial ability skills
Recognizing that course instruction may not address domain specific skills necessary to reason with spatial information they advise chemical educators to familiarize themselves with the current research based knowledge in this area and utilize it to strengthen their instruction (Harle and Towns, 2011). There is however, very little research to substantiate teaching methodologies that are useful in teaching these skills within the discipline of chemistry or even more broadly in science education. Our research seeks to expand our knowledge of effective methodologies by exploring the use of guided activities to support students learning to reason with content specific spatial information.
This study carried out training with external representations† through the use of guided activities that required students to view hand-held molecular models from different sight lines, visualize in their minds eye, represent 3D models, and translate between different representations of molecules. The significance of training students to use external representations is supported in the research (Kozma and Russell, 2005; Cohen and Hegarty, 2007; Hegarty, 2012). They encourage educators to carefully consider how to structure learning opportunities that support effective student use of external representations. This idea is in keeping with previous learning research by Schwartz and Heiser (2005), which emphasizes that educators cannot assume students will extract the necessary spatial information simply through visual exposure to representations and imagery. Research in cognitive science has shown that students ability to construct accurate internal representations is enhanced through training with external representations (Wu and Shah, 2004; Gilbert, 2005; Yilmaz, 2009; Eysenk and Keane, 2010), thus to improve overall spatial understanding, it makes sense to place emphasis on training that assists students in how to use, create, and interpret external representations (Wu and Shah, 2004; Mohler, 2008; Hegarty, 2012).
Skill areas
Symmetry planes
When learning about different molecular shapes it helps to have an understanding of symmetry, this may be because symmetrical patterns and shapes have been found to “cohere easily in the mind and require less time for processing” (Ramadas, 2009, p. 304). In chemistry, symmetry features of molecules assist students reasoning with early VSPER concepts, which then carry over to their understanding of polarity. Students that acquire a solid understanding of molecular geometry gain a deeper sense of how structure influences function. It is in general chemistry that students first begin to make these connections. For example, students may employ their understanding of the symmetry relationships of a tetrahedron, to decide how a dipole moment in a particular region of a molecule, would influence the intermolecular force of attraction. Much of students' reasoning with molecular shapes relies on their ability to conjure an accurate mental image of the structure as well as their ability to mentally rotate it, or imagine it from different perspectives. To find a plane of symmetry chemistry students need to be able to assess 2D and 3D molecular representations for symmetry relationships. The development of this skill requires practice with simple geometric structures, which will later enable them to assess more complex structures. The significance of students learning to identify symmetry planes was influenced by the work of Taagepera et al. (2011), whose research underscored the importance of integrating an understanding of symmetry and symmetry planes for students in introductory organic chemistry where it is helpful for conformational analysis, and the identification of chiral centers. Their research with knowledge space theory‡ clearly showed that students' ability to locate a plane of symmetry came later than expected in their knowledge structure pathway, indicating that students found it difficult to locate symmetry planes on simple organic molecules (Taagepera et al., 2011). Due to it's usefulness in understanding molecular geometry, symmetry plane analysis is recognized as a tool, or analytic strategy, which may simplify or obviate the need to use mental rotation to identify and/or compare stereo-isomers (Ramadas, 2009; Stieff et al., 2012). Symmetry continues to be of further importance in more advanced topics such as NMR spectroscopy and group theory.Visualizing molecules
For students to comprehend the particulate nature of matter, at the sub-micro level, they need to be able to visualize molecular structures and their interactions (Gabel, 1999; Johnstone, 2000). The term “to visualize” or the process of visualization in different contexts has broad meaning, here we use the term to denote both visual perception and visual imagery, as suggested by J. K. Gilbert in Visualization in Science Education (2005). The ability to visualize is recognized as an essential skill in chemistry, and much research has been devoted to finding useful ways to develop and strengthen students' abilities in this area (Wu and Shah, 2004; Gilbert, 2005; Ramadas, 2009). In fact, researchers frequently use examples from chemistry to explain the significance of these skills more broadly in science education. To learn effectively students need to be capable of visualizing what is meant by a variety of chemical and structural formulae. For example, when provided with the chemical formula for difluoromethane, CH2F2, students may be asked to draw the Lewis structure and use it to determine the corresponding VSPER geometry, reasoning from the 2D Lewis structure to the 3D geometry, requires students to visualize spatial relationships among the atoms in the molecule. Frequently students are then asked to apply their understanding to determine whether the molecule is polar. This step not only requires the visualization of the three-dimensional shape in their minds eye, it may also involve mental rotation of the structure to assess whether the polar bonds cancel one another, thus eliminating a net dipole moment on the molecule. In this example, it is their understanding of the spatial orientation of the bonded atoms that leads them to the correct answer. It is in general chemistry that students first learn to make these kinds of assessments. This is a big step for most students, because it requires them to apply their knowledge of molecular geometry to consider molecular interactions, and it represents an initial exposure to structure and function relationships.Research findings in science education show that the manipulation of molecular models adds a multimodal feature to learning about the spatial attributes of 3D shapes, while encouraging interaction and discussion, which has been shown to enhance understanding of visuospatial information (Ferguson et al., 2008; Donaghy and Saxton, 2012). Further, cognitive science has shown that touch plays an important role in the construction of mental imagery (Wesson, 2012) and thus augments the process of visualization. The ability to visualize is also enhanced through the process of sketching discussed below.
Representation
The need for chemistry students to be trained in the art of representation was emphasized by Kozma and Russell (2005) and was based in part on their findings that students frequently made sketches that were incorrect, and this adversely affected their problem solving strategies. Kozma and Russell identified six general skill areas, of “representational competence” which they felt were critical for students becoming chemists (Kozma and Russell, 2005, p. 121). Of these areas we focused on two: students ability to create accurate representations, and their ability to analyze features of representations, while working across different representations. An earlier pilot study, carried out by the primary author, found that chemistry majors had little training in the construction of 2D sketches and that their understanding of sketching stemmed mainly from copying sketches made by the professor (Carlisle, 2013). Research has shown that students who are successful at solving spatial problems often make sketches and diagrams to assist them with their reasoning processes. Representations are thought to decrease cognitive load by opening working memory space, thus facilitating the process of problem solving (Kozma and Russell, 2005). Not surprisingly, research has also shown that the students who are able to make accurate representations are the ones who problem-solve correctly and through representation students strengthen their ability to visualize (Kozma and Russell, 2005; Stieff, 2007). For these reasons, practice with representation was recognized as an important skill area related to the development of students' visualization and translational processes.Translation
Chemistry students are asked to reason with a variety of different representations (e.g. Lewis structures, VSEPR shapes with dash/wedge cues, ball and stick models), and this requires them to make meaningful associations between these various representations. Here we use the term translation to mean the ability to reason from one of these representations to another. For example, consider a molecule of methanol, reasoning from a 2D VSEPR representation with dash/wedge cues to a 3D ball and stick model involves understanding the spatial meaning of dash/wedge cues, so that students may evaluate the positioning or orientation of a ball and stick model relative to that of the 2D representation on paper. Performing this kind of translation successfully allows students to gain a better sense of molecular structure and strengthens their ability to use discipline specific tools to reason with spatial information. Frequently, students do not have a physical molecular model available, and they have to reason with a 3D mental image of a 2D VSEPR representation, in this case students ability to correctly visualize the structure is an important factor, in the translation process. Research has shown that students have trouble interpreting depth cues and identifying axes and planes, which in turn affects their ability to visualize these structures, limiting their ability to translate between representations effectively (Wu and Shah, 2004). Kozma and Russell (2005) list the ability to “make connections across different representations, and transfer features of one type of representation onto those of another” as one of seven core skills that students must develop to use visual representations effectively. Further, translational reasoning may be one of the initial steps in learning to use transformational reasoning in science (Ramadas, 2009). Visual-spatial images are known to be manipulated either internally in the mind and/or externally through various representations, and cognitive science suggests that one's ability to reason across representations may be tied to the ability to transform these representations (Ramadas, 2009). Interestingly, visual-spatial transformations have been shown to be an important component in all areas of scientific discovery, therefore providing practice and training with translation skills may also serve as a precursor to the development of students ability to transform representations.Constructing spatial knowledge through experience
Realizing that students in large undergraduate general chemistry courses have opportunities to develop their spatial understanding in lecture, often through direct instruction, and on homework assignments using their textbook and on-line resources, this study sought to provide further opportunities for students to construct their spatial knowledge based on meaningful hands-on activities. This approach was influenced by constructivist teaching practices that “help learners to internalize and reshape, new information” (Brooks and Brooks, 1999, p. 15) leading to a meaningful form of learning that is likely to be recalled and transferred to new situations. What students think is important, and interesting is influenced by what they already know, thus making associations to prior knowledge allows students to store information in their LTM networks in such a way that it is more easily retrieved. Constructivist learning theories place emphasis on understanding students prior knowledge, and encourage the use of methodologies that make connections to student's prior knowledge, while at the same time drawing out alternative conceptions (Brooks and Brooks, 1999). Strengthening spatial reasoning ability requires that students build the necessary memory associations, allowing them to use spatial information appropriately. In our study, skill development took place through activities that encouraged students to create, analyze, and compare molecular structures, rather than as a set of helpful facts that explain important spatial features of molecules. These activities were also designed to guide students such that they would discover the relevance of these skills as they worked to answer the guiding questions.Rationale
The purpose of this study was to engage students in chemistry-specific guided activities designed to strengthen their spatial reasoning skills. The goal of these activities was to help general chemistry students strengthen their understanding of 3D molecular structures. General chemistry plays a central role in the undergraduate curriculum at our university, and we felt this would be an appropriate place to provide training with spatial skills that have been identified as beneficial for chemistry learning (Wu and Shah, 2004; Harle and Towns, 2011; Hegarty, 2012; Carlisle, 2013). Further, we wanted to support students initial experience reasoning with spatial concepts within the discipline, as this is where the majority of students would benefit the most from skill training. Given the fact that all students in this study were STEM majors, we felt they all would benefit from engaging in spatial skill training regardless of whether they continued on with more advanced chemistry coursework.Research design
This study used a quasi-experimental design, in which a pre-test was used to establish the equivalency of the experimental and control groups. Following the intervention, both groups participated in a post-test, which was used to assess their performance on chemistry questions requiring spatial knowledge. The quasi-experimental design is commonly used in educational research where intact classes must be assigned to a condition (Gall et al., 2008). In this study, the same professor taught two sections of the same general chemistry course, these two sections were randomly assigned to the experimental and control treatments, however, as individual students were not randomly assigned to each treatment this is a quasi-experiment (Gall et al., 2008). The professor randomly assigned his first section of the day, Section 2, as the experimental group and his second section, Section 3, as the control group. Random assignment was accomplished by writing the section numbers down on a slip of paper and performing a draw to determine the section to be assigned to the intervention. The assignment to condition was done prior to the start of the semester, with no knowledge of the students themselves or their performance on spatial tasks. The experimental group voluntarily participated in three spatial activities during their laboratory period. The control group did not participate in any of the activities. Both groups studied the same units during lecture and performed the same laboratory experiments. The spatial activities were connected to relevant general chemistry topics covered in this course, including polar molecules, intermolecular forces, and solutions. The study was conducted in the spring term so that students could revisit and apply their basic knowledge of VSEPR geometries, learned in the fall, while considering how molecular structure affects behavior in these content areas. Three intervention activities were administered to students in the experimental group during their lab periods and were spaced evenly over the semester. A post-test was administered two weeks after the last intervention activity. The instructor administered the pre and post-tests, while the researcher (DC) carried out the spatial intervention activities during the laboratory periods to provide a consistent approach and similar support for each group of students. While informed by the relevant literature it should be noted that the guided activities carried out in our study specifically addressed skill areas identified in an earlier year-long pilot study carried out by the primary author at the same university, with a group of chemistry majors enrolled in organic chemistry (Carlisle, 2013).Intervention activities
The focus of the intervention was to provide students with engaging activities that would help them reason with spatial information required within the discipline of chemistry. These activities were designed to provide students with experiences that would enhance their ability to use and create external representations of molecules, as well as improve their ability to form accurate mental images of common molecular structures. According to the literature (Schwartz and Heiser, 2005; Ramadas, 2009; Hegarty, 2012) this is an important area in which to train our students. Activities were designed such that students would be able to construct their own knowledge through participation in guided activities and discussion with their peers (Brooks and Brooks, 1999). The intervention activities were administered monthly during the students' laboratory periods for a total of three interventions (February, March, and April). As this was a large lecture course, student's laboratory period provided the most effective environment for skill training. The activities were carried out in self-selected groups of 2–4 students, and took 15–20 minutes to complete. These activities were developed and piloted with a group of chemistry majors (n = 30) at the beginning of their organic chemistry course at the same university (Carlisle, 2013). We chose to pilot with this group to discover whether the activities were engaging for students vested in the discipline (i.e. students who had self-identified with chemistry by choosing it as their major) or whether students considered them to be review of prior learning. The rationale being that if chemistry majors found them helpful they would be beneficial to learn, establishing credibility for the activities. On the other hand, if they found them easy and intuitive, perhaps students had already acquired these skills in their general chemistry coursework. Further, these students had just completed the general chemistry course for which we had designed the activities.Following brief initial instruction by the researcher (DC), students carried out the activities independently, with the researcher present to answer questions and observe student participation. Students were at liberty to choose to participate in any or all intervention activities, and thus created three groups, allowing information to be gathered on how many activities were necessary for students to improve their spatial skills in these areas.
The intervention activities were administered in the form of a worksheet with guiding questions that required students to:
• Interpret sketches containing dash/wedge cues
• View molecular models from different sight lines
• Sketch using dash/wedge notation from these different perspectives
• Compare and contrast molecular models in different orientations
• Determine whether 2D sketches of molecular structures were identical, both with and without the use of hand-held molecular models
• Locate symmetry planes
• Consider intermolecular interactions using molecular models
• Translate between 3D molecular models and 2D sketches with dash/wedge notation
Examples of the intervention questions are provided in Appendix I.
The guiding questions were structured to develop each of the spatial skills highlighted previously.
(1) Symmetry plane identification: students analyzed 2D VSEPR shapes and 3D molecular models for symmetry, which provided practice and allowed them to become more skilled in finding a symmetry plane on a 3D model, and in imagining one on the 2D representation of the molecule. The identification of symmetry planes provided students with a reason to assess structures from different perspectives, which instinctively augmented their spatial understanding.
(2) Visualization: physical molecular models were used to assist students' ability to visualize common molecular shapes in the following ways:
• Perspective taking
• Handling/Kinesthetic use of molecular models
• Representation of molecular models
Viewing molecular models from different sight lines, involves perspective taking, which allowed students to gain experience with three-dimensionality in a concrete manner. Perspective taking also encourages the retention of a mental image of each view to assist in the relative comparison of atom positioning and overall molecular orientation. The kinesthetic aspects of molecular models have been shown to support visual perception and enhance understanding of three-dimensionality (Coleman and Gotch, 1998; Ferguson et al., 2008; Wesson, 2012).
(3) Representation: familiarity of spatial relationships was established through the process of making 2D sketches with dash/wedge cues. The making of 2D sketches created a need to critically consider how to represent the spatial information that was apparent in a 3D molecular model, and it is through this interpretation that visual understanding may be improved.
(4) Translation: the guided activities required students to work back and forth between 2D sketches containing dash/wedge cues and 3D ball and stick molecular models to provide practice and experience.
Note: the interpretation required for representation is in fact one type of translation, the other type would be positioning (matching) a molecular model to the relative orientation shown in a 2D sketch.
All intervention activities were related to relevant course content, including the topics of polarity, intermolecular forces, and solution chemistry. A more detailed description for each intervention activity is summarized in Table 1. While answering questions, students shared their thinking and visualization processes with each other.
| Intervention | Content | Skills |
|---|---|---|
| 1 | Perspective taking, sketching a physical molecular model from different views using dash/wedge, identify/locate symmetry planes using a molecular model, relationship of symmetry to polarity, compare and contrast molecular shapes |
1. Visualizing from different perspectives, understanding view is relative.
2. Sketching: practice with representation and translation 3. Identifying symmetry planes 4. Observe common features of molecules |
| 2 |
Sketch from a physical molecular model using dash/wedge, identify symmetry planes, determine whether molecules are the same using
(1) 2D sketches with dash/wedge cues (2) Physical molecular models |
1. Sketching molecules
2. Translating spatial information from 3D to 2D, and visa versa 3. Identifying symmetry planes 4. Identifying similar molecules in 2D and 3D 5. Performing molecular rotations both mentally and physically |
| 3 |
Position physical models to match 2D sketch with dash/wedge, rotate molecular models around imaginary x, y axes, sketch physical molecular models incorporating dash/wedge, position models as though interacting, determine whether molecules are the same using
(1) 2D sketches with dash/wedge cues (2) Physical molecular models |
1. Visualizing molecular orientations and molecular interactions 2. Performing molecular rotations both mentally and physically 3. Sketching molecules 4. Identifying similar molecules in 2D and 3D 5. Efficient comparison strategies |
While sketching was not measured on the post-test it was an important piece of the intervention activities. Templates and dot matrix paper were provided to support the sketching process (Sorby, 2009).
Participants
The numbers of students participating in the intervention activities and in the post-test are given in Table 2. Students did not receive credit or other reward for participating in the study. The experimental group participated in the intervention activities on a volunteer basis. All research was approved by the internal review board of the university and followed institutional guidelines. Each participant signed an informed consent form prior to participating in the intervention activities.| Female | Male | Total | |
|---|---|---|---|
| *Note 6 students in the experimental group did not report their gender information and 16 did not participate in the study. | |||
| Post-test | 138 | 65 | 209 |
| 1 Intervention | 20 | 12 | 32 |
| 2 Interventions | 38 | 12 | 50 |
| 3 Interventions | 68 | 37 | 105 |
Of note is the fact that the experimental group contained about twice as many females (138) as male (65) students, while the control group contained 110 female and 95 male students. The professor was unaware of these gender differences at the time the groups were randomly assigned to the conditions. Laboratory sections contained up to 16 students and met every other week for at total of five times per semester. The same male professor with 7 years of teaching experience taught both the control and experimental groups.
Pre- and post-tests
To assess the impact of the intervention activities on students' ability to reason with spatial information both a pre and a post-test were designed by the researcher (DC). These tests were developed out of the necessity to probe students understanding of general chemistry content that required them to reason with spatial relationships within and between molecules. To the best of our knowledge there are no tests currently available for this purpose. Due to class time constraints, both tests were comprised of 21 multiple-choice questions and designed for completion within a 25 minute time frame. Both of the pre and post-tests are provided in appendices III and IV, and a summary table of the questions from each test is provided in Appendix II.The pre-test contained six questions that were randomly selected from the Purdue Spatial Visualization Test: Rotations (PSVT:R) developed by Guay (1977), to assist in establishing homogeneity between groups with an established measure. The PSVT is thought to be the best cognitive measure of spatial ability, because it is the least influenced by analytic techniques (Bodner and Guay, 1997). The other 15 questions were developed to probe student understanding of spatial content relevant to chemistry (Carlisle, 2014). These latter questions concerned molecular geometry based on VSEPR theory, intermolecular forces, identification of similar molecules, interpretation of the dash/wedge convention, the comparison of spatial orientations of molecules, and the identification of a symmetry plane. These areas were selected, because they made relevant connections to the spatial content within the course. Some questions required students to imagine molecular interactions in three dimensions given only a chemical formula, while others provided a picture of a 3D molecular model. These questions included two typical traditional test questions, but were primarily creative questions probing students understanding of molecular structure and 3D orientation. The pre-test was used to assess the homogeneity of the experimental and control groups with respect to their spatial reasoning ability, and establish a baseline for the intervention effectiveness. The internal consistency and reliability for the 15 newly developed items on the pre-test, is given by the reliability coefficient, Cronbach's alpha α = 0.66.
The post-test included 15 spatial questions whose content was similar to that described above for the pre-test. Examples of post-test items are shown in Fig. 2 and 4, with all questions being presented throughout the results section. Of note is the fact that we decided not to use questions from the PSVT on the post-test, due to the fact that 80% to 90% of the students in both groups correctly answered these questions on the pre-test, we felt this did not leave enough margin to measure change between the groups on the post-test. Thus further discipline specific questions were developed for the post-test. The post-test also included six likert-type questions, asking about the number of interventions performed, gender, and self-perception, while these are not the subject of this paper you may refer to Appendix IV for these post-test questions. To establish content validity, experienced chemistry teachers, including two chemistry professors at the same university, and two high school AP chemistry teachers, independently reviewed all test questions. These questions were found to be appropriate and relevant to the current subject matter covered in the course. Suggested revisions to questions included clarifying solution choices, and simplifying choices for timing purposes; these were discussed and changes made accordingly. The post-test was used to compare the performance of the experimental group to that of the control group, who did not receive the intervention activities, for the purpose of assessing the effectiveness of the intervention (Gall et al., 2008). Additional content validation of test questions was established through the use of think-aloud protocol as students responded to similar questions during interviews (Carlisle, 2014). As an example, we provide an excerpt from this study, where students were asked to answer a question similar to question 7 on the post-test, the only difference being that a chlorine atom, Cl and not a bromine atom, Br was attached in position 2. Refer to Fig. 4 or Appendix IV for this post-test question.
Student, F1 reads the question and looks at the molecular representation. “I think they are the same.”
“Can you explain why you think so?” (Interviewer)
“Yes, basically I imagine taking the molecule on the right and flip it over to the left. This helps me to imagine what it would look like laid on-top of the molecule on the left.” (Student, F1)
“And how does this tell you that they are the same?” (Interviewer)
“Because now the dash goes to being a wedge, coming out, so the molecules match.” (Student F1)
This data shows that the student visualized how the molecule would look in a new orientation, if the chlorine (or bromine) atom, which was oriented back behind the plane of the paper, comes to the front as the structure is rotated to the left. We used this data, as well as other similar student responses, to confirm that students answered question 7 on the post-test by performing a mental rotation. Similar data was used to confirm that students were responding to the questions using the intended spatial knowledge, and also led to question clarification. Clarification edits included rephrasing of questions, and changes in molecular representations to remove ambiguity in student responses.
Construct validity was established by tying the questions to cognitive factors for spatial ability based on theory proposed by Lohman (1979), while recognizing that spatial ability is a complex and comprehensive construct. The cognitive factors used to operationalize spatial ability in this study represent the three major factors generally accepted as common attributes, spatial relations (SR), spatial orientation (SO), and visualization (VZ), as defined in the literature review. The content of each question was evaluated against the operational definitions and the intercorrelation between items was high suggesting questions were measuring the same construct. Questions related to the construct of spatial ability were developed through a pilot study, and scoring showed significant differences for these items (Carlisle, 2013). The internal consistency and reliability, of the 15 content items on the post-test, is given by the reliability coefficient, Cronbach's alpha α = 0.75. The summary table, shown in Appendix II, lists each test question, the cognitive factor and associated spatial skill.
The same pre and post-test was administered to both sections on the same day with the experimental group receiving it first. Tests were announced one week prior to administration. All questions were projected in PowerPoint on large screens at the front of the lecture hall, and students responded to the multiple-choice questions via their own individual audience response devices. Rapid response times minimized the opportunity for “guess and check” and increased the chance that students answered with efficient spatial strategies. A response choice of “not sure” was also included to minimize guessing. Students were not allowed to discuss their answers, and were observed not doing so. Responses were transferred from the audience response system software to Microsoft Excel for analysis.
Results
Pre-test for homogeneity
The mean pre-test scores for the experimental and control groups were not significantly different (experimental mean = 9.91 s.d. 2.10, control mean = 9.99, s.d. 2.21) with t(561) = 0.435, p = 0.664. Both groups were confirmed to be homogeneous based on Levene's test for homogeneity of variance. The results of the six PSVT:R test questions (Bodner and Guay, 1997) for both groups were all quite high, with 80 to 90% of students answering correctly in both sections. This suggests that both groups were quite capable of mentally rotating figures, or changing their egocentric reference frame to adopt different perspectives. The results of the remaining 15 questions were very similar between groups, as revealed by the mean scores.Post-test results
The post-test scores, shown in Table 3, indicate a significant improvement for the experimental group with t(419) = 5.76, and p < 0.000. A Cohen's d value of 0.56 confirmed that the intervention had a moderate effect on the entire experimental group. This effect size needs to be considered in light of the fact that the experimental group was comprised of three groups of students each of whom participated to different extents in the intervention activities, see Table 1. The research questions can be best evaluated by comparing the results for the group of students who performed each of the intervention activities (n = 105) with those of the control group (n = 212), for which t(315) = 6.36, and p < 0.000. This group, performing three activities, had a large effect size with a Cohen's d of 0.80. These results show that the intervention activities were effective, and that the best performance was achieved by engaging in repeated structured learning activities, allowing for greater skill acquisition.| Scores | N | Gender | N | Mean | Std deviation | p value between genders | p value between groups | Effect size |
|---|---|---|---|---|---|---|---|---|
| a Note: six students in the experimental group and seven students in the control group took the post-test, but did not report their gender information. | ||||||||
| Experimental | 209 | Female | 138 | 7.23 | 2.51 | 0.346 | 0.000 | d = 0.56 |
| Male | 65 | 7.58 | 2.43 | |||||
| Control | 212 | Female | 110 | 5.51 | 2.29 | 0.004 | ||
| Male | 95 | 6.46 | 2.36 | |||||
While test score means show a statistically significant difference between the groups overall, a clearer understanding of student performance can be obtained from a comparison of correct item responses between the following two groups: the students who participated in all 3 intervention activities (n = 105) and the control group (n = 212), which are shown in Fig. 1.
As Fig. 1 shows, the experimental group performed better on most questions than the control. However, given the correct response rate was still relatively low, close to 50% for many questions, the data suggests that more than three activities are required to boost students' spatial skills to a level of solid proficiency. We also feel this underscores the importance of incorporating spatial training activities into the general chemistry curriculum.
The questions showing the greatest gains, 10% or more for the experimental group, were analyzed and grouped according to the skill focus of the interventions, see Table 4. Three skill areas appeared to transfer well to the post-test: identification of symmetry plane(s), visualization of molecules, which included comparison of molecular structures with dash/wedge cues, and translation between a 3D image and a 2D sketch with dash/wedge cues.
| a Note: each question represents an increase of 10% or more for the experimental group. The parameter of 10% was chosen on a scale of 100% suggesting the difference of approximately a grade. |
|---|

|
The questions comprising these three skill areas, where students showed the greatest improvement, are analyzed in more depth below.
Discussion
Symmetry planes
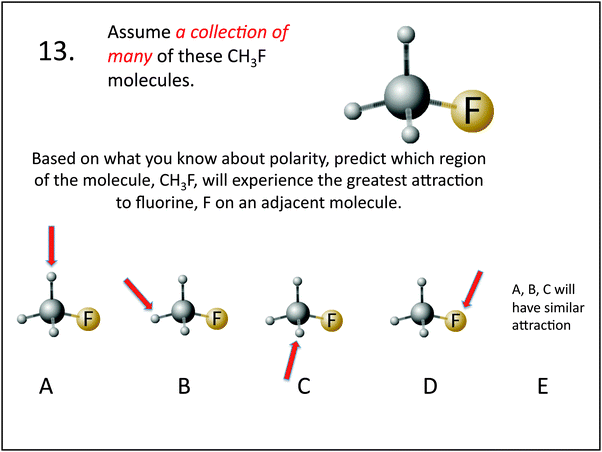
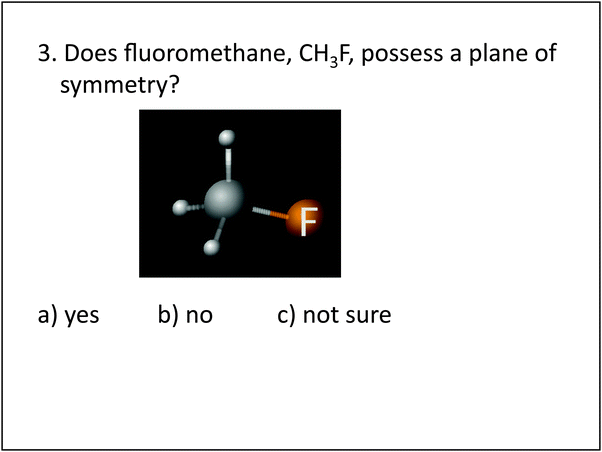
In general, a larger percentage of students in the experimental group were capable of correctly identifying molecules that contained a symmetry plane. Symmetry plane identification is a skill that was focused on during interventions 1 and 2, where students practiced locating and sketching symmetry planes on different molecules. An understanding of symmetry and symmetry planes was considered an important skill to develop for two reasons: (1) it helped students assess the 3D structure of a molecule for balance and evenness, which in turn helped them to grasp the important concept of molecular polarity; and (2) it provided students with a reference point allowing the relative in and out positions of attached atoms to become more obvious, and this assisted with sketching and dash wedge understanding. During the intervention activities, students gained first-hand experience with symmetry and symmetry planes by viewing the structures of molecular models. The intervention activities began with students exploring symmetrical molecules containing one central atom, such as methane, CH4, and progressed to the consideration of ethanol and butane shown in intervention two, question 2, see Appendix I. In these activities, students practiced perspective taking by viewing stationary molecular models from different places (intervention 1), and by rotating hand-held molecular models (intervention 2), which allowed them to observe that the models looked symmetrical in some orientations while in others they did not. Additionally, students were asked to position molecular models along a line, drawn on a piece of paper with a black sharpie. The use of this concrete line provided a reference from which to assess the relative in and out positions of atoms, assisting students in the identification of symmetrical orientations. Students also practiced imagining a place where an index card could be passed through a molecule to bisect it into two equal halves.The post-test questions pertaining to symmetry planes, are items 2, 3, and 5, see Table 4. Question 2 required students to imagine the tetrahedral shape of CCl4, in their minds eye, and then correctly identify the number of atoms within a plane of symmetry. For questions 3 and 5, see Fig. 2, students were asked whether a molecule possessed a plane of symmetry, and approximately half of the experimental group responded correctly as compared to 30% of the control group. The 3D picture provided in question 3, appeared to assist some students in the experimental group, as they scored slightly higher on question 3 than on question 5 where only the chemical formula was provided. In contrast, the control group scored exactly the same on both questions. Both groups performed better on question 2 than they did on questions 3 and 5, indicating that it was easier for them to determine the number of atoms located in a symmetry plane, for CCl4, than it was to identify a symmetry plane on a tetrahedral molecule with one substituted group (e.g. CH3F, CH3OH). One explanation for this could be that the symmetry of the CCl4 molecule makes it easier to reason with, because all sight lines are similar, and therefore the structure does not necessarily require any mental manipulation to determine the number of atoms within a plane from all directions. On the other hand, both CH3F, CH3OH require the consideration of different sight lines, achieved by either changing ones egocentric reference frame or by mentally rotating the structure, to determine whether the molecules have a plane of symmetry.
Visualizing molecules
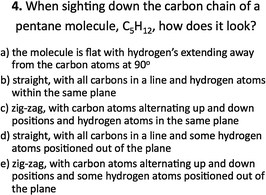
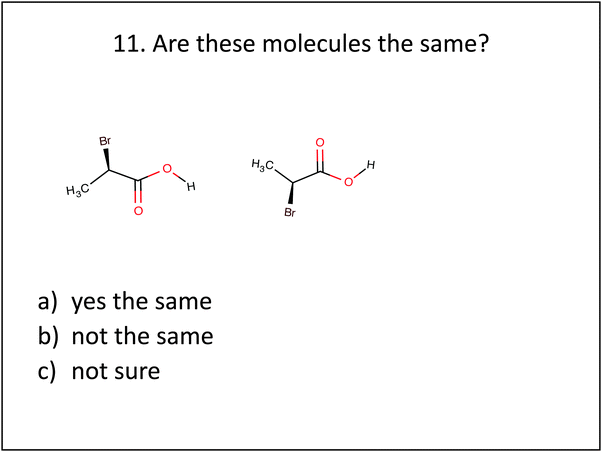
The experimental group also performed better on post-test questions requiring the visualization of molecules. For example, when students were asked to describe what they would see when looking down the carbon–carbon chain of pentane, as in question 4 shown in Fig. 3, the experimental group was more successful in correctly describing the spatial arrangement of atoms.Students in both groups were familiar with small hydrocarbon chains such as propane, butane and pentane. However, their spatial understanding of atom arrangement for these structures was tied to their visual knowledge of Lewis structures' and 3D computer images. In addition to this, the experimental group used hand-held molecular models of ethane and butane to answer questions during intervention activities 1 and 2, and this may have assisted them in creating a mental image to correctly visualize the carbon chain of pentane. Students were also asked to compare similar molecular structures, which required the visualization of molecules in different orientations, see Fig. 4. Students in the experimental group were much better at correctly identifying similar molecular structures. These students practiced comparing similar 2D molecular structures containing dash–wedge cues in both the second and third intervention activities, where they answered questions with the use of hand-held molecular models to scaffold their visualization. Successful comparisons required mental rotation of one or both of the structures and/or the ability to imagine the molecules from a different sight line by mentally changing one's egocentric reference frame. Molecular models assisted student's visualization of the mental rotation process and provided a concrete way for them to assess the new orientation of atoms attached with a dash or a wedge.
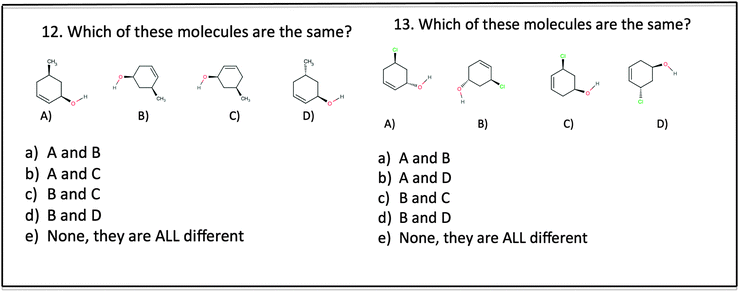
Question 7 was the most straightforward, because the carbon-to-carbon chain is oriented in the same manner, making an M, which allows the structures to be easily compared. The red double-bonded oxygen atom helps to draw the eye to similarities in structure and orientation. By contrast, question 11 required either a rotation up or down around the x-axis to orient the carbon-to-carbon chain in the same direction, before students could compare structures. Questions where students needed to employ spatial skills requiring visualization and comparisons of molecular structures showed some of the greatest difference (over 25%) between the experimental and control groups, see Fig. 1.
Although cyclic structures were not part of the intervention activities, the experimental group also performed better when asked to compare cyclic molecular structures, see Fig. 5. To answer question 12 only one rotation was required, either clockwise or counter clockwise within the plane of the paper, and a little more than half (55%) of the experimental group selected the correct response. Neither group did well on question 13, which required two rotations, one out of the plane and another clockwise or counter clockwise within the plane. These results suggest that students require substantial training with challenging questions similar to 13, where more than one rotation is required to match molecular structures.
Translation
Translation was an important area of focus in this study, because an earlier pilot study demonstrated that students had difficulty interpreting the spatial information provided in a 2D sketch, and therefore could not use this information to reason with (Carlisle, 2013). During each of the three intervention activities students practiced interpreting the spatial information in 2D sketches containing dash/wedge cues with the assistance of molecular models. Questions were structured such that students had to reason back and forth between a 2D sketch containing dash/wedge cues and a physical molecular model to interpret the necessary information. While this was an important skill development area, it was difficult to assess with the multiple-choice post-test format, primarily because true 3D molecular models were not available for student use. During the post-test, students were asked to reason with the pictures of molecular models shown in Fig. 6, and while these provided some 3D information and possible priming, they were presented in 2D. However, we felt these questions did probe students spatial knowledge of common bond angles as well as the use of dash/wedge notation to represent different spatial arrangements.The experimental group performed better on questions that required the association of spatial information provided in a picture of a 3D molecule to a 2D sketch with dash/wedge cues. The post-test results show that about half of the students in the experimental group were able to interpret the information provided by a picture of a 3D molecular model, as compared to about one-third of the control group.
Similar performance
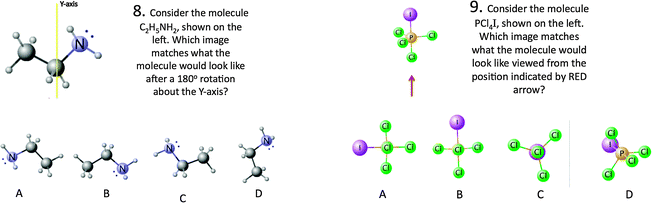
Both groups performed approximately the same on questions 1, 6, 8, 9, analysis of these questions suggests that they may have lent themselves to memorization, use of heuristic, and/or simply require further practice. It appears that roughly 50% of students in the experimental and control groups require assistance on questions 1 and 6, see Fig. 7, which require an understanding of polar molecules and VSEPR Theory. Students may have selected the correct answers to questions 1 and 6, by applying heuristic thinking, which involves using learned strategies as shortcuts, rather than using knowledge of geometric structure. For example, questions 1 and 6 on the post-test could be answered by assessing the Lewis Structures and recognizing the pattern of 3 bonding pairs and one lone pair around the central atom(s), indicating polarity and a pyramidal structure that is not planar/flat. It is also possible that students answered these questions through memorization. However, these questions were included, because they are representative of traditional assessment questions used on exams.Questions 8 and 9, shown in Fig. 8, are interesting to consider for two reasons, both the control and the experimental groups' performance was about the same, and both groups scored above 70%. In considering why both groups did so well on these questions, we felt there were 2 important features: (1) both questions provided pictures of 3D ball and stick molecules to support student visualization and (2) both questions included a reference to assist students in where to look on the molecule.
In contrast, both groups had their lowest performance on question 10, shown in Fig. 9. This question provided a picture of a 3D ball and stick model to assist students with the spatial arrangement of atoms and lone pairs on the methanol structure. Question 10 was intended to probe students spatial understanding of how many water molecules could arrange themselves, in 3D, around one methanol molecule. These results show that both groups of students require more focused training in the area of intermolecular forces and hydrogen bonding. The experimental group only had one activity during the third intervention that focused on hydrogen bonding. In this activity student groups were asked to arrange molecular models to mimic hydrogen-bonding interactions between ethanol and water, and this did not appear to be enough practice to assist students in answering this question.
Interventions
The results show that students who completed three activities outperformed those who completed only one or two, see Table 5, providing evidence that each additional activity strengthened students' skills. A one-way ANOVA was used to test for differences among the three spatial interventions and the control group. Student performance differed significantly across the three interventions and the control group F(3.410) = 15.29, p = 0.000. Tukey post hoc comparisons of the four groups indicate that both students in the second intervention (M = 7.34, 95% CI[6.59, 8.08]) and in the third intervention (M = 7.74, 95% CI[7.27, 8.21]) had significantly higher performance than that of the control group (M = 5.89, 95% CI[5.61, 6.24]), p = 0.001. While each group showed improvement in their mean score, the 105 students performing all three activities showed a 2-point improvement over the control group (7.74 vs. 5.84).| Participants | Total | Mean | Standard deviation | Female | Male | ||
|---|---|---|---|---|---|---|---|
| Experimental: number of interventions | N | N | Mean | N | Mean | ||
| a Note: six students in the experimental group and seven students in the control group took the post-test, but did not report their gender information. | |||||||
| 1 | 32 | 6.75 | 2.05 | 20 | 7.05 | 12 | 6.25 |
| 2 | 50 | 7.34 | 2.60 | 38 | 7.21 | 12 | 7.33 |
| 3 | 105 | 7.74 | 2.46 | 68 | 7.48 | 37 | 8.22 |
| p = 0.35 | p = 0.041 | ||||||
| Control | 212 | 5.84 | 2.38 | 110 | 5.51 | 95 | 6.46 |
Conclusions
There is evidence that students are inadequately prepared in terms of their spatial skills for entry into an organic chemistry course sequence (Stieff, 2007; Harle and Towns, 2011; Taagepera et al., 2011; Carlisle, 2013), and as it is clear that the acquisition of spatial skills is important for student success in chemistry (Wu and Shah, 2004; Gilbert, 2005; Kozma and Russell, 2005; Terlecki et al., 2008; Stieff et al., 2012), this study implemented guided activities as a step towards improving student understanding of the spatial relationships that exist between bonded atoms and among molecules. The post-test results show that the spatial skills of general chemistry students in a large lecture section, containing a broad range of STEM majors, improved. These findings indicate that three short activities performed approximately every four weeks during the semester, strengthened students abilities to reason with spatial information. In particular, our results indicate that students improved their ability to identify symmetry planes, visualize in 3D, mentally rotate molecules, and translate spatial information between 2D representations and 3D models. Earlier studies have highlighted the significance of training chemistry students in these areas (Wu and Shah, 2004; Cohen and Hegarty, 2007; Ramadas, 2009; Harle and Towns, 2011; Taagepera, et al., 2011).As chemical educators we utilize many visual modes of instruction to expand on our students spatial understanding, such as 2D sketches, computer generated images, and hand-held molecular models, and it is important to realize that students require assistance in learning how to interpret these representations in order to build solid foundational knowledge (Schwartz and Heiser, 2005; Gilbert, 2005). Teaching students how to reason with these external visual-spatial representations has been recognized as an essential component of spatial training (Cohen and Hegarty, 2007; Hegarty, 2012). In our study, the intervention activities provided guidance for students, directing them to look at particular features, sketch models from different perspectives, perform specific rotations, and position physical models to match 2D sketches with dash/wedge cues, tasks which require critical consideration of the spatial information embedded in 2D sketches and 3D models. These spatial activities bring to life the particulate nature of matter, and therefore have the potential to enhance student understanding of submicroscopic properties, properties of chemicals that cannot be seen, by making them more tangible (Gabel, 1999; Johnstone, 2000). The results show that engaging in these activities strengthened students' ability to reason with external representations effectively. Therefore, we suggest that chemical educators integrate some of these activities into their instruction. Our results also suggest that educators do not need to devote a large amount of class time to strengthen students skills, as we found short, guided activities to be effective. This study showed that students who engaged in multiple learning opportunities performed better, with each additional intervention. However, our results also show that further skill development is necessary. The design of curricular materials to support student learning in this area, together with the development of tests to assess the acquisition of discipline specific spatial skills for general chemistry are on-going research topics (Carlisle, 2014).
Our study contributes to the existing research by providing additional evidence that spatial skills are indeed malleable. It also strengthens the case for the utility of spatial training by responding to the need for longer studies, lasting for a semester or more, with an adequate control group to support the effectiveness of spatial training (Uttal and Cohen, 2012). This study also makes pre and post-test resources available, and as there are no validated tests yet available to assess students' spatial knowledge related to general chemistry content, these may contribute to the design of suitable spatial tests. Additionally, some questions may prove useful for assessment in other undergraduate general chemistry courses.
Overall, there is much interest in supporting students' spatial understanding in chemistry (Wu and Shah, 2004; Kozma and Russell, 2005; Terlecki et al., 2008; Stieff et al., 2012), and while a few lessons have been developed that incorporate activities (Taagepera et al., 2011; Donaghy and Saxton, 2012) future studies should continue to explore various learning progressions for the spatial knowledge required in chemistry. These studies should specifically consider how to best enhance student understanding through skill training in these areas over time.
Limitations
This study was limited by the number of activities students chose to participate in, as well as the amount of laboratory time the course instructor was able to devote to the study. The fact that students could choose to participate throughout the course of the term meant that there was inconsistent practice, while the activities were designed to cumulatively strengthen students' skills. It is also possible that this may have led to differential selection (Gall et al., 2008), because students choosing to participate in all three activities may have done so for reasons that may have influenced the outcome. For example, students may have participated in all three interventions because they enjoyed the activities, felt they were important, and were particularly motivated individuals. Having enthusiastic and motivated participants could have affected the overall results of the study, and it would be beneficial to carry out future studies where all students are required to participate in each intervention activity.Two other limitations of this study relate to the assessment. First, students' skill acquisition was assessed through the use of multiple-choice questions, which were not an authentic means for measuring student learning in some of the skill areas. However, this method allowed us to gather data on two relatively large lecture sections of general chemistry, which was useful for an overall evaluation our activities. It is quite likely that students learned more from the intervention activities than is accounted for in the assessment. To date, we have captured student learning in these areas with qualitative methods and these results will also be used to further refine future intervention activities. Secondly, we note that increasing the number of test items associated with some of the skills could strengthen the reliability of the pre and post-tests. Future test revisions will take this into consideration and increase the number of items related to each skill accordingly. The length of each pre and post-test was restricted to 25 minutes of lecture time, which contributed to this limitation.
Appendix I
Sample intervention questions provided to students
(d) Holding your molecule of dichloromethane, CH2Cl2, rotate it to inspect it from different angles. What is the maximum number of atoms that can lie within a plane at any one time?
(a) Take turns holding and rotating these molecules. Are these molecules symmetrical?
(b) Can you find a plane of symmetry for both molecules?
(c) How do you think symmetry affects polarity? Use your molecular models to help explain. You may make a sketch to support your answer
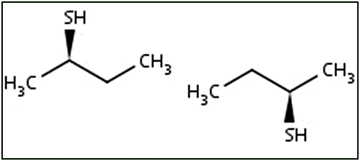
(b) Next you will view these structures in 3D. Move to the laboratory bench with the molecular models of these structures (named 2-butanethiol). Look carefully at these structures, without manipulating them. Are you able to confirm your answer in part A? Explain.
(c) Now you will use the molecular models to see if these structures are alike. Select ONE of these molecules and try to reposition it to match the other structure. Are they alike? Explain.
Appendix II
Pre and post-test questions and skill summary
| Pre-test | Post-test | ||
|---|---|---|---|
| Question | Spatial skills | Question | Spatial skills |
| Note: all spatial skills may involve overlap. For example, to identify a plane of symmetry, students may need to visualize the 3D structure, mentally rotate it, picture it in a new orientation and/or translate spatial cues. The overlap of skills for spatial reasoning tasks is widely recognized and is a factor that may confound spatial ability tests (Wai et al., 2009; Harle and Towns, 2011). | |||
| 1. PSVT:R | Visualization | 1. Which of these molecules is polar? | Visualization: mental image or possible memorization or use of heuristic |
| 2. PSVT:R | Visualization | 2. What is the maximum number of atoms that can lie w/in a symmetry plane? |
Symmetry plane identification
Visualization: mental image |
| 3. PSVT:R | Visualization | 3. Does fluoromethane, CH3F, possess a plane of symmetry? |
Symmetry plane identification
Visualization: mental rotation |
| 4. PSVT:R | Visualization | 4. When sighting down the C–C chain of pentane, C5H12, how does it look? | Visualization: mental image |
| 5. PSVT:R | Visualization | 5. Does methanol, CH3OH, possess a plane of symmetry? |
Symmetry plane Identification
Visualization: mental image, or memorization |
| 6. PSVT:R | Visualization | 6. Which of these molecules is NOT flat? (VSEPR) | Visulalization: mental image or possible memorization or use of heuristic |
| 7. Paper folding | Visualization | 7. Are these molecules the same? | Visualization: mental rotation |
| 8. Rotate the molecule 180° in the clockwise direction. Which image matches what the molecule would look like? | Visualization: mental rotation | 8. Consider molecule (rendered in 3-D) C2H5NH2 what would it look like after a rotation about the Y-axis? | Visualization: mental rotation orientation |
| 9. Which atom lies behind the plane of the screen? | Translation (interpretation of dash/wedge) | 9. Consider molecule PCl4I, (rendered image) how would this look when viewed from the bottom (arrow points to spot)? | Visualization: mental rotation orientation |
| 10. How many water molecules could hydrogen bond to one molecule of acetic acid? | Visualization: mental image orientation | 10. How many water molecules could H-bond to 1 methanol molecule? | Visualization: mental image orientation |
| 11. Does this molecule posses a plane of symmetry? | Symmetry Plane Identification | 11. Are these molecules the same? | Visualization: mental rotation |
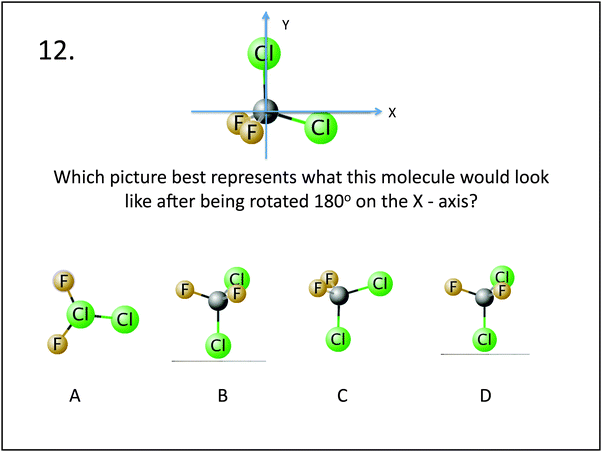
| 12. Which picture best represents what this molecule would look like after being rotated 180° on the x-axis? | Visualization: mental rotation | 12. Which of these molecules are the same? | Visualization: mental rotation |
| 13. Predict which region of CH3F will experience the greatest attraction to F on an adjacent molecule | Visualization: of polar interaction | 13. Which of these molecules are the same? | Visualization: mental rotation orientation |
| 14. Select the 3D shape that best corresponds to the Lewis formula | Translation, visualization | 14. Which of these sketches correctly represents the molecule, NH2F as shown below? | Translation (of 3D molecular image to 2D sketch with dash/wedge) |
| 15. Which of these molecules is not flat? | Visualization (or memorization) | 15. Which of these sketches correctly represents the molecule shown below when viewed down the F to C bond? | Translation (of 3D molecular image to 2D sketch with dash/wedge) |
| 16. Imagine a molecule of ammonia, NH3. If you carefully inspected it, what is the maximum number of atoms that would lie within one plane? | Visualization: mental imagery | ||
Appendix III: pre-test
Appendix IV: post-test
Acknowledgements
We would like to thank Professor Sarmad Hindo for his invaluable assistance with this study.References
- Bodner G. and Guay R., (1997), The Purdue visualization of rotations test, Chem. Educ., 2(4), 12–37.
- Brooks J. and Brooks M., (1999), In search of understanding – the case for constructivist classrooms, Alexandria VA: ASCD.
- Carlisle D., (2013), Spatial reasoning in organic chemistry: from novice to expert the missing links, paper presented at the New England Educational Research Association, Portsmouth, NH.
- Carlisle D., (2014), Developing spatial reasoning skills in general chemistry students, Doctoral dissertation, University of Massachusetts Amherst, Amherst, MA, 01003.
- Carroll J., (1993), Human Cognitive Abilities: A Survey of Factor-Analytic Studies, Cambridge England: Cambridge University Press, pp. 304–363.
- Cohen C. and Hegarty M., (2007), Individual differences in the use of external visualisations to perform an internal visualisation task, Appl. Cogn. Psychol., 21, 701–711.
- Coleman S. and Gotch A., (1998), Spatial perception skills of chemistry students, J. Chem. Educ., 75(2), 206–209.
- Donaghy K. and Saxton K., (2012), Connecting geometry and chemistry: a three-step approach to three-dimensional thinking, J. Chem. Educ., 89, 917–920.
- Eysenk M. and Keane M., (2010), Cognitive Psychology: A students handbook, N.Y.: Psychology Press.
- Ferguson C., Ball A., McDaniel W. and Anderson R., (2008), A comparison of instructional methods for improving the spatial visualization ability of freshman technology seminar students. IAJC-IJME International Conference, ISBN 978-1-60643-379-9.
- Gabel, D., (1999), Improving teaching and learning through chemistry education research: a look to the future, J. Chem. Educ., 76, 548–554.
- Gall M., Gall J. and Borg W., (2008), Educational Research, An Introduction, NY: Allyn and Bacon.
- Gilbert J., (2005), Visualization: a metacognitive skill in science and science education, in Gilbert J. K. (ed.), Visualization in Science Education, The Netherlands: Springer, pp. 9–27.
- Guay R. B., (1977), Purdue spatial visualization test: rotations, West Lafayette, IN: Purdue Research Foundation.
- Harle M. and Towns M., (2011), A review of spatial ability literature, it's connection to chemistry, and implications for instruction, J. Chem. Educ., 88(3), 351–360.
- Hegarty M., (2012), Meta-representational competence as an aspect of spatial intelligence, Cognit. Sci., 1240–1241.
- Hegarty M. and Waller D., (2004), A Dissociation between mental rotation and perspective-taking spatial abilities, Intelligence, 32, 175–191.
- Johnstone A., (2000), Chemical education research: where from here? Univ. Chem. Educ., 4(1), 34–39.
- Kozma R. and Russell J., (2005), Students becoming chemists: developing representational competence, in Gilbert J. K. (ed.), Visualization in Science Education, Netherlands: Springer, pp. 121–146.
- Lohman D., (1979), Spatial Ability: A Review and Reanalysis of the Correlational Literature, Technical Report No. 8, Aptitiude Research Project, Sanford University, Palo Alto CA.
- Mohler J., (2008), The impact of visualization methodology on spatial problem solutions among high and low visual achievers, J. Industrial Tech., 24(1), 2–9.
- National Science Board (2010), Preparing the next generation of STEM innovators: identifying and developing our nation's human capital, NSB-10-33.
- Presidents Council of Advisors on Science and Technology, (2010), Report to the President, Prepare and inspire: K-12 education in science, technology, engineering and math (STEM) for America's future.
- Ramadas J., (2009), Visual and spatial modes in science learning, Int. J. Sci. Educ., 31(3), 301–318.
- Schwartz D. and Heiser J., (2005), Spatial Representations and Imagery in Learning, in Sawyer K. (ed.), Cambridge University handbook of the learning sciences, Cambridge University Press: New York, pp. 283–298.
- Sorby S., (2009), Educational research in developing 3-D spatial skills for engineering students, Int. J. Sci. Educ., 31(3), 459–480.
- Stieff M., (2007), Mental rotation and diagrammatic reasoning in science, Learn. Instr., 17(2), 219–234.
- Stieff M., (2010), When is a molecule three dimensional? A task-specific role for imagistic reasoning in advanced chemistry, in Ford, M. and Varelas, M. (ed.), Learn. Sci. Educ., Wiley Periodicals, pp. 310–336.
- Stieff M., Ryu M., Dixon, B. and Hegarty M., (2012), The role of spatial ability and strategy preference for spatial problem solving in organic chemistry, J. Chem. Educ., 89, 854–859.
- Taagepera M., Arasasingham R. D., King S., Potter F., Martorell I., Ford D., Wu J. and Kearney A. M., (2011). Integrating symmetry in stereochemical analysis in introductory organic chemistry, Chem. Educ. Res. Pract., 12, 322–330.
- Terlecki M., Newcome N. and Little M., (2008), Durable and generalized effects of spatial experience on mental rotation: gender differences in growth patterns, Appl. Cogn. Psychol., 22, 996–1013.
- Uttal D. and Cohen C., (2012), Spatial thinking and STEM education: when, why, and how? Psychol. Learn. Motiv., 57, 147–178.
- Wai J., Lubinski D. and Benbow C., (2009), Spatial ability for STEM domains: aligning over 50 years of cumulative psychological knowledge solidifies its importance, JPN J. Educ. Psychol., 101(4), 817–835.
- Wesson K., (2012), Brain-sight: can touch allow us to “see” better than sight? Brain World magazine, online url: http://brainworldmagazine.com/brain-sight-can-touch-allow-us-to-see-better-than-sight/.
- Wu H. and Shah P., (2004), Exploring visuospatial thinking in chemistry learning, Sci. Educ., 88(3), 465–492.
- Yilmaz H. B., (2009), On the development and measurement of spatial ability, Int. Elect. J. Elem. Educ., 1(2), 83–96.
Footnotes |
| † External representations are concrete representations, outside of one's mind, with which we can interact, some examples include: sketches, hand-held molecular models, and computer images. |
| ‡ Knowledge space theory is a mathematical theory in cognitive science that explains how particular elements of knowledge can be gathered to form distinct knowledge spaces and describe possible states of learning. |
| This journal is © The Royal Society of Chemistry 2015 |