Implementation of 5E inquiry incorporated with analogy learning approach to enhance conceptual understanding of chemical reaction rate for grade 11 students
Saksri
Supasorn
*a and
Vinich
Promarak
b
aUbon Ratchathani University, Faculty of Science, Ubon Ratchathani, 34190, Thailand. E-mail: saksri.supasorn@gmail.com
bSuranaree University of Technology, Institute of Science, Nakhon Ratchasima, 30000, Thailand
First published on 29th October 2014
Abstract
The main purpose of this study was to enhance student understanding of the scientific concepts of chemical reaction rate. Forty-four grade 11 students were the target group. The treatment tools were seven learning plans of 5E inquiry incorporated with an analogy learning approach during 15 hours of class time. In each learning plan, the students (1) addressed a scientific question regarding chemical reaction rate, (2) explored evidence to answer the question by carrying out a corresponding experiment, (3) drew explanations from collected evidence to answer the question, (4) elaborated their understanding by studying the given analogy and the target, and (5) evaluated their conceptual understandings by creating their own analogy and identifying similarities and differences of their analogies and the targets. The data collecting tool was a conceptual test of chemical reaction rate, consisting of 30 two-tier three-choice questions. The normalized learning gain for the whole conceptual test was at the medium gain level (0.64). The dependent samples t-test analysis indicated that the post-conceptual test score (mean 45.32, SD 6.46) was statistically higher than the pre-test score (mean 19.70, SD 3.10), but was statistically lower than the retention test score (mean 48.03, SD 9.04) at the significance level of 0.05. In the pre-conceptual test, the percentages of students in the good-, alternative-, and misconception categories were 13.69, 38.45, and 47.86, respectively. In the post-conceptual test, the percentages of students in these categories were 64.72, 24.6, and 10.63, respectively. This finding indicates that this implementation was an effective means to enhance and retain students' conceptual understanding of chemical reaction rate.
Introduction and background
Chemical reaction rate, or chemical kinetics, has been found to be one of the most difficult chemistry topics to understand because it involves mathematical calculations and because there are many factors influencing the reaction rate (Justi, 2003). Thai students exhibit the same learning difficulties as those reported for other students (Chairam et al., 2009). Some students have an alternative understanding of concepts which are not consistent with the consensus of the scientific community (Mulford and Robinson, 2002; Taber, 2002). Their understanding of concepts may be partially right, but incomplete or just simply wrong (Piquette and Heikkinen, 2005). Requiring students to generate their own analogues (also called analogical models) and to identify how their analogues are similar to and/or different from the targets (also called target concepts) of the corresponding concepts can reveal their conceptual understandings and identify some of their alternative conceptions. This information is useful for devising corresponding analogues that best support students' concept acquisition.Chemical reaction rate
The term “reaction rate” is not a property of chemical species themselves it is a property of the extent of a reaction (Schmitz, 2005). Cunningham (2007) commented that descriptions of methods for helping students to understand reaction rate have been presented in many textbooks, but there has been little discussion of how to gain students' understanding by asking them to find their own meaning of reaction rate. He designed the following assignments to help students enhance their understanding of reaction rate and to assess that understanding:(1) Can the student identify a change that is clearly chemical, as opposed to physical, in nature?
(2) Can the student identify a chemical reaction whose increased or decreased rate is of some interest or practical importance?
(3) Can the student correctly identify the reactants and products of the chemical change they have selected?
(4) Can the student clearly and correctly explain the mechanism by which the factor identified increase, and
(5) Can the student effectively apply the standard conventions of written English?
Many studies have been carried out on students' conception of the topic of chemical reaction rate or chemical kinetics. For example, Van Driel (2002) attempted to develop grade 10 students' ideas of macroscopic chemical phenomena together with their views of the particulate nature of matter. The students were requested to carry out chemical experiments and explain their experimental results. He concluded that students of this age in the Netherlands have limited abilities for reasoning in corpuscular terms. His approach has the potential to aid students to move from primitive corpuscular to more scientifically acceptable views. Although, student explanations may be deficient from a scientific perspective, students gradually learn to become more proficient in using corpuscular models as explanatory tools.
Another example is the investigation carried out by Chairam et al. (2009) on the study of chemical kinetics by Thai students. They reported that chemical kinetics is an extremely important concept taught in introductory chemistry courses. The teaching of chemical kinetics to high school and undergraduate students in Thailand generally begins with an emphasis on qualitative aspects. Students are often introduced to the rate of reaction and factors (such as temperature, concentration, and catalysts) which influence the rate of a reaction. They also investigated the effect of inquiry-based learning activities in which the first year undergraduate science students at a public university in Thailand were requested to design and carry out an experiment to investigate the reaction of acids and bases. They found that the students were able to develop a good conceptual understanding of chemical kinetics from participation in this more active and enjoyable teaching approach.
The conceptual changes of Turkish grade-11 students was studied by Çalik et al. (2010). They examined some previous studies and identified some problems encountered in learning the concept of chemical reaction rate. Some of these problems are (1) an inability to define the rate of reaction, (2) misunderstanding, misapplying or misinterpreting of the relationship between the rate of reaction and its influencing factors, and (3) a lack of understanding of how activation energy and enthalpy relate to the rate of reaction. They also investigated the effects of conceptual change pedagogy on students' conceptions. They found that conceptual change pedagogy intervention helped students to notice and correct their alternative conceptions. They suggested that a combination of various conceptual change methods may be more effective for decreasing students’ alternative conceptions. Çalık and his colleague (Kolomuç and Çalık, 2012) also explored the alternative conceptions generated by Turkish chemistry teachers and students (grade 11) for the topic of chemical reaction rate. They found that chemistry teachers and students tended to have similar alternative conceptions, which may be have been transmitted from the chemistry teachers. Examples of some alternative conceptions include: (1) a lack of understanding of the effect of enthalpy on the rate of reaction and mechanism of reaction, and (2) misunderstanding/misapplying of the relationship between temperature or concentration and the rate of reaction.
Actually there are more studies about student conceptions on the concept of ‘chemical reaction rate’ or ‘chemical kinetics’ (Bektaşlı and Cakmakcı, 2011; Cakmakci et al., 2006; Çalık et al., 2009b; Cakmakci, 2010), however, details of these studies are not presented in this article.
5E inquiry learning activities in chemistry
There are a number of models of inquiry in learning science. The 5E learning cycle has been proven to be one of the most effective inquiry learning model for chemistry and other sciences and can be applied at several levels in the instructional sequences within lessons (Bybee et al., 2006). The 5E learning cycle involves the following steps:(1) Engagement – students are engaged in inquiry questions,
(2) Exploration – students plan, design, and carry out their experiment, and record the experiment data,
(3) Explanation – students give explanations from the experimental data to answer the questions,
(4) Elaboration – students extend and apply their findings in a new context, especially a daily life one, and
(5) Evaluation – students evaluate their experimental process and results in a variety of ways, such as an activity report, instructor observation during the activity, and student presentations.
Although other learning cycle models (3E and 7E) have been introduced for chemistry instruction, these models are adapted directly from the 5E instructional model. The 5E learning cycle has many advantages, for example, it promotes active learning process, supports the processing of new information by students based on the extent of their personal knowledge, and improves students’ attitudes to chemistry instruction. Inquiry not only supports students' understanding of science concepts but also illustrates how they can construct knowledge themselves through the inquiry learning cycle. In addition, the 5E learning cycle can help students edit their alternative conceptions rather than rely only on textbook-oriented instruction. However, students' alternative conceptions and existing knowledge prior to the inquiry instruction should be explored. This information can be used in designing inquiry activities that support student efforts to correct their alternative conceptions (Balci et al., 2006; Bybee et al., 2006).
Inquiry learning activities have been found to be effective in teaching chemistry and have been widely advocated in the last few decades (Sanger, 2009). These types of activities possess advantages over traditional activities. Students are challenged to practice using learning resources and working in groups to enhance their higher-order cognitive skills (HOCS) or the skills of interpretation, analysis, prediction, and synthesis (Zoller and Tsaparlis, 1997; Bybee et al., 2006; Zoller and Levy Nahum, 2012). The instructors tend to play a role as facilitators who motivate and challenge students to carry out the activities through a science inquiry process (Deters, 2005). Moreover, instructors who continuously implement a 5E learning cycle tend to ask higher-order cognitive skill (HOCS) questions more often than non-5E instructors, who ask recognition and recall questions (Bybee et al., 2006).
Based on the findings of the studies detailed above, the topic of chemical reaction rate or chemical kinetics can be said to play an important role in learning subsequent related chemistry topics but students in many countries (both secondary and undergraduate students) tend to have alternative conceptions (Kolomuç and Çalık, 2012). Inquiry-based experiments or activities are proven to be effective means to help students overcome their alternative conceptions and change to more correct conceptions (Chairam et al., 2009; Van Driel, 2002).
Analogies in chemistry learning
Based on the assumption of Sarantopoulos and Tsaparlis (2004), an analogy is a system of relations (correspondences) between parts of the structure of two domains: the analogue and the target. The analogue domain, also called the source or base domain, is a domain that exists in memory, from which the analogy is drawn. The target domain contains the scientific concept, the learning objective of the analogy. An analogy involves the transfer of relational information from the analogue to the target, which consists of finding the correspondences between the two systems.Previous research studies suggested some instructional models for teaching with analogies. The FAR (Focus, Action, and Reflection) guide is one of the most common models used in analogy learning in science (Harrison and Treagust, 2006; Harrison and Coll, 2007). This model was proposed to maximize the benefits and minimize the problems encountered in analogy instruction (Venville, 2008). In the Focus phase, the scientific (target) concept and student familiarity with the analogue are considered. This can guide pre-lesson planning by focusing attention on issues of concept complexity, prior student knowledge, and experience with the analogy. In the Action phase, students experience the analogical model and identify the similarities and dissimilarities (or differences) of the analogue and the target concept. Various methods can be used to help the students identify similarities and differences between the analogue and target concept. The Reflection phase takes place after the presentation of the analogy in this phase the instructor reflects upon the clarity and usefulness, and conclusions drawn from the analogue. This phase prompts the teacher to consider the clarity and usefulness of the analogy and to re-focus on the previous phases as necessary (Venville, 2008; Davis, 2013). The three typical phases in the FAR guide model for teaching with analogies are illustrated in Table 1 (Venville, 2008; Davis, 2013).
| Focus phase | Pre-lesson planning |
|---|---|
| Concept | Is the concept difficult or abstract? |
| What is difficult about the concept? | |
| Students | What ideas do students currently have about the concept? |
| Experience | What familiar experiences do students have that I can use? |
| Action phase | In-lesson action |
|---|---|
| Similarities | Cue the student memory of the analogy |
| Discuss ways in which the analogue is like the target | |
| Are there surface features or deep relations? | |
| Differences | Discuss ways in which the analogue is unlike the target |
| Summary | Conclude by summarising the outcomes of using the analogy |
| Reflection phase | Post-lesson reflection |
|---|---|
| Conclusions | Was the analogy clear and useful, or confusing? |
| Improvements | What changes are needed for the following lesson? |
| What changes are needed the next time I use this analogy? |
In previous decades many outstanding studies have been carried out on analogy learning in chemistry. For example, Çalık and Ayas (2005) devised an analogy learning activity based on students' alternative conceptions about solution chemistry from their previous study to address students' alternative conceptions of solution chemistry. They found that this alternative teaching method was generally successful, however, its applicability has not been investigated. They finally suggested that analogies can effectively make intangible concepts tangible for students when the used analogies support students to clearly connect between the analogue and target concepts. Çalik further investigated the effectiveness of an analogy activity in improving students' conceptual change of solution chemistry concepts with his colleagues (Çalik et al., 2009a). They used ‘travel on a public bus’ as the analogy activity. They found that most of the students' pre-test responses were in the No Understanding (NU) category. Some of the students' alternative conceptions were about using incorrect scientific terms (i.e., use of the words ‘less saturated’ or ‘diluted’ instead of ‘unsaturated’, and ‘concentrated’ instead of ‘saturated’) and difficulty in differentiating the terms (i.e., the terms ‘melting’ and ‘dissolving’). However, the majority of their post-test and delayed post-test responses moved to the more understanding categories, Partial Understanding with Specific Alternative Conception (PU + AU), Partial Understanding (PU), and Sound Understanding (SU). They then suggested that in such analogy learning activities if student self-assessment is to be used, the intervention time should be planned carefully.
Orgill and Bodner (2004) reported that analogies can be powerful teaching tools because they can make new material intelligible to students. Many students enjoy, pay particular attention to, and remember the analogies that their instructors provide. Although some analogies are not as effective as others, these analogies do help students to understand, visualize, and recall what they have learned in class. This is consistent with the findings of Harrison and Coll (2007), who reported that analogies are often used in science to engage student interest and to explain difficult and abstract ideas. While some analogies effectively clarify difficult concepts, many are inadequate or can cause further confusion. Eskandar et al. (2013) also suggested that teaching chemistry with textual elaborated analogies can also enhance students' logical thinking ability. However, they reported that although all the students stated that they were familiar with analogy concepts in science textbooks, it is likely that some were less familiar than others.
Çalik et al. (2009a) reviewed previous studies on teaching chemistry with analogies and concluded that teaching using multiple analogies is better than teaching using a single analogy. They also suggested some key features for effective analogy instruction: (1) ensuring the analogy is familiar to the students, (2) mapping as many shared attributes as possible, and (3) identifying where the analogy breaks down.
The previous studies suggested that learning chemistry by using familiar analogies is usually effective at promoting student conceptual changes (Çalik et al., 2010). Analogies allow students to understand even intangible chemistry concepts since they aid students to relate between the analogue and target concepts (Çalık and Ayas, 2005).
Inquiry learning activities are effective demonstrations of tangible chemistry (i.e., macroscopic) concepts, and the analogies make it possible for students to understand intangible chemistry (i.e., molecular or sub-microscopic) concepts. Therefore, the combination of inquiry and analogy learning approaches could enhance students' understanding of both tangible and intangible chemistry concepts.
Research questions
The main purpose of this study was to develop inquiry activities that incorporate analogies and to use these activities as a means of enhancing and retaining students' conceptual understanding of chemical reaction rate. When the activities were implemented, the following questions were posed:1. How does the implementation of inquiry activities that incorporate analogies enhance and retain students' conceptual understanding of chemical reaction rate?
2. How do the percentages of students having good conceptions, alternative conceptions, or misconceptions of chemical reaction rate change after they complete inquiry activities that incorporate analogies?
Research methodology
The details of the methodology for this study are as follows.Treatment tools
The treatment tools consisted of seven learning plans (totalling 15 hours) of inquiry combined with analogy learning activities for instruction, as shown in Table 2, while an example of the FAR guide model for the topic of “effect of a catalyst or a retarder on chemical reaction rate” is illustrated in Table 3.| Learning plans (hours) | Key activities (E = experiment, A = analogy) |
|---|---|
| 1. Definition and calculation of reaction rate (3) | – A: running various distances within a limited time. |
| 2. Theories of reaction rate (2) | – A: blowing a clay ball up various slopes. |
| 3. Effect of nature of substances on reaction rate (2) | – E: reactions of various shells (egg, crab, or mollusc) with various acids. |
| – A: sailing paper boats of various-thickness. | |
| 4. Effect of surface area on reaction rate (2) | – E: reactions of acid and various-sized shells. |
| – A: dissolving table and crystalline sugars in water. | |
| 5. Effect of concentration on reaction rate (2) | – E: reactions of various-concentrations of acids and a specific shell (egg, crab, or mollusc). |
| – Analogy: increasing number of identical images in the image matching game. | |
| 6. Effect of catalyst and retarder on reaction rate (2) | – E: effects of manganese sulfate (MnSO4) and sodium fluoride (NaF) on the reaction of oxalic acid (H2C2O4) and sulfuric acid (H2SO4). |
| – A: blowing a clay ball up various slopes. | |
| 7. Effect of temperature on reaction rate (2) | – E: reactions of acid and a specific shell at various temperatures. |
| – A: cooking popcorn at various temperatures. |
| Focus phase | Pre-less on planning |
|---|---|
| Concept | – How a catalyst and retarder affect the chemical reaction rate is difficult to understand. |
| Students | – Already understand that a catalyzed reaction occurs faster or a non-catalyzed reaction occurs slower than a normal reaction, but do not understand the mechanism of how a catalyst or a retarder affect the rate. |
| Experience | – Riding a bicycle, riding a motorcycle, driving a car uphill, and walking up stairs. |
| Action phase | In-lesson action |
|---|---|
| Similarities | – Slope of hill and amount of activation energy (Ea). |
| – Time for riding and reaction time. | |
| – Power used for riding and reaction energy. | |
| Differences | – Slope of hill may remain the same but the amount of activation energy (Ea) can be decreased or increased. |
| – Riding, driving, or walking to the top is a physical change (no product), but a reaction is a chemical change (product generated). | |
| – People who are biking and walking uphill may feel tired, but reactants do not have feelings. |
| Reflection phase | Post-lesson reflection |
|---|---|
| Conclusions | – Riding a motorcycle and driving a car uphill do not involve feeling tired (clear analogies). |
| Improvements | – Riding a bicycle uphill and walking up stairs are tiring and this should not be taken into account in these analogies. |
| – More explanation about decreasing or increasing of Ea caused by a catalyst or a retarder. |
In each learning plan, the intervention process of the 5E inquiry cycle incorporated an analogy with FAR guide learning as shown below:
(1) Students were presented with a scientific question regarding chemical reaction rates.
(2) Students explored evidence (or data) to answer the question by planning and carrying out a corresponding experiment.
(3) Students came up with an explanation from collected evidence (or data) to answer the question.
(4) Students elaborated their understanding through a corresponding analogy by identifying similarities and differences between the given analogue and the target, following FAR guide analogy instruction (Harrison and Coll, 2007).
(5) Students were asked to generate their own analogy and then identify the similarities and differences between their analogues with the targets to evaluate their conceptual understanding.
Data collecting tool
The data collecting tool was a conceptual test on chemical reaction rates and consisted of 30 two-tier three-choice questions. The two-tier multiple choice questions were developed specifically for the purpose of identifying students' alternative conceptions about various concepts in limited and clearly defined content areas (Chandrasegaran et al., 2007). The items were content-validated by two senior lecturers of chemistry and one professor of chemical education. Each question comprised two tiers and the students were required to make their choice of answer for the content question in the first tier, and then select the explanation or reason for that choice in the second tier (22 items out of 30 items or 73.33%). Examples of the conceptual test questions are shown in Fig. 1. For some questions, students were asked to supply calculation methods for the response that they had selected instead of selecting the explanation choice (8 items out of 30 items or 26.67%), see also Fig. 2 (Treagust, 1988).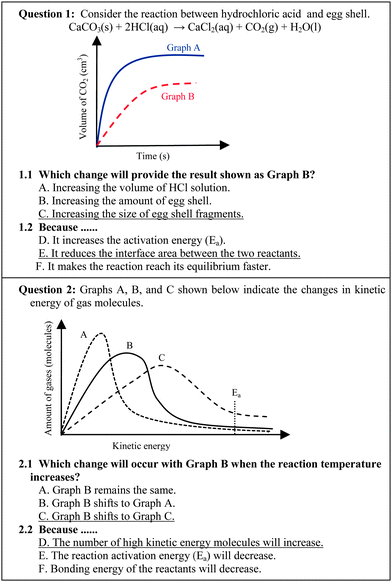 | ||
| Fig. 1 Examples of two-tier three choice questions (selecting choices of answer for both content question and explanation tiers). | ||
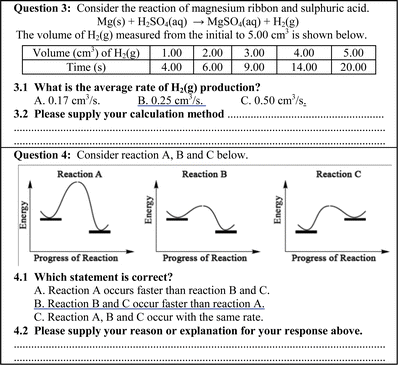 | ||
| Fig. 2 Examples of two-tier three choice questions (selecting choices of answer for content question tier and supply calculation method or reason for explanation tier). | ||
The difficulty index (P), discrimination index (r), and reliability were calculated by using the Simple Item Analysis or SIA software, which is generally used in many schools in Thailand. The difficulty index (P) for each item was in the range of 0.20–0.80, in which the percentages of items with P in the ranges of 0.20–0.39, 0.40–0.59, and 0.60–0.80 were 20.00, 70.00, and 10.00, respectively. The discrimination index (r) for each item was in the range of 0.27–1.00, in which the percentages of items with r in the ranges of 0.20–0.39, 0.40–0.59, 0.60–0.79, and 0.80–1.00 were 6.67, 36.67, 46.67, and 10.00, respectively. In addition, the reliability based on the Kuder–Richardson formula 20 or KR20 for the entire test was 0.85.
Note that all of the research tools including the treatment tools (lesson plans) and data collecting tools (conceptual tests, analogies, and interview) were in Thai. The class was taught in Thai and all the examples included in this article involved translation into English.
Participants
With prior permission from the school principal and the instructor of the chemistry course taught during the second semester of academic year 2013, 44 students out of 61 voluntary students (two classrooms) who attended all of the activities throughout the study were purposively selected as the participants of this study. They were grade 11 students attending the Chiangkaew Pittayakom School, a medium-sized public high school in the Ubon Ratchathani province of Thailand.Implementation
Prior to the implementation, the students spent an hour completing the pre-conceptual test on chemical reaction rates, also called the pre-test. They then participated in seven inquiry/analogy learning plans on chemical reaction rate for five weeks, three hours a week, totalling 15 hours. Right after the implementation, they spent an hour to completing the post-conceptual test, also called the post-test. Thirty days after the implementation, they spent another hour completing the delayed-post conceptual test, also called the retention-test. Please note that the pre-, post-, and retention-tests were the same test but the item questions and choices had been rearranged. In addition, these students were studying the topic of chemical equilibrium during the time between the post- and retention-tests. Finally, participants who provided interesting explanations in the good-, alternative-, and misconception were purposively selected for an informal interview.Data analysis
The data collected in this study were pre-, post, and retention-conceptual scores. Each two-tier three-choice item was worth 2 points (1 point for each tier). Therefore, the available score for each test was 60 points. The test scores were also analyzed by using the paired-samples t-test to identify the differences between the means of pre- and post-conceptual test scores and between the means of post- and retention-conceptual test scores. Class normalized learning gain or 〈g〉 was applied to minimize the floor and ceiling effect. That is a student can get no less than 0% and no more than 100% correct with such an instrument. Hake (1998) explained that a student who gains a small pre-test score may have more chance of attaining a large percentage gain, while a student who begins with a large pre-test score may gain only a small percentage score. In other words, it is common for students who attain a higher pre-test score to attain a smaller absolute gain (post-test score minus pre-test score). The floor and ceiling effect can be minimized by using normalized gain 〈g〉 analysis. The topics with 〈g〉 ≤ 0.30, 0.30 < 〈g〉 > 0.70, and 〈g〉 ≥ 0.70 were classified into low-, medium-, and high gain categories, respectively (Hake, 1998).The students were also categorized into good- (sound understanding, aligned to scientific consensus), alternative- (partial understanding, on the right track, but incomplete), and misconception (illogical or incorrect information, simply wrong) groups according to their answers, see categories of student conceptions in Fig. 3 (Mulford and Robinson, 2002; Çalik et al., 2009a). Student answers were used as the criteria for categorizing them into groups. If the student answered correctly for both tiers, correctly for either the first or the second tier, or incorrectly for both tiers, they were categorized in the good-, alternative-, or misconception group, respectively.
 | ||
| Fig. 3 Levels of student conceptions compared to scientific consensus, adapted from Çalik et al. (2009a) and Mulford and Robinson (2002). | ||
Results and discussion
The study results were divided into three parts: students' pre-, post-, retention–conceptual test scores, percentages of students in the good-, alternative-, and misconception categories, and students' analogies of chemical reaction rate.Students' pre-, post-, retention-conceptual test scores
Prior to the implementation of inquiry incorporated with analogy learning plans on chemical reaction rate, the mean of the students' pre-conceptual test score was 19.70 (SD 3.10), as shown in Table 2. The students obtained high scores for the topics of the effect of catalyst and retarder (50.25%) and the effect of concentration (43.12%), while they obtained low scores for the topics of the effect of the nature of the substance (22.75%) and the surface area (26.75%), definition and calculation (23.60%) and the theories of chemical reaction rate (23.57%). The higher scores may have arisen because these students had learned about the effects of catalysts, concentration, and temperature during the basic chemistry course taught in the previous year. Right after the implementation, the mean of the students' post-conceptual test score was 45.32 (SD 6.46). The students obtained the highest post-test percentage scores and actual gains for the topics of definition and calculation (84.50 and 60.90) and the nature of substances (79.50 and 56.75). These high actual gains may have occurred because this topic is not complicated and once the students had understood the concepts and theories, they were able to calculate the chemical reaction rate. Moreover, the analogies that the instructor used in these topics (running various distances within a limited time and sailing paper boats of various-thickness) were perfectly matched to the target concepts (see Table 4). The lowest post-test percentage score was in the topic of the effect of surface area (68.50%) possibly because some students found the relation between size and surface area to be confusing. The student interviews revealed that some of them had misunderstood that an object or substance with a larger size has a larger surface area, which was correct when compared one smaller object to one larger object. However, they did not notice that the amount (weight or mole) of substances must be considered. Therefore, smaller sized substances have a larger total surface area than larger sized substances when the mole numbers were equivalent. In other words, the total surface area of equal amounts of substances increases as the size is reduced (Normand and Peleg, 2014).| Topics (score) | Conceptual test score (points) | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre- | Post- | Gain | ||||||
| Mean | SD | % | Mean | SD | % | Actual | 〈g〉 | |
| 1. Definition and calculation (10) | 2.36 | 0.89 | 23.60 | 8.45 | 1.25 | 84.50 | 60.90 | 0.80 |
| 2. Theories (14) | 3.30 | 1.34 | 23.57 | 9.93 | 2.04 | 70.93 | 47.36 | 0.62 |
| 3. Nature of substances (4) | 0.91 | 0.96 | 22.75 | 3.18 | 0.66 | 79.50 | 56.75 | 0.73 |
| 4. Surface area (8) | 2.14 | 0.95 | 26.75 | 5.48 | 1.41 | 68.50 | 41.75 | 0.57 |
| 5. Concentration (8) | 3.45 | 0.82 | 43.12 | 6.57 | 1.56 | 82.21 | 39.09 | 0.69 |
| 6. Catalyst and retarder (8) | 4.02 | 1.75 | 50.25 | 5.66 | 1.60 | 70.75 | 20.50 | 0.41 |
| 7. Temperature (8) | 3.52 | 1.21 | 44.00 | 6.05 | 1.89 | 75.62 | 31.62 | 0.56 |
| Total (60) | 19.70 | 3.10 | 32.83 | 45.32 | 6.46 | 75.53 | 42.68 | 0.64 |
The normalized learning gain for the whole conceptual test was 0.64 (medium gain). The students were classified as high gain for the topics of definition and calculation of chemical reaction rate (0.80) and the effect of the nature of substances (0.73), while the remaining topics were classified as medium gain. The students obtained the lowest normalized gain (0.41) for the topic of the effect of catalyst and retarder, possibly because this topic is complicated and abstract and they could not understand how the mechanism of a catalyst and retarder affect the reaction rate. It was concluded from the student interviews that some of them had misunderstood that a catalyst increases the energy of reactants, while a retarder decreases the energy so the catalyzed reaction proceeds at a faster rate than a normal reaction. Some misunderstood that catalysts and retarders affect the amount of exothermic or endothermic energy of the reaction. Some of them supplied the correct explanation that the catalyzed reaction proceeds at a faster rate than a normal reaction because the catalyst decreases the activation energy (Ea) of the reaction. This is consistent with the problems in learning about chemical reaction rates identified by Çalik et al. (2010) who identified that students lack an understanding of the effect of surface area and catalyst on the rate of reaction. In addition, the dependent samples t-test analysis indicated that the means of the post-conceptual test scores in every topic were statistically higher than those of the pre-test scores at the significance level of 0.05. This finding indicates that the incorporation of a combined inquiry and analogy learning approach was effective at enhancing students' conceptions of chemical reaction rate. This finding confirm that intervention of inquiry activities (Van Driel, 2002; Chairam et al., 2009) and corresponding and familiar analogies (Çalık and Ayas, 2005; Çalik, et al., 2010) are powerful for promoting student conceptual changes and moving to the more correct conceptions of reaction rate.
Students' retention of the concept of chemical reaction rate
Thirty days after the implementation, the retention-conceptual test was administered. The mean total score of the retention-conceptual test was 48.03 (SD 9.04). The dependent samples t-test analysis indicated that the retention scores in the topics of the effects of surface area, concentration, and temperature were statistically higher than those for the post-conceptual scores at the significance level of 0.05. However, no statistical difference was found for the topics of effect of the nature of substances and catalysts and retarders, definition and calculation, and theories involving chemical reaction rate, as shown in Table 5.| Topics (score) | Post-test | Retention-test | T-test | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | % | Mean | SD | % | T | Sig* | |
*![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Statistically difference at significance level of 0.05. Statistically difference at significance level of 0.05. |
||||||||
| 1. Definition and calculation (10) | 8.45 | 1.25 | 84.50 | 8.55 | 1.27 | 85.50 | 0.46 | 0.65 |
| 2. Theories (14) | 9.93 | 2.04 | 70.93 | 10.68 | 2.92 | 76.29 | 1.96 | 0.06 |
| 3. Nature of substances (4) | 3.18 | 0.66 | 79.50 | 3.34 | 0.83 | 83.50 | 1.16 | 0.25 |
| 4. Surface area (8) | 5.48 | 1.41 | 68.50 | 6.09 | 1.52 | 76.12 | 2.47 | 0.02* |
| 5. Concentration (8) | 6.57 | 1.56 | 82.21 | 7.07 | 1.44 | 88.38 | 2.33 | 0.02* |
| 6. Catalyst and retarder (8) | 5.66 | 1.60 | 70.75 | 6.02 | 2.02 | 75.25 | 1.11 | 0.27 |
| 7. Temperature (8) | 6.05 | 1.89 | 75.62 | 6.55 | 1.58 | 81.88 | 2.67 | 0.01* |
| Total (60) | 45.32 | 6.46 | 75.53 | 48.03 | 9.04 | 80.05 | 2.07 | 0.04* |
Since the retention scores were higher than or not less than the post-test scores, the findings indicate that there was retention of knowledge of all the topics of chemical reaction rate. The high increase of performance in the retention test compared to the post-test may have arisen because analogy instruction may be one of the effective tools for promoting student conceptual changes and then storing in their long-term memories (Çalik et al., 2010). The other explanation is that during the time between the post- and retention-tests the participants were studying the topic of chemical equilibrium, which is highly related to the chemical reaction rate. In addition, the participants also had access to additional instruction and did additional homework before the retention test.
Percentages of students in the good-, alternative-, and misconception categories
Prior to the implementation of inquiry incorporated with analogy learning plans on chemical reaction rate, the percentages of students in the good-, alternative-, and misconception categories of the pre-conceptual test were 13.69, 38.45, and 47.86, respectively (Table 6). They were mostly in the alternative- and misconceptions (86.31). Most of the students were in the misconception category for the topics of the effect of the nature of substances (75.00), theories (62.99), and definition and calculation (57.39).| Topics (number of items) | Pre-test categories (%) | Post-test categories (%) | ||||
|---|---|---|---|---|---|---|
| Good- | Alternative- | Mis- | Good- | Alternative- | Mis- | |
| 1. Definition and calculation (10) | 9.66 | 32.96 | 57.39 | 81.44 | 9.84 | 8.71 |
| 2. Theories (14) | 10.06 | 26.95 | 62.99 | 54.87 | 31.49 | 13.64 |
| 3. Nature of substances (4) | 20.45 | 4.55 | 75.00 | 60.23 | 37.50 | 2.27 |
| 4. Surface area (8) | 1.14 | 51.70 | 47.16 | 56.25 | 24.43 | 19.32 |
| 5. Concentration (8) | 18.75 | 48.86 | 32.39 | 69.89 | 23.86 | 6.25 |
| 6. Catalyst and retarder (8) | 22.73 | 55.68 | 21.59 | 54.55 | 27.27 | 18.18 |
| 7. Temperature (8) | 17.05 | 53.98 | 28.98 | 59.09 | 32.95 | 7.95 |
| Total (30) | 13.69 | 38.45 | 47.86 | 64.72 | 24.65 | 10.63 |
Right after the implementation, the percentages of students in these categories were 64.72, 24.65, and 10.63, respectively. Most students (more than 50%) were in the categories of good-conception for all of the topics. The highest percentage of students with good conceptions was in the topic of definition and calculation (81.44). However, some students (35.28%) were still classified in the alternative- and good-conceptions, especially in the topics of the effect of catalyst and retarder (45.45%), the theories of chemical reaction rate (45.13%), and the effect of surface area (43.75%). Since the percentages of students in the good conception category increased and the percentages in the alternative- and misconception categories decreased, it appears that this implementation was successful in enhancing students' conceptual understanding of chemical reaction rate.
Since the corresponding inquiry learning activities deeply engaged and challenged students in all of the steps of the activity process, their conceptual understanding was enhanced (Green et al., 2004). Therefore, the instructors were no longer the main source of knowledge about activities, but were the facilitators who guided their students through the inquiry process (Deters, 2005). In addition, the analogy activities were often enjoyable and interesting for students as some students commented that they favour analogies with social relevance (Sarantopoulos and Tsaparlis, 2004) and familiar analogies from science textbooks (Eskandar et al., 2013). Analogies can engage students’ interest and make it possible for them to understand difficult and intangible concepts (Harrison and Coll, 2007).
Students' analogies of chemical reaction rate
Students analogies generated during each topic of chemical reaction rate were also investigated, as shown in Table 7. These analogies contain student conceptions which may be correct (good-conceptions), partially correct but incomplete (alternative-conceptions), or simply wrong (misconception) when compared to scientific consensus about the concepts (Mulford and Robinson, 2002). However, even partially correct analogies can be powerful tools to help students to understand, visualize, and recall what they have learned in class (Orgill and Bodner, 2004).| Topics | Students' analogies (analogue = A, target concept = T) |
|---|---|
| a Note: ✓, ✓/✗, and ✗ indicate the analogies that are correct, partially correct but incomplete, and simply wrong, respectively, when compared to the targets. | |
| 1. Definition and calculation | 1. Driving a car at different speeds, but an equal distance. |
| 2. Peeling palm fruits at different speeds, but equal time. | |
| 3. Marathon runners running at different speeds, but over an equal distance. | |
| ✓ A: speed of driving, peeling, or running, T: reaction rate | |
| ✓ A: driving, peeling, or running time, T: reaction time | |
| 2. Theories | 1. Driving a car uphill. |
| 2. Riding a bicycle uphill. | |
| ✓ A: slope of hill, T: amount of activation energy (Ea) | |
| ✗ A: time for driving or riding uphill, T: reaction time | |
| 3. Riding a surfboard over different heights of waves. | |
| ✓ A: heights of waves, T: amount of activation energy (Ea) | |
| ✗ A: time for driving or riding uphill, T: reaction time (partly about staying or standing still on a surfboard) | |
| 3. Nature of substances | 1. Drying hair with a fan and an electronic dryer. |
| ✓ A: a fan and an electronic dryer, T: nature of reactants | |
| ✓ A: time for hair drying, T: reaction time | |
| 2. Riding a motorcycle and biking a bicycle. | |
| ✓ A: motorcycle and biking a bicycle, T: nature of reactants | |
| ✓ A: time for riding or biking to stop point, T: reaction time | |
| 3. Running with running shoes and slippers. | |
| ✓ A: running shoes and slippers, T: nature of reactants | |
| ✓ A: time used for running to stop point, T: reaction time | |
| 4. Surface area | 1. Baking small and large sized cupcakes. |
| ✓ A: large and small cupcakes, T: small and large surface area | |
| ✓ A: raw and baked cupcake, T: reactants and products | |
| ✓ A: cooking time, T: reaction time | |
| 2. Dissolving curry cube and powder in water. | |
| ✓ A: curry cube and powder, T: small and large surface area | |
| ✗ A: curry solution (physical change), T: reaction product | |
| ✓ A: dissolving time, T: reaction time | |
| 3. Boiling small and large sizes of starch bubbles. | |
| ✓ A: large and small bubbles, T: small and large surface area | |
| ✓ A: boiling time, T: reaction time | |
| ✓ A: cooked starch bubbles, T: reaction product | |
| 5. Concentration | 1. Making fire balls with different amount of gunpowder. |
| ✓ A: amount of gunpowder, T: concentration of reactant | |
| ✗ A: power of fire balls, T: reaction rate | |
| 2. Fishing catfish in the natural and farm ponds. | |
| ✓ A: amount of catfish, T: concentration of reactant | |
| ✗ A: natural and farm ponds, T: high and low concentrations (partly about nature of substances) | |
| 3. Feeding a bird and a flock of birds with same amount of rice. | |
| ✓ A: amount of birds, T: concentration of reactant | |
| ✓ A: time used for bird-feeding, T: reaction time | |
| 6. Catalyst and retarder | 1. Riding geared and non-geared bicycles over the same distance. |
| ✓/✗ A: geared and non-geared bicycles, T: catalysed and non-catalysed reactions (partly about nature of substance) | |
| ✓ A: time for riding bike to stop point, T: reaction time | |
| 2. Driving a car on paved and unpaved roads. | |
| ✓/✗ A: paved and unpaved roads, T: catalysed and non-catalysed reactions (partly about nature of substance) | |
| ✓ A: time for driving to stop point, T: reaction time | |
| 3. Walking home with and without a shortcut. | |
| ✓ A: shortcut route, T: catalysed reaction | |
| ✓ A: time used for walking, T: reaction time | |
| 7. Temperature | 1. Cooking rice at high and low temperatures. |
| 2. Boiling eggs at high and low temperatures. | |
| 3. Baking rice popcorn at high and low temperatures. | |
| ✓ A: temperature used for cooking rice, boiling eggs, or baking rice popcorn, T: reaction temperature | |
| ✓ A: cooking, boiling, or baking time, T: reaction time | |
| ✓ A: cooked rice, eggs, or rice popcorn, T: reaction product | |
Since there might not have been analogies which perfectly matched the target concepts, the information expressed in the generated analogies may not have been powerful enough to really identify their conceptions. However, the authors attempted to categorize their conceptions into correct (✓), partially correct or alternative- (✓/✗), or misconception (✗) to promote group discussion to be more powerful at promoting student conceptual changes (Çalik et al., 2010; Davis, 2013). Examples of student identification of similarities and differences in their generated analogies are shown in Table 8.
| Analogy | Analogue | Target |
|---|---|---|
| 1. Definition of reaction rate: driving a car at different speeds, but over an equal distance. | ||
| Similarities | – Speed of driving | – Reaction rate |
| – Driving time | – Reaction time | |
| Differences | – Physical change (no product) | – Chemical change (product generated) |
| – People may feel tired | – Reactants have no feelings | |
| 2. Theories of reaction rate: riding a bicycle uphill. | ||
| Similarities | – Slope of hill | – Amount of Ea |
| – Time for riding | – Reaction time | |
| – Power used for riding | – Reaction energy | |
| Differences | – Slope of a hill remains the same | – Amount of Ea can be decreased or increased |
| – Physical change | – Chemical change | |
| – People may feel tired | – Reactants have no feelings | |
| 3. Nature of substances: riding a motorcycle and riding a bicycle. | ||
| Similarities | – A motorcycle and a bicycle | – Nature of reactants |
| – Time for riding | – Reaction time | |
| Differences | – Physical change | – Chemical change |
| – Fuel (chemical) for riding and energy for biking | – Reaction energy | |
| 4. Boiling small and large sized starch bubbles. | ||
| Similarities | – Large bubbles | – Small surface areas |
| – Boiling time | – Reaction time | |
| – Cooked starch bubbles | – Reaction products | |
| Differences | – Sticky cooked bubbles often stick together | – Reaction products may not stick together |
| – Eatable food | – Uneatable | |
| 5. Effect of concentration: making fire balls with different amounts of gunpowder. | ||
| Similarities | – Amount of gunpowder | – Concentration of reactant |
| – Power of fire balls | – Reaction rate | |
| Differences | – Increasing gunpowder may not increase the power of the fire balls (improper mixing ingredients) | – Increasing the concentration always increases the rate |
| – Fire ball explosion is an exothermic process | – A reaction may be an exothermic or an endothermic process | |
| 6. Effect of catalyst and retarder: driving a car on paved and unpaved roads. | ||
| Similarities | – Paved and unpaved | – Catalysed and non-catalysed |
| – Time for driving | – Reaction time | |
| Differences | – Slope of road is not considered | – Amount of Ea involved |
| – Physical change (no product) | – Chemical change | |
| – Unreliable of paved and unpaved roads in rural districts | – Catalyzed-always faster than non-catalyzed reactions | |
| 7. Effect of temperature: boiling eggs at high and low temperatures. | ||
| Similarities | – Temperature for boiling | – Reaction temperature |
| – Boiling time | – Reaction time | |
| – Cooked eggs | – Reaction product | |
| – Different types of eggs | – Different reactants | |
| Differences | – Boiling eggs is an endothermic process, and the evaporation of water is an exothermic process | – A reaction may be an exothermic or an endothermic process |
For example, some students gave a correct analogy (✓) about boiling small and large sized starch bubbles for the effect of surface area on chemical reaction. One of the similarities of the target and analogue is that small sized and large sized starch bubbles represent large and small surface areas, respectively. Some students gave a partially correct analogy (✓/✗), which is dissolving a curry cube and powder in water. One of the differences in this case is that sugar dissolving in water is not a chemical change, but a physical change. In another example, some students gave a correct analogy (✓) about making fire balls with different amounts of gunpowder for the effect of concentration on chemical reaction. One of the similarities of the target and analogue is that the amount of gunpowder represents the concentration of gunpowder in the fire ball mixture. Some students gave a partially correct analogy (✓/✗), which is fishing for catfish in natural and farm ponds. One of the differences in this case is that it cannot be confirmed that the amount of catfish in the farm-pond is equal to the amount in the natural-pond.
Conclusion, implications and limitations
Despite the limitations of this study that involved students from a single school, this study verified that the implementation of inquiry supported by analogy learning activities was an effective means of enhancing and retaining students' conceptual understanding of chemical reaction rate. The normalized learning gain from pre- to post-conceptual tests showed a medium gain in understanding. The dependent samples t-test analysis indicated that the post-conceptual test score was statistically higher than the pre-test score, but was statistically lower than the retention test score at the significance level of 0.05. Prior to the implementation, students were mostly in the alternative- and misconception categories. After the implementation of the corresponding inquiry incorporated with analogy learning activities, the majority of the students moved to the good-conception categories. However, some students still held alternative- and misconceptions, which were expressed when they were asked to create their own analogy and to identify similarities and differences between their analogies and the targets for each chemical reaction rate topic.This study may have implications for chemistry instructors, because inquiry activities may be an effective means of enhancing and retaining students' conceptual understanding, but may not be effective for helping them recognize their alternative- or misconceptions. The implementation of inquiry activities in conjunction with the corresponding analogies may be a more effective means of helping learners correct their alternative conceptions. It is advisable that instructors should design tasks or assignments to find out their students' understanding of reaction rate (Cunningham, 2007) and various cooperative learning methods (classroom discussion, argumentation, or negotiation) can enable better understanding of the concepts of reaction rate and improve students' motivation to study chemistry (Venville, 2008; Kırıka and Bozb, 2012). These learning methods could truly enhance and retain students' understanding of reaction rate. The instructors should keep in mind that while many analogies are useful and do convey useful information, the message of the analogies is not always obvious to all students. They may misinterpret the main points of the analogies which can lead students to have alternative conceptions (Orgill and Bodner, 2004). Therefore, instructors have to be assured that the students understand the scientific concepts, and do not develop alternative conceptions from the analogy (Venville, 2008). Analogy instruction can inform teachers how analogies can be used effectively in classrooms. It is advisable that providing teachers opportunities to practice and experience teaching with analogies will enhance the successful enhancement of students' conceptual understanding in their classes (Harrison and Coll, 2007; Venville, 2008).
The retention test score obtained in this study was higher than the post-test score. This limitation arose because after the implementation and post-conceptual test on chemical reaction rate, the participants had access to additional instruction and did additional homework before the retention test. In addition, they were studying the topic of chemical equilibrium which relates to the topic of chemical reaction rate, before the retention test. To avoid this limitation, the retention test should be completed before the participants begin to study the next topics which may relate to the topic being studied. The other limitation is that the instructor did not spent enough time organising a group discussion between the students having similar and different conceptions. Instructors should enable students to recognise when they have unacceptable conceptions and should help them to correct their understanding (Chandrasegaran et al., 2007).
Acknowledgements
This study was part of the TRG5680024 project entitled “Development of Inquiry Chemistry Experiments in conjunction with Molecular Animations (ICEMA) to Promote High School Students' Conceptual Understanding and Conceptual Change at the Molecular Level”, co-funded by the Thailand Research Fund (TRF) and Ubon Ratchathani University (UBU). The authors thank Loretta Jones of the University of Northern Colorado for assistance with English editing and a detailed review of the manuscript. The authors thank Panida Kanyakarn (class instructor) and her grade 11 students during academic year 2013 at Chiangkaew Pittayakom School for their active participation.References
- Balci S., Cakiroglu J. and Tekkaya C., (2006). Engagement, exploration, explanation, extension, and evaluation (5E) learning cycle and conceptual change text as learning tools, Biochem. Mol. Biol. Educ., 34(3), 99–102.
- Bektaşlı O. and Cakmakcı G., (2011), Consistency of students' ideas about the concept of rate across different contexts, Educ. Sci., 36(162), 273–287.
- Bybee R. W., Taylor J. A., Gardner A., Van Scotter P., Powell J. C., Westbrook A. and Landes N., (2006), The BSCS 5E instructional model: Origins, effectiveness, and applications, Colorado Springs: BSCS.
- Cakmakci G., (2010), Identifying alternative conceptions of chemical kinetics among secondary school and undergraduate students in Turkey, J. Chem. Educ., 87(4), 449–455.
- Cakmakci G., Leach J. and Donnelly J., (2006) Students' ideas about reaction rate and its relationship with concentration or pressure, Int. J. Sci. Educ., 28(15), 1795–1815.
- Çalık M. and Ayas A., (2005), An analogy activity for incorporating students' conceptions of types of solutions, Asia-Pacific Forum on Science Learning and Teaching, 6(2), Article 6, 1–13.
- Çalik M., Ayas A. and Coll R. K., (2009a), Investigating the effectiveness of an analogy activity in improving students' conceptual change for solution chemistry concepts, Int. J. Sci. Math., 7, 651–676.
- Çalık M., Ayas A. and Ebenezer J., (2009b), Analogical reasoning for understanding solution rates: students' conceptual change and chemical explanations, Research in Science and Technological Education, 27(3), 283–308.
- Çalik M., Kolomuc A. and Karagolge Z., (2010), The effect of conceptual change pedagogy on students' conceptions of rate of reaction, J. Sci. Educ. Technol., 19(5), 422–433.
- Chairam S., Somsook E. and Coll R. E., (2009), Enhancing Thai students' learning of chemical kinetics, Research in Science and Technological Education, 27(1), 95–115.
- Chandrasegaran A. L., Treagust D. F. and Mocerino M., (2007), The development of a two-tier multiple-choice diagnostic instrument for evaluating secondary school students' ability to describe and explain chemical reactions using multiple levels of representation, Chem. Educ. Res. Pract., 8(3), 293–307.
- Cunningham K., (2007), Applications of reaction rate, J. Chem. Educ., 84(3), 430–433.
- Davis J., (2013), Use of the FAR guide to present a pedagogical analogical model of gel electrophoresis in year 10 science, Teaching Science, 59(1), 28–31.
- Deters K. M., (2005), Student opinions regarding inquiry-based labs, J. Chem. Educ., 82(8), 1178–1180.
- Eskandar F., Bayrami M., Vahedi S. and Ansar V., (2013), The effect of instructional analogies in interaction with logical thinking ability on achievement and attitude toward chemistry, Chem. Educ. Res. Pract., 14, 566–575.
- Green W. J., Elliott C. and Cummins R. H., (2004), Prompted inquiry-based learning in the introductory chemistry laboratory, J. Chem. Educ., 81(2), 239–241.
- Hake R. R., (1998), Interactive engagement vs. traditional methods: a six-thousand-student survey of mechanics test data for introductory physics courses, Am. J. Phys., 61(1), 64–74.
- Harrison A. G. and Coll R. K., (2007), Using analogies in middle and secondary science classrooms: The far guide – an interesting way to teach with analogies, CA: SAGE Publications.
- Harrison A. G. and Treagust D. F., (2006), Teaching and learning with analogies, in Aubusson P., Harrison A. and Ritchie S. M. (ed.), Metaphor and analogy in science education, Dordrecht, The Netherlands: Springer, pp. 11–24.
- Justi R., (2003), Teaching and Learning Chemical Kinetics, in Gilbert J. K., De Jong O., Justi R., Treagust D. F. and Van Driel J. H. (ed.), Chemical Education: Towards Research-based Practice, Netherlands: Springer, pp. 293–315.
- Kırıka Ö. and Bozb Y., (2012), Cooperative learning instruction for conceptual change in the concepts of chemical kinetics, Chem. Educ. Res. Pract., 13, 221–236.
- Kolomuç A. and Çalık M., (2012), A comparison of chemistry teachers' and grade 11 students' alternative conceptions of ‘rate of reaction', J. Balt. Sci. Educ., 11(4), 333–346.
- Mulford D. R. and Robinson W. R., (2002), An inventory for alternate conceptions among first-semester general chemistry students, J. Chem. Educ., 79(6), 739–744.
- Normand M. D. and Peleg M., (2014), Surface area increase by size reduction, the Wolfram Demonstrations Project, Retrieved Oct 29, 2014 from http://demonstrations.wolfram.com/.
- Orgill M. and Bodner G., (2004), What research tells us about using analogies to teach chemistry, Chem. Educ. Res. Pract., 5, 15–32.
- Piquette J. S. and Heikkinen H. W., (2005), Strategies reported used by instructors to address student alternate conceptions in chemical equilibrium, J. Res. Sci. Teach., 42, 1112–1134.
- Sanger M., (2009), How does inquiry-based instruction affect teaching majors' views about teaching and learning science?, J. Chem. Educ., 85(2), 297–302.
- Sarantopoulos P. and Tsaparlis G., (2004), Analogies in chemistry teaching as a means of attainment of cognitive and affective objectives: a longitudinal study in a naturalistic setting, using analogies with a strong social content, Chem. Educ. Res. Pract., 5, 33–50.
- Schmitz G., (2005), What is a reaction rate? J. Chem. Educ., 82(7), 1091–1093.
- Taber K. S., (2002), Chemical misconceptions – prevention, diagnosis and cure, Theoretical background, London: Royal Society of Chemistry, vol. 1.
- Treagust D. F., (1988), Development and use of diagnostic tests to evaluate students' misconceptions in science, Int. J. Sci. Educ., 10, 159–169.
- Van Driel J. H., (2002), Students' corpuscular conceptions in the context of chemical equilibrium and chemical kinetics, Chem. Educ. Res. Pract., 3(2), 201–213.
- Venville G. J., (2008), The Focus-Action-Reflection (FAR) guide-science teaching analogies, in Harrison A. G. and Coll R. K. (ed.), Using Analogies in Middle and Secondary Science Classrooms, California: Corwin Press, pp. 22–31.
- Zoller U. and Levy Nahum T., (2012), From teaching to Know to learning to Think in science education, in Fraser B. J., Tobin K. and McRobbie C. J. (ed.), The Second International Handbook of Science Education, New York, NY: Springer, pp. 209–229.
- Zoller U. and Tsaparlis G., (1997), Higher and lower-order cognitive skills: the case of chemistry, Res. Sci. Educ., 27(1), 117–130.
| This journal is © The Royal Society of Chemistry 2015 |
