The effect of case-based instruction on 10th grade students' understanding of gas concepts
Eylem
Yalçınkaya
a and
Yezdan
Boz
*b
aTunceli University, Faculty of Engineering, Department of Chemical Engineering, Tunceli, Turkey
bMiddle East Technical University – Faculty of Education, METU Üniversiteler Mah. Dumlupınar Blv. No:1, Ankara çankaya 06800, Turkey. E-mail: yezdan@metu.edu.tr
First published on 6th November 2014
Abstract
The main purpose of the present study was to investigate the effect of case-based instruction on remedying 10th grade students' alternative conceptions related to gas concepts. 128 tenth grade students from two high schools participated in this study. In each school, one of the classes was randomly assigned as the experimental group and the other class, instructed by the same chemistry teacher, was assigned as the control group. The students in the experimental groups were instructed by case-based instruction based on conceptual change conditions while the control group students received traditionally designed chemistry instruction. As pre-tests, the science process skills test, the attitude and motivation towards chemistry and the gas concept test were applied to both groups of students. As a post-test, the gas concept test was administered to both groups of students to determine their alternative conceptions and understanding of gas concepts. One-way ANOVA was used to assess the effect of case-based instruction on students' understanding of gas concepts. The results revealed that case-based instruction was an effective method for overcoming students' alternative conceptions about the gas concepts.
Introduction
Constructivism explains learning as an active process, where students construct their own knowledge by making links between their existing and new concepts. In the case of a contradiction between new knowledge and existing ideas, it is hard to make sense of it since learners cannot make meaningful connections. This indicates that a person's prior knowledge plays a critical role in the process of learning (Driscoll, 2005). Research studies on science learning indicated that students come to classes with their own conceptions, which are often different from the scientifically accepted view. These alternative conceptions hinder students' subsequent learning since they interpret new knowledge in the light of these alternative conceptions (Gilbert et al., 1982). Therefore, instruction that considers students' prior knowledge and that allows students to modify their conceptions of scientific ideas would be beneficial to remedy students' alternative conceptions. As Wassermann (1994) states, case-based instruction serves this purpose by embedding learning in a realistic and social environment where students are actively involved in the process of knowledge construction. Although case-based instruction has been used in medicine, law, and business, there have been few research studies in science education. Moreover, there is almost no reported research study investigating the effectiveness of case-based instruction for overcoming students' alternative conceptions about the gas concepts. The present study aims to contribute to the literature by finding out whether case-based instruction is effective in enhancing students' understanding of gases and remedying their alternative conceptions. The related research questions are;1. Is there a significant mean difference between the groups exposed to case-based instruction based on conceptual change conditions and traditionally designed chemistry instruction with respect to tenth grade students' understanding of gas concepts and alternative conceptions?
2. What are the tenth grade students' alternative conceptions about gases after being exposed to case-based and traditional instruction?
Literature review
Most of the studies in science education indicated that students at all levels have difficulty in understanding the basic properties and behavior of gases (Novick and Nussbaum, 1978, 1981; Ben-Zvi et al., 1982; Brook et al., 1984, 2003; Gabel et al., 1987; Stavy, 1988; Benson et al., 1993; Hwang, 1995). For example, research studies showed that students face problems in understanding the notion of empty space between particles. Students stated that dust, other particles, gases such as oxygen and nitrogen, air, dirt, unknown vapors exist between particles (Novick and Nussbaum, 1978, 1981). In addition, students thought that gases have no mass or that substances in the gas phase are lighter than in the liquid or solid state (Stavy, 1988, 1990; Mas et al., 1987; Lee et al., 1993). Another common misconception is that students attribute macroscopic properties to particles, such as “expand” and “contract”, “get hot”, and “melt” (Brook et al., 1984, 2003; Novick and Nussbaum, 1981; Gilbert et al., 1982; Lee et al., 1993). For example, students believed that gas particles increase in size with the change from solid to liquid to gas (Haidar and Abraham, 1991).Students believed that air flows from one place to another like water but is unevenly distributed. According to some students, atmospheric pressure pushes the gas molecules down (Lin et al., 2000); air does not exert the same pressure in different directions (Brook et al., 2003); and gas particles are unevenly scattered in any enclosed space (Novick and Nussbaum, 1981; Lee et al., 1993; Cho et al., 2000). Moreover, students supposed that when the air is compressed, particles are compacted like a solid and do not move or they stick together (Lonning, 1993). Some of the students thought that when the air is compressed in a syringe, air moves toward the opening of the syringe (Lee et al., 1993). She (2002) examined the process of conceptual change related to air pressure and reported that most of the students believed that air cannot be compressed. They also thought that air pressure has a direction.
Moreover, some naive conceptions have been identified regarding cold and warm air. Most students thought that a balloon would blow up or get larger due to hot air or heat instead of thermal expansion. Hence, they believed that hot air would rise and cold air would go down in a bottle, so there was hot air or heat in the top and cold air in the bottom. In addition to these, some students believed that when a substance evaporates, it becomes invisible and it no longer exists (Lee et al., 1993). Moreover, the stereotypical views like ‘air is everywhere’ and ‘hot air rises’ were often stated by pupils (Séré, 1986). Related to the kinetic theory of gases, students had some major alternative conceptions; for example, they considered that atmospheric pressure pushes gas molecules down; gas molecules rise and stay away from heat; and molecules expand when they are heated (Lin et al., 2000). Students believed that when the temperature is lowered, gas particles sink to the bottom of a container and the majority of the high school students explained the decrease in volume of a gas on cooling not in terms of decreasing particle motion but in terms of increasing attractive forces (Novick and Nussbaum, 1981). Sometimes students' intuitive thinking can be one of the sources of misconceptions; for example as a cause of deflation of the balloon students said, “The energy gradually dies, so the gas motion stops and balloon deflates” (Haidar and Abraham, 1991). Accordingly, most of the students were not able to draw the appropriate representation of gas particles inside a flask (Lin et al., 2000). Nussbaum (1985) asked how the distribution of gases would be after evacuating some of the air from a flask. While some of the students (14 years old) thought that the upper part of the flask is filled with air, the others believed that the lower part of the flask is filled with air.
Students also had difficulties in understanding and applying the ideal gas law appropriately. Students memorized the ideal gas formula, PV = nRT without understanding it conceptually (Lin et al., 2000). Many of the students focused on the relationship between two variables in the ideal gas law regardless of the others. For instance, they assumed that “Pressure is always inversely proportional to the volume” and “Pressure is always directly proportional to the temperature” (Kautz et al., 2005a). From the microscopic viewpoint, some of the students believed that the density of a gas decreases as a result of expansion and so in order to keep the pressure constant, the speed of the particles must increase. Some of them thought that when a gas is enclosed in a smaller volume, gas particles are more likely to come together and collide with each other frequently. Consequently, the temperature and then average kinetic energy increases; that is, they “Mistakenly assume that molecular collisions generate kinetic energy” (Kautz et al., 2005b). Some students even thought that these collisions may result in a change of atomic size (Griffiths and Preston, 1992). Similarly, in the context of the diffusion concept, students thought that molecular motion of gases stops at an ending point in the diffusion. In addition, students believed that the diffusion rate of gases increases with increasing molecular weight (Cho et al., 2000).
Consequently, research findings about students' conceptions of gases indicated that gases are one of the abstract subjects in which students have difficulties in understanding. As stated before, the constructivist approach considering the students' prior knowledge and stressing the active engagement of students in the learning process is influential in providing meaningful learning (Mayer, 1999). Accordingly, case-based learning environments providing both real life examples and social experience promote constructivist learning (Jonassen, 1994). Case-based instruction aims to teach the topic through cases. Cases are composed of two main parts: one of them is the case situation for the study and the other is the questions related to the case. Cases are complex teaching instruments in the form of narratives. The narratives are generally based on real life situations. Teacher and students study the problems related to daily life cooperatively (Wassermann, 1994). Cases can vary from a paragraph or two to a dozen pages but it is suggested that long cases be distributed and read before the class to prevent students getting confused and becoming lost in details. Learners solve the presented problem using their background knowledge (DeYoung, 2003).
At the end of each case, some study questions related to the cases help students to evaluate outcomes, concepts, and subjects of the case. The purpose of the study questions is to facilitate student understanding, rather than simply asking for the names, dates, or labels. Case-based teaching provides opportunities for students to study in small groups and discuss their responses before the whole-class discussion session occurs. During examination of the case, the teacher manages the class discussion by promoting the critical analysis of the real life problems with the students and helping students to discover the meaning. The teacher avoids imposing his or her own thoughts. Rather s/he lets students interpret their own understanding during the period of discussion (Wassermann, 1994).
Though studies regarding case-based instruction in science education are limited, some research studies showed that case-based instruction was effective in improving students' critical thinking skills and increasing their interest in learning science (Gabel, 1999); making laboratory courses interesting and relevant to daily life (Frerichs, 2012); establishing a link between science and non-science classes (Richmond and Neureither, 1998); and increasing students' performance and academic knowledge regarding the nervous system (Çakır, 2002) and the human reproductive system (Saral, 2008). Moreover, case-based learning was found to be an effective method for remediating students' misconceptions in the context of solubility equilibrium (Çam, 2009); solids, liquids and gases (Ayyıldız and Tarhan, 2013); and gene biodiversity (Gallucci, 2007). Consequently, it can be said that case-based instruction is a teaching strategy for promoting students' engagement in learning science and making improvements toward conceptual change. In the present study, the aim was to provide four conditions for conceptual change that Posner et al. (1982) identified; dissatisfaction, intelligibility, plausibility, and fruitfulness.
Although the related literature suggests that case-based instruction would be more effective compared to the traditional instruction, the success of any teaching instruction depends on several issues. To illustrate, both students and teachers in Turkey are not accustomed to any active teaching methods, i.e. case-based instruction. Teachers in Turkey are used to teaching in a traditional way. However, in case-based instruction, the role of the teacher will change from the disseminator of information to the facilitator that guides students to construct their own knowledge. Similarly, the role for the students will change from listening passively to participating in discussions to reveal their ideas explicitly. As Airasian and Walsh (1997) suggest, it would take some time for students and teachers to get accustomed to these roles. Woods (1994) observed that teaching habits can make it difficult for teachers to accept change. Moreover, Gallucci (2007) claimed that instructors' enthusiasm using the case-method is an important factor contributing to the effectiveness of this method. In addition, more class time is needed for students to construct knowledge, and this presents a difficulty for teachers who need to complete a topic in an allocated period of time determined by the curriculum. This also affects the application of case-based instruction. If a teaching method was not applied genuinely in the classroom, even if it is effective theoretically, one cannot obtain positive results in practice. Similarly, success of a teaching approach may depend on the nature of the topic and the characteristics of students. Students with an external locus of control, where success or failure is attributed to external issues such as luck, fate etc., rather than their personal control, tend to do better when instructed in a teacher-centered way (Peterson, 1979). Moreover, it is not clear if suggested teaching methods based on constructivism would be successful for different subjects or contents (Airasian and Walsh, 1997). Cobern et al. (2010) also mentioned the nature of a topic does influence the choice of the most effective method of instruction. Therefore, we thought that it would be meaningful to compare case-based instruction with traditional instruction in order to reveal which one would be more effective for the students in our country in the context of the gases topic.
Methodology
Design of the study
Non-equivalent control group design was used in this study. In this design, although the groups being compared are randomly assigned as control and experimental, the subjects are not randomly assigned to these groups; instead already formed groups are used (Gay and Airasian, 2000). Two schools participated in the current study; one of them was a public high school, the other was an Anatolian high school. In Turkey, after elementary education, students enter a nation-wide examination to be placed at different high school types. Based on the scores achieved from this exam, students make some preferences. Students getting higher scores were placed at Anatolian high school; however, there are also score differences among Anatolian high schools. In the present study, students enrolled in the Anatolian high school scored more than the public high school students, but there was not a big score difference between these students. The same National Curriculum is followed in these schools and schools were similar in terms of the school facilities and the way teachers deliver chemistry lessons. Moreover, these schools were in the same district and the socio-economic backgrounds of students were similar. To control for the various variables that can influence students' achievement, students' pre-scores regarding attitude, motivational beliefs, understanding of gas conceptions and science process skills were taken into account (Pintrich et al., 1993; Brotherton and Preece, 1995; Harlen, 1999; Koballa and Glynn, 2007). Therefore, prior to the treatment, a science process skills test (SPST), an attitude scale towards chemistry (ASTC), the motivation section of motivated strategies for learning questionnaire (MSLQ) and the gas concept test (GCT) were administered to both experimental and control group students before instruction to determine whether there was a significant mean difference between two groups in terms of students' knowledge about gas concepts, students' attitude towards chemistry, science process skills and their motivation. After treatment, the gas concept test (GCT) was distributed to both groups of students in order to reveal whether there is significant mean difference in terms of students' conceptions of gas concepts.Sample
All 10th grade students in Ankara, the capital city of Turkey, were determined as the target population. However, since it is hard to get in touch with the whole target population, all the 10th grade students in Çankaya, which is the one of the districts in Ankara, were identified as an accessible population. After conversations with high school chemistry teachers in Çankaya, the schools in which the teachers volunteered to use a new teaching method in their chemistry lessons were chosen as implementation schools. Therefore, one public high school and one Anatolian high school were selected from the identified accessible population by the convenience sampling technique. In each school, one of the classes was randomly assigned as the experimental group and the other class instructed by the same chemistry teacher was assigned as the control group. Therefore, two classes from each school were assigned randomly as the experimental and the control group. Random selection was done by flipping a coin. In each school, classes were labeled as class A and class B. Heads side of the coin was matched with the experimental group while tails side of the coin determines the control group. For example, for class A, we flipped a coin and if it were heads, that class was assigned as the experimental group. Two instructional methods; case-based instruction on conceptual change conditions (CBCC) and traditionally designed chemistry instruction (TDCI) were assigned to the experimental and control groups respectively. Forty five tenth grade students (22 boys and 23 girls) from the Anatolian high school and 83 tenth grade students (44 boys and 39 girls) from the public high school participated in this study. There were 63 students instructed by CBCC in the experimental groups (31 girls and 32 boys) and there were 65 students (31 girls and 34 boys) instructed by TDCI in the control groups in total. The age range of the participants was 15–16. In each school, students were instructed by the same chemistry teacher for 12 weeks.Instruments
The science process skills test (SPST), the attitude scale towards chemistry (ASTC), the motivation section of the motivated strategies for learning questionnaire (MSLQ) and the gas concept test (GCT) were used as pre-test measuring instruments in order to determine the pre-existing differences between control and experimental group students before instruction. After treatment, in order to determine the effect of case-based instruction on overcoming alternative conceptions about gas concepts, GCT was administered to both groups as a post-test. In addition to GCT, after instruction semi-structured interviews were conducted with students from both groups in order to obtain deeper information regarding their conceptions about gas concepts. Finally, after treatment, a feedback form for case-based instruction was used as a research instrument in order to reveal experimental group students' opinions about the effectiveness of case-based instruction.Gas concept test (GCT)
The Gas concept test included 26 multiple choice questions with five alternatives. Many of the questions in GCT were taken and adapted from the earlier studies related to the gas topic (Azizoglu, 2004; pek, 2007). These questions were based on common alternative conceptions about gas concepts in the literature (Novick and Nussbaum, 1978, 1981; Brook et al., 1984; Séré 1986; Mas et al., 1987; Stavy, 1988, 1990; Rollnick and Rutherford, 1990; Benson et al., 1993; Lee et al., 1993; De Berg, 1995; Cho et al., 2000; Lin et al., 2000; Niaz, 2000; Sanger et al., 2000; She, 2002; Givry, 2003). At the beginning of the development stage of the test, the instructional objectives for gas concepts were stated based on the national curriculum. This test covered the following subtopics: (1) properties of gases, (2) volume of gases, (3) kinetic theory of gases, (4) diffusion of gases, (5) pressure of gases, (6) gas laws (Charles law, Boyle Marriott, Dalton, Avogadro, Gay Lussac), (7) ideal gas laws, (8) partial pressure of gases.Each item of the GCT was examined in detail by four chemistry educators and six chemistry teachers in terms of content validity and format. Based on these recommendations, the corrections on the test were made. Afterwards, GCT was piloted with 332 high school students from different schools who had learned the gas concept previously. The Cronbach-alpha value of the multiple-choice test was 0.70 in the reliability analysis. There was not any major change to the items on the test after the pilot study. Some questions involved diagrams related to representations of submicroscopic particles in order to make the questions more comprehensible. The final form of the test was administered to both group of students (control & experimental) as a pretest and a posttest in order to evaluate their understanding of concepts related to gases (see some examples of test items in Appendix I). Please note that all questions in the GCT were written in Turkish and the present article reports translated versions. The first author, who was a PhD candidate in chemistry education at the time of the study, translated questions in the GCT into English independently. Then, the equivalence of the translated and original version was checked by the second author, who is an instructor in the chemistry education department. They discussed any disagreements together in order to reach a consensus for the final English version of the GCT.
The science process skills test (SPST)
The test, which included 36 multiple choice questions related to identifying variables, operationally defining variables, identifying appropriate hypotheses, interpreting data and designing experiments, was originally developed by Okey et al. (1982). It was adapted into a Turkish version by Geban et al. (1992) found Cronbach's alpha to be 0.85 which indicated that the instrument is reliable enough. Some sample items are as follows (Table 1).| Sample items in SPST |
|---|
| A police chief is concerned about reducing the speed of autos. He thinks several factors may affect the automobile speed. Which of the following is a hypothesis he could test about how fast people drive? |
| (A) The younger the drivers, the faster they are likely to drive. |
| (B) The larger the autos involved in an accident, the less likely people are to get hurt. |
| (C) The more policemen on patrol, the fewer the number of auto accidents. |
| (D) The older the autos the more accidents they are likely to be in. |
| A science class is studying the effect of wheel width on ease of rolling. The class puts wide wheels onto a small cart and lets it roll down an inclined ramp and then across the floor. The investigation is repeated using the same cart but this time fitted with narrow wheels. |
| How could the class measure ease of rolling? |
| (A) Measure the total distance the cart travels. |
| (B) Measure the angle of the inclined ramp. |
| (C) Measure the width of each of the two sets of wheels. |
| (D) Measure the weight of each of the carts. |
The motivated strategies for learning questionnaire (MSLQ)
MSLQ is a self-report questionnaire developed for a college course by Pintrich et al. (1991) to evaluate students' motivational orientations and their use of different learning strategies. It uses a 7-point Likert scale from “not at all true of me” to “very true of me” measuring students' motivational and learning strategies constructs. Basically there are two main sections in MSLQ, a motivation section and a learning strategies section. In the current study, only the motivation section of MSLQ was used for both experimental and control group students to determine students' perceived motivation before treatment. In the motivation part, students' goals and belief values for a course, their beliefs about their skills to succeed, and their anxiety about tests in a course were evaluated by 31 items. MSLQ contains six sub-headings: (1) intrinsic goal orientation (IGO), (2) extrinsic goal orientation (EGO), (3) task value (TV), (4) control of learning beliefs (CLB), (5) self-efficacy for learning and performance (SELP), (6) test anxiety (TA). Sungur (2004) adapted and translated MSLQ into Turkish for a biology lesson. In the current study, minor changes were made to the instrument developed by Sungur (2004) and it was used for the chemistry lesson. Table 2 shows sample items for each sub-section of the questionnaire.| Sub-headings | Sample items |
|---|---|
| Intrinsic goal orientation | In a class like this, I prefer course material that arouses my curiosity, even if it is difficult to learn. |
| Extrinsic goal orientation | If I can, I want to get better grades in this class than most of the other students. |
| Task value | I think the course material in this class is useful for me to learn. |
| Control of learning beliefs | If I try hard enough, then I will understand the course material. |
| Self-efficacy for learning and performance | I'm confident I can learn the basic concepts taught in this course. |
| Test anxiety | When I take tests I think of the consequences of failing. |
This instrument was piloted with 324 tenth, eleventh and twelfth grade science students. As seen from Table 3, the instrument was found reliable enough.
| N (sample size) | IGO | EGO | TV | CLB | SELP | TA | |
|---|---|---|---|---|---|---|---|
| ENG | 356 | 0.74 | 0.62 | 0.90 | 0.68 | 0.93 | 0.80 |
| TUR (Sungur's) | 488 | 0.73 | 0.54 | 0.87 | 0.62 | 0.89 | 0.62 |
| TUR (current) | 324 | 0.69 | 0.75 | 0.64 | 0.69 | 0.70 | 0.77 |
Attitude scale towards chemistry (ASTC)
This scale included 15 items and was developed by Geban et al. (1994) to determine students' attitude toward chemistry as a school subject. It is a 5-point Likert scale as follows: “strongly agree, agree, undecided, disagree, and strongly disagree”. Cronbach's alpha was found to be 0.88 indicating that ASTC has good reliability. Some sample items from ASTC are: “I like reading books related to chemistry”, “Chemistry is not important in our daily life” and “I get bored when I study chemistry.”Interview questions
After treatment, semi-structured interviews were conducted with a total of sixteen students from both experimental and control groups. Eight students from the control group (3 girls, 5 boys) and eight students from the experimental group (4 girls, 4 boys) were interviewed. The criterion for selecting interviewees from each group of students was based on post-GCT scores. Two high, four medium and two low scorers from each group attended the interview.Interviewees were selected using the technique that Thompson and Soyibo (2002) used in their study in order to categorize students' attitudes into categories using the mean of the posttest scores and standard deviations. Similarly, in the present study, students whose scores were above one standard deviation from the mean were regarded as high achievers. Students whose scores were within one standard deviation below the mean and one standard deviation above the mean were considered as being moderate or neutral achievers. Students with scores below one standard deviation from the mean were classified as low or poor achievers. From the pool of high, moderate and low achiever students, two high achievers, two low achievers and four moderate achievers for both experimental and control groups were randomly selected by using the option (select random sample of cases) in SPSS. Extra questions were not prepared; instead test items used in GCT were used during the interviews. The purpose of the interviews was to probe the questions asked in the concept test and detect the reasons for selecting the wrong alternative. Also, both control and experimental group students were compared after treatment in terms of their conceptions about the gas topic in the light of the interviews. The elapsed time between the gas concept test and the interviews was about one to two weeks. The first author of the present study who was not a teacher of the students interviewed the students. Each interview was conducted individually and lasted about 50 minutes. All interviews were audio-taped and transcribed later.
Treatment (CBCC vs. TDCI)
Before the implementation of the current study, necessary legal permissions were received and all the materials used during the instructions were examined by the ethics committee of the university. During the implementation of the study, students were not harmed in any way (physically or mentally). All the students consented to participate in the study. Besides, the issue of confidentiality was emphasized in a way that names of the students would not be reported anywhere and the accessible data would be seen just by the researcher. Students were told that they would not be graded according to their responses and that it was important to respond to the presented tests as sincerely as possible.One hundred and twenty-eight tenth grade students from a public high school (N = 83) and an Anatolian high school (N = 45) were the participants in the present study. In each school, one of the classes was randomly selected as experimental and one class as the control group. The experimental group students were instructed by case-based instruction based on conceptual change conditions (CBCC) whereas the control group students were instructed by traditionally designed science instruction. All groups of students in the two schools followed the same National Curriculum and the same concepts for the same amount of time. For example, similar daily life examples and alternative conceptions were mentioned in both groups, and no laboratory work or demonstrations was conducted in both groups. However, in the control group, the teacher transmitted information while in the experimental group the students tried to construct this knowledge. In each school, the same teacher instructed both control and experimental group students. The teacher was male in one school while the other teacher was female.
A month before treatment, a teacher manual containing theoretical information about case-based instruction and the conceptual change model, cases that students will carry out in groups, and some directing questions for the teacher to guide students' discussion were given to the teachers. After a week, for three weeks, approximately one-hour meetings were conducted with the teachers. In these meetings, case-based instruction, the conceptual change model, the roles of the teacher and the students in case-based instruction were again explained to the teachers. Teachers were trained about the new method and how to implement case-based instruction based on conceptual change conditions for the gas unit in chemistry. For this purpose, the researcher explained a sample lesson using the atmospheric pressure case. Moreover, how the teachers would provide four conditions of conceptual change was discussed in teacher training sessions. To clarify, it was mentioned that discussion of study questions in the case was designed to create dissatisfaction among students. Therefore, the teacher should take students' opinions about these questions and discuss the reasons for different answers with the class. At that stage, the teacher may ask some more questions to promote students' dissatisfaction with the ideas. For the intelligibility and plausibility steps, the teacher was to ask some prompting and challenging questions and guide students to find the intelligible and plausible conclusion. For the fruitfulness step, the teacher was to encourage students to link the concept with daily life. Actually, classroom observations showed that teacher training sessions were successful because teachers took these suggestions into account and could apply the conceptual change conditions successfully in the classroom.
Experimental group students received case-based instruction based on conceptual change conditions. Prior to treatment, in each school experimental group students were divided into groups of four or five students by their chemistry teachers so as to be as heterogeneous as possible in terms of their chemistry achievement and general attitude toward chemistry. Then, the teachers gave information about the new teaching method; what the case-based instruction is and how it is applied in classroom settings emphasizing the roles of students in detail. The role of students in each group was to read and discuss the given problem and scenario under teacher guidance. The role of teachers was to provide an arrangement of groups and avoid giving the direct answers of the case-based learning questions during group discussions. The same content was covered in experimental group classes as in control group classes but experimental group students were instructed basically by means of the presented cases, problems or scenarios working in small groups. Students' alternative conceptions of gas concepts and remedies based on conceptual change conditions were taken into account while preparing the cases. In this study, a total of fifteen cases generally based on real-life events and experiments were used for gas concepts (see a case example in Appendix II). The cases were about atmospheric pressure, Avogadro's law, Boyle's law, Charles's law, Gay-Lussac's law, Dalton's law, a combination of Boyle–Avogadro–Charles's laws, diffusion rate (two cases), partial pressure, properties of hot and cold air, and properties of gases (four cases) (for more information on cases, please see Appendix III). For the preparation of cases, researchers investigated daily life applications and some experiments related to gas concepts from the chemistry books and internet resources. Then, case scenarios were developed from these sources. Three cases were taken from the studies of İpek (2007) and Bilgin et al. (2009). Similarly, study questions related to them were prepared based on students' alternative conceptions about gas concepts found in the literature. To illustrate, some examples of the study questions related to the case given in Appendix II were: “Compare the inside pressure of a deflated bicycle tire with the outside pressure”; “What can be said about the motion/state of gas particles in the deflated bicycle tire?”; “Can using up the energy of the gas particles in time and ceasing their motion be the reasons for deflation of the bicycle tire? Why?”; “Draw the distribution of the gas particles in the inflated and the deflated bicycle tire.” These study questions were formed in the light of the alternative conceptions: “the pressure inside a deflated bike tire or balloon is different from the pressure outside”: “the energy gradually dies, so the gas motion stops and balloon deflates”: and “gas particles are unevenly scattered in any enclosed space.”
After the preparation, for the content validity, these case materials were given to two teachers who were involved in the implementation of them and two chemistry educators. After the feedback, cases were reorganized if required. Before treatment, the cases were discussed with the chemistry teachers to decide where the cases will fit in the class schedule.
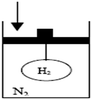
In experimental groups, students in small groups analyzed the given cases and answered the related questions. Afterward, group members shared their ideas with the whole class and class discussion began. Discussion continued until a reasonable or plausible answer(s) were found to the case questions. Meanwhile, experimental group teachers guided students by asking open-ended and challenging questions and prompting further thinking. Since the discussion of cases began and ended in class, students did not have opportunities to search or investigate the subjects from different resources such as books, internet, and library. Therefore, sometimes the needed information or clues was provided in the class materials required to solve the given problem. For example, during the implementation of the air bag case, the reaction equation (2NaN3(s) + heat → 2Na(s) + 3N2(g)) was given by the teacher after students' discussion about swelling of the air bag. And then one of the group members wrote the answers. This active learning environment allowed students to work in groups, identify learning issues, share related information with their classmates and develop critical thinking ability about events.
Since one of the purposes of the present study was to remedy students' alternative conceptions about gas concepts by case-based instruction based on conceptual change conditions, scenarios or problems were prepared by considering these alternative conceptions. The teaching strategy was planned by considering the conceptual change principles needed to assist students in removing their alternative conceptions, the conceptual change principles being dissatisfaction, intelligibility, plausibility, fruitfulness.
For example, experimental group students were presented with the atmospheric pressure case and asked why water boils faster above sea level. The dissatisfaction created in students' minds by this case and learning environment provided students opportunities to discuss the given scenario or event with both their group mates and classmates. While studying the case about atmospheric pressure, each group began to discuss the reasons for boiling water faster on the mountain. At first, some groups could not relate that event to the atmospheric pressure; they just said that the boiling point should be lower. At that point, the teacher tried to prompt students' further thinking, making them think about the relationship between the reason for boiling water faster on the mountain and atmospheric pressure using an open-ended, challenging question: Teacher: Think about the atmospheric pressure. How does it change with the altitude?
After this clue, students stated their reasoning
Some students: Water boils faster at the mountain since atmospheric pressure increases with increase in altitude.Most of the students: Water would boil faster due to the decrease in atmospheric pressure with altitude.
After group discussions, each group revealed its ideas and tried to convince the other groups with different viewpoints to accept its viewpoints. Most of the group had the correct reasoning
Most students: In the mountains, there is less atmospheric pressure, in order to boil faster, water should boil at a lower boiling point, less atmospheric pressure causes the water boil faster.
Some students: As you go towards the mountains, pressure increases. Since pressure increases, water molecules collide more and water boils at a lower temperature, that is why water boils faster at the mountain
A group told: You are confusing vapour pressure with atmospheric pressure. You say that pressure increases on the mountains, this is atmospheric pressure not vapor pressure. Atmospheric pressure is the pressure exerted by air
Teacher: What is boiling?
A student: Boiling occurs at the temperature where atmospheric pressure is equal to the vapor pressure
Teacher: What do you say; in order to boil faster, water should boil at a lower temperature or higher temperature than 100 °C, right?
Students: Lower temperature
Teacher: If you say that boiling occurs when atmospheric pressure is equal to vapor pressure. Should we decrease or increase atmospheric pressure in order to make water boil at a lower temperature
Students: Decrease
Teacher: So what do you think? What should atmospheric pressure be for the water to boil faster at the mountain?
Students: If the water boils faster, the pressure on it should be lower hence the atmospheric pressure should decrease when you go higher
The whole class discussion continued until the intelligible and plausible answer(s) were found by the students. As understood from the dialogues, the teacher had an important role in guiding the discussion and helping students to construct the knowledge correctly. Sometimes students gave extraneous or irrelevant responses. In this case teachers asked challenging questions to extend the thinking about the topic without changing the direction of the discussion. In order to make the information more meaningful and permanent, other questions related to the topic were asked. For example, the teacher asked another question “why do the climbers make a camp at certain altitudes while climbing the mountain or why do their nose bleed while climbing?”. Besides, he asked them whether they have had such an experience or not. None of the students had such an experience but one of them said that he saw it in a movie and explained the cause of this event as pressure change. After that, students were asked “Why people living in uplands or plateau are ruddy-cheeked?” The responses were interesting. While some groups of students thought that it was the consequences of the healthy diet, most of them specified it was due to the atmospheric pressure. The teacher also initiated several discussions about the oxygen amount in uplands as well as adaptations and transport of oxygen in human blood. Through this case, students not only learned how the atmospheric pressure changes with altitude, they gave an explanation to some real life events. In this way, the fruitfulness (or usefulness) stage of the conceptual change was provided. At the end of the instruction, GCT was administered to both groups of students as a posttest to measure the change in alternative conceptions about gases.
Students in the control group were instructed by traditional instruction in which a teacher-centered learning strategy was adopted. In traditionally designed classes, teachers defined and explained the concepts and solved related or similar questions for students. For example, during teaching the atmospheric pressure concept, a control group teacher mentioned that atmospheric pressure decreases with altitude. At that point, he asked whether water boils at the mountain at a lower or higher temperature or the same temperature. Different answers came from students. The teacher told the students that water boils at a lower temperature than 100 degrees Celsius on the mountains. Further questions used in experimental group classes were also asked but a discussion platform was not created in these classes. In some cases, students failed to respond to the questions. In this case, the teacher, himself, gave the answer to the question. For instance, he clearly defined that the people living in the uplands look pink since the number of red blood cells in their blood increases after a while. In control groups, students were only motivated by teacher-directed questions, and there were not any activities like group work included during the teaching of a gas topic. Students in traditional classes asked very few questions. They generally responded to the questions asked by the teacher. Teachers allowed a certain amount of time to solve the presented question. Meanwhile he or she sat on his or her table or walked around the class. Then students' opinions about questions were usually taken verbally and their questions were solved on the board by the teachers. When students asked any questions about the subject matter, the teacher answered them. However, students simply acted as passive listeners taking notes. Instruction in the control group was based on informing students about gas concepts. Similar daily life examples and alternative conceptions to those presented to the experimental groups were also mentioned in the control groups by the teachers.
After completing the case activities, the researcher and a PhD student in chemistry education observed the instruction in the experimental classes once a week and completed the treatment verification checklist which was prepared by the researcher in order to check whether the case-based instruction method was applied as required. The treatment verification checklist consisted of two parts: the first part included “yes” or “no” type items and the second part included items with a 5-point Likert-type scale (always, usually, sometimes, rarely, and never). The percentages of items marked as “usually” and “yes” were 75%. This checklist indicated that case-based instruction was implemented in accordance with the purpose of the study. Thus, treatment fidelity was provided with the help of a treatment verification checklist.
Limitations of the study
As limitations, the students were not randomly assigned to the groups. This may affect the representativeness of the sample. Another limitation may be that the GCT was not distributed to students as a delayed test so we could not assess the effect of case-based instruction on knowledge retention. Moreover, it should be noted that the novelty effect, which means the increased interest, motivation, or engagement of participants because of doing something different, not because it is effective or better may threaten the external validity of the study. In addition, the expectancy effect, where researchers expect the effectiveness of case-based instruction to be greater than traditional instruction, may have an influence on the effect level of case-based instruction (Taber, 2008).Results
Statistical analysis of pre-scores
All statistical analysis was carried out at the 0.05 significance level by using the statistical package for the Social Sciences (SPSS) 18. Before treatment, pre-ASTC, pre-SPST, pre-GCT and pre-MSLQ scores of students in both control and experimental group were compared to check the equality. After meeting the assumptions of normality, independence of observations and equal variances, independent samples t-test was used in order to check the equality of both experimental and control group students' pre-ASTC, SPST and GCT scores. Before treatment, it was found that experimental and control group students were not significantly different from each other with respect to their pre-attitude towards chemistry (ASTC) scores t(126) = 0.95, p = 0.34, their science process skills (SPST) scores t(126) = −0.45, p = 0.65, and pre-existing knowledge about gas concepts (pre-GCT) scores t(126) = −0.24, p = 0.16. Table 4 gives information about mean values of pre-ASTC, pre-SPST, pre-GCT and post-GCT scores for both control and experimental group students.| Group | Pre-ASTC | Pre-SPST | Pre-GCT | Post-GCT |
|---|---|---|---|---|
| Mean | Mean | Mean | Mean | |
| CG | 54.50 | 17.18 | 10.21 | 11.74 |
| EG | 52.88 | 17.60 | 10.33 | 16.84 |
Moreover, after meeting normality, homogeneity of covariance matrices and independence of observations assumptions, one-way MANOVA was conducted before treatment to check whether control and experimental students were different with respect to motivational variables. It was found that there was no statistically significant mean difference between experimental and control group students with respect to the students' motivational collective dependent variables of intrinsic goal orientation (IGO), extrinsic goal orientation (EGO), task value (TV), control of learning beliefs (CLB), self-efficacy for learning and performance (SELP), test anxiety (TA), Wilks λ = 0.92, F(6,121) = 1.68, p = 0.13. Table 5 describes mean values of students' pre-motivational scores across both groups of students.
| Dependent variables | CG | EG |
|---|---|---|
| Mean | Mean | |
| Intrinsic goal orientation | 19.31 | 20.68 |
| Extrinsic goal orientation | 22.11 | 21.73 |
| Task value | 30.38 | 30.82 |
| Control of learning beliefs | 22.06 | 21.63 |
| Self-efficacy for learning and performance | 40.20 | 40.01 |
| Test anxiety | 20.95 | 19.63 |
Statistical analysis of post-GCT scores
After meeting normality, homogeneity of variance and independence of observations assumptions, one-way ANOVA was performed to answer the first research question. A significant mean difference (F(1,126) = 49.91, p = 0.000) between experimental and control group students with respect to the treatment effect on students' understanding of gas concepts was found. The Eta-Squared value of 0.28 indicated the difference between experimental and control groups was not small. In other words, 28% of the variance of the dependent variable was associated with the treatment. Also, the power value of 1.000 showed the difference between experimental and control groups aroused from the treatment effect. As seen from Table 4, mean scores of post-GCT scores were 11.74 and 16.84 for control and experimental group students respectively. In sum, case-based instruction based on conceptual change conditions was an effective method for promoting students' understanding about gas concepts.Results of gas concept test (GCT) and interview
Eight experimental and eight control group students were the interviewees. Frequency analysis of students' responses for each item in GCT and interview results indicated that experimental group students had better understanding of gas concepts than their control group counterparts and they performed better reasoning in their answers than that given by control group students. However, some alternative conceptions were still present for both groups of students. Table 6 shows some alternative conceptions detected by GCT.| Alternative conceptions | Experimental group | Control group |
|---|---|---|
| When the gas in the container is cooled, each of the gas particles shrinks or gets smaller | 25.8 | 27 |
| When the gas is cooled, gas particles accumulated at the bottom of the container like liquids | 8.1 | 42.9 |
| When the gas is heated in a constant-volume container, gas particles condense in the wall of the container | 3.2 | 25 |
| With decreasing temperature, air molecules are getting closer to each other and accumulated in the middle of the container | 16.1 | 52.3 |
| Air molecules are accumulated at the bottom of the container with decreasing temperature | 9.7 | 36.9 |
| Heated air is accumulated on the walls of the balloon | 9.5 | 14.1 |
| Air is located between the particles of a gas | 12.9 | 55.4 |
| Hot air is lighter than cold air | 30.6 | 28.1 |
| Hot air is heavier than cold air | 1.6 | 12.5 |
Substance between the particles of a gas
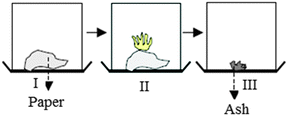
When students were asked what is present between the particles of a gas, item analysis of GCT showed that 74.2% of the experimental group students believed that there was nothing between gas particles, however only 16.9% of the control group students answered this question correctly. Interview findings also confirmed the difference between experimental and control group students' understanding. For example, six out of eight experimental group students knew that there is nothing between the particles of a gas. On the other hand, six of the control group students and two of the experimental group students believed that since air is present everywhere, it might be also present between the particles of a gas: “Air must be found among the particles of a gas since it is available in everywhere.”Conservation of mass
For the conservation of mass, GCT results showed that 48.4% of the control and 82.3% of the experimental group students were aware that mass was conserved. Interviews with students indicated that most of the experimental group students (six students) had better understanding than control group students since they knew that the total mass was conserved as a result of the burning of paper in a closed container. They expressed their reasoning by means of the law of conservation of mass in chemical reactions. However, two of the control group students and two of the experimental group students believed that the total mass of the container was the smallest in condition III where ash was formed after burning paper because they only paid attention to the picture ignoring the chemical reaction inside the container: “If the mass of the paper reduces while burning, the mass of the container also decreases. So, condition III has less weight.”According to three control group students, condition III has the biggest mass due to the increase in pressure in the container: “In condition III, the container is the heaviest due to the fact that it contains gas. Paper is not a heavy substance, and when it is in gas state it accumulates, so it is likely to make an effect on the pressure. The pressure would affect the mass of the container, but we cannot measure the pressure within the container and I think the mass of the container increased.”
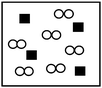
Partial pressure
From the interviews, it was noticed that control group students were confused about the concept of partial pressure. They could not select the correct figure that represents the partial pressure of oxygen when a mixture of helium and oxygen gases was placed in a closed container. Instead, four of them tried to remember the related formula or have no idea about the concept of partial pressure. In addition to these, three of the students from the control group expected to see the chemical reaction as an answer to the partial pressure of oxygen. GCT results also confirmed the superiority of experimental group students' understanding with respect to the partial pressure concept. Majority of the experimental (71.0%) and some control group (32.8%) students gave the correct response by selecting the correct figure.Distribution of air particles at 0 °C and 60 °C
For the distribution of air molecules at 0 °C and 60 °C, both interview and GCT results showed that experimental students had better understanding compared to control group students. For example, analysis of GCT revealed that 66.1% of experimental group students and 10.8% of control group students selected the scientifically correct answer, which represents homogeneous distribution of air with decreasing temperature when the temperature is lowered to 0 °C. Similarly, when the temperature is increased to 60 °C, a large difference between experimental (71.0%) and control (28.1%) group students giving the correct answer was detected. However, both groups of students had some alternative conceptions (see Table 6). Interviews with students showed that, as for the cooling of gas, four of the control group students believed that gas particles are collected in the middle of the container due to some reasons like temperature is not too cold for sinking, gravitational force has no effect on them, or they stick to each other when cooled down. Two experimental and four control group students thought that gas particles sink to the bottom of the container when the temperature was lowered to 0 °C though they were told that the gas was still a gas at that temperature. Moreover, one of the interesting answers came from experimental group lower achiever student. He stated that; “When the gas is cooled down, the gas particles move towards the upper part of the container since the upper part remains warm, the particles move towards upward”.When the temperature is increased to 60 °C, two experimental and five control group students thought that gas particles may condense on the walls of the container. They mainly think that when the temperature is increased, gas particles move away from each other, they are pushed towards the sides of the container and so the pressure increases. Increase in the pressure in the container leads students to have the idea that gas molecules may condense on the walls of the container. One of the control group medium achievers expressed the accumulation of particles on the walls like this: “The gas particles condense on the walls of the container when the gases are heated because the middle part of the container will be firstly affected from the heat since the heat is given from below”.Properties of cold and hot air
For the properties of cold and hot air, though experimental group students had better understanding of the properties of cold and hot air than control group students, both control and experimental group students had some alternative conceptions. For example, “Hot air is lighter than cold air” was found to be a common alternative conception and it was resistant to change. Apart from three students from the experimental group, none of the students thought that hot and cold air have the same mass but different volume. Students generally believed that particles of hot air must be lighter than the cold air gas particles: “Warm air rises and cold air sinks in a bottle, therefore warm air is lighter since it rises”. On the other hand, three students (two experimental group students and one control group student) thought that hot air is heavier than cold air due to daily life experiences. To clarify, two experimental group students thought that since the electric wires are loose in summer and stretch in winter, hot air is heavier than cold air. Additionally, one of the control group students stated, “Hot air is heavier than cold air. I think from the example that foots of the mountain are hotter, we feel the cold air more as we get upper and at the top it is seen that snow does not melt easily in any way therefore at the lower part of the mountain, there is hot air, at uppers there is cold air. Hot air is at lowers because hot air is heavier than cold air”. This finding is also confirmed by the GCT results. In GCT, 54.8% of experimental group students and 6.3% control group students selected the correct alternative stating that hot and cold air may have different volumes but they have equal masses. However, both groups of students still had some alternative conceptions regarding hot and cold air (see Table 6).Gas pressure in a non-constant volume container
For gas pressure in a non-constant volume container, students were asked to predict the shape of the balloon when the cylinder is pushed downward without touch of elastic balloon to the surface of the vessel (as shown in the Fig. 1).The answers given to GCT indicated that most of the experimental group students (51.6%) made correct interpretation about the shape of the balloon when the pressure inside the cylinder is increased compared to the control group students (34.9%). Similarly, interviews revealed that experimental group students' conceptions were more adequate. Experimental group students had better reasoning when they stated that a gas exerts pressure in each direction. On the other hand, about half of the control group students (four students) and a few of the experimental group students (two students) deduced that the balloon shrinks only from the bottom or only from above because they thought that pressure shrinks the balloon in the direction in which they exert the force to the cylinder or the exact opposite direction to the force applied: Since we push the piston downward, only the bottom of the balloon may shrink, there will be no change on the sides.
Gas pressure in a closed constant-volume container
For the gas pressure in a closed constant-volume container, students were asked to explain the reason for increase in the gas pressure in a closed constant-volume container with increase in temperature. Item analysis of GCT revealed that most of the experimental (84.1%) and control group students (60.9%) selected the scientifically correct response as stating that when the gas is heated in a constant-volume container, the number of collisions increase and so does the pressure. However, interview findings indicated that both control and experimental group students had some alternative conceptions related to the reasons for increasing pressure with increasing temperature in a constant volume container. For example, two of the experimental group students believed that the size of the gas particles increases due to heating and so does the pressure and three of the control group students thought that the size of the gas particles decreases with increase in temperature. Moreover, three of the control group students supposed that gas particles might become heavier due to taking heat. However, as with the other concepts, experimental group students' understanding was better.Change in speed of particles with the change in the volume of container
Interviews with students also revealed that when students were asked whether the speed of gas particles changes with changing the volume of the container, nearly all students gave incorrect responses. Only one of the high achievers from the experimental group and one of the high achievers from the control group associated the speed of the particles with the temperature. However, while five experimental and three control group students believed that speed of the gas particles increases due to the compression or increase in the number of collisions, two of the experimental and three control group students thought that speed of the particles of X(g) decreases with decrease in volume. An alternative conception revealed by experimental and control group students was that if the volume decreases, the speed of the particles increases due to the increase in the number of collisions. Students made a connection between the volume and the change of the speed of the gas particles because of the change in pressure in a container.Charles law
In the application of Charles's law where students were asked to select the environment that would decrease the volume of an inflated balloon tied by a rope, GCT results indicated that relatively more control group students answered this question more correctly; 76.9% of experimental and 79% of control group students gave the correct response. However, interviews showed that experimental group students' understanding was superior compared to that of the control group students. Interviews revealed that, though some students gave the correct answer based on their daily life observations, they could not give any further scientific explanations. For instance, one of the control group students stated: “The same pressure and colder because I have done it at home and I saw the balloon shrinking. In fact, I do not know the reason”. Interview results also indicated that some students provided the correct answer to this question but sometimes with wrong reasoning. Some of the control group students thought that in order to decrease the volume of the balloon, external pressure must be increased. They believe that since external pressure increases in a cold environment, the balloon shrinks. One of the control group students thought that in the cold, gas molecules accumulate in the middle and so the volume of the balloon decreases. Some of the experimental group students (three students) and one control group student gave the correct answer with correct reasoning. They associated the reason for the decrease in the volume of the balloon with the decrease of the speed, or kinetic energy of the particles or the distance between them.Gay Lussac law
For the Gay Lussac law, the following question was asked: there is a drop of mercury in the glass container as shown in Fig. 2. The mercury drop moves to the right or left depending on the pressure and temperature changing inside the glass container. The apparatus of the room temperature (25 °C) is put into environment 5 °C, students were asked to predict the direction of the movement of the drop of mercury.Item analysis of GCT showed that most of the control (62.3%) and some experimental group (42.6%) students gave the correct answer by stating the direction of movement as a result of decreasing pressure within the container with decreasing temperature. The interviews related to the effect of temperature on gas pressure in a constant volume container showed that though most of the experimental (six students) and control group students (six students) predicted the direction of movement of the mercury droplet correctly, some control group (three students) and some of the experimental group students (two students) had the wrong reasoning. The following excerpts indicate one of the control and experimental group students' ideas related to the direction of the mercury droplet as shown in the above figure regarding the cooling of the system:
When the temperature is lowered, the pressure inside the container decreases. Gas particles might be getting smaller or clustered. Since the pressure decreases, the mercury moves to left.
I think it moves to the left because when the temperature decreases, mercury droplet will move towards the particles due to shrinkage. Mercury comes close to gas.
Students believed that gas pressure decreases in the container due to the shrinking or getting smaller of the gas particles or clustering of them in the container. As well, one student from each group claimed that the volume changes due to a change in the pressure ignoring the constant volume container in the given system and misusing the ideal gas law. The results concerning the application of Gay Lussac law indicated that some students were unable to establish the relationship between temperature and the pressure of a gas when all the other variables that affect the pressure of a gas are kept constant.
To conclude, both GCT and interview results indicated that experimental group students had superior understanding of gas concepts compared to control group students. However, some of the alternative conceptions were still existent among students from both groups even after instruction.
Discussion and implications
In the present study of gas properties, case-based instruction based on conceptual change conditions promoted students' understanding of gas concepts and was effective in remedying the alternative conceptions of many of the students. This finding was supported by other researchers (Çakır, 2002; Mayo, 2002, 2004; Rybarczyk et al., 2007; Saral, 2008; Çam, 2009). In the current study, real-life events and illustrations were used in the construction of the cases and the list of study questions included alternative conceptions to create contradiction in students' minds related to the subject matter. Concepts associated with real life may have facilitated students' understanding and visualization of concepts. Furthermore in-group and whole-class discussions helped to reveal alternative conceptions because during those discussions, the existence of different ideas or points of view stimulated students' thinking and awareness and hence played an important role in remedying alternative conceptions. This is parallel with Gallucci's (2006, 2007) suggestions that small group discussions can be used to construct students' own conceptions. These features of case-based instruction seem to have provided better understanding of gas concepts compared to traditional instruction.In addition, a frequency analysis of students' responses for each item in GCT and interviews with students indicated that case-based instruction based on conceptual change conditions helped to overcome students' alternative conceptions and remedied most of them compared to the traditionally designed instruction. However, though most of the experimental group students had better understanding on the gas concepts, it was impossible to remedy all the alternative conceptions. This means that students are persistent in their use of alternative conceptions even after instruction designed to address these alternative conceptions (Champagne et al., 1985; Anderson and Smith, 1987; Wandersee et al., 1994). However, still, case-based instruction was found to promote students' conceptual understanding of gas concepts.
This study has implications for chemistry teachers, teacher education programs and textbook writers. Firstly, teachers should be aware of possible alternative conceptions students may have and consider students' existing knowledge when designing lessons since new knowledge is constructed upon the existing one. Since this study reported the effectiveness of case-based instruction on improving students' understanding, we can recommend that teachers use case-based instruction to support meaningful learning. So, teachers should be trained about how to write and implement the cases in their routine classes. They should also be encouraged to use new teaching techniques like case-based instruction in order to enrich their lessons. Secondly, teacher training programs in universities should include this method of learning and present examples of the implementation of cases so that graduate pre-service teachers can know how to implement case-based instruction in their future classes. Lastly, textbook writers should include effective cases in textbooks since instruction with cases was found to be effective in enhancing students' understanding.
We can also make some recommendations for future study. In the present study, cases were not supported by use of laboratory work or demonstrations. As a future study, effectiveness of the use of laboratory work or demonstration with cases could be evaluated with respect to written cases. Besides, as a future study, we can suggest that the effectiveness of case-based instruction on students' retention of knowledge should be assessed.
Appendix I
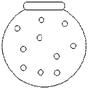
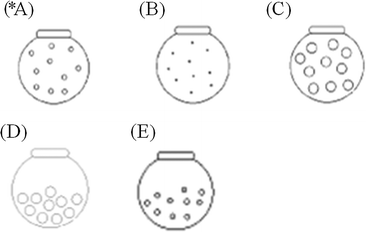
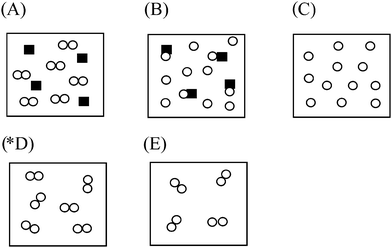
Q1. The distribution of hydrogen gas molecules in a closed container at 25 °C and 1 atm pressure was given in the next. (the circles (○) represent the distribution of hydrogen molecules), Which of the following diagrams illustrate the distribution of H2 molecules when the temperature of the container is lowered to −15 °C? (note: before responding to this problem students were told that at −15 °C hydrogen is still gas).
Q2. When a constant-volume closed container filled with a gas is heated, increase in pressure is observed. In which of following alternative explains the reason of this event most accurately?
(A) Increase the size of gas particles
(B) Increase in the numbers of particles when the gas is heated
(C) Becoming heavier of the gas when it is heated
(*D) Increase in the number of the collisions when the gas is heated
(E) When the gas is heated, gas particles condense in the wall of the container
Q3. A constant-volume container filled with air is connected to a balloon as shown in the figure. When the tap of the container is opened and the container is heated, it is observed that balloon is swelling. Which of the following illustrate the distribution of air the best after swelling the balloon? (dots (.) represent the molecules within air.)
Q4. What exists between the particles of a gas?
(A) Air
(B) Water vapor
(C) Other gases
(*D) Nothing
(E) Foreign substances (dust, dirt, etc.)
Q5. As shown in following figure, a piece of paper is put in a closed glass container in condition I. In condition II paper is burning and in condition III ash is formed. In all three cases, glass container is weighted. Accordingly, which one of the following is true?
(A) Condition I has the biggest mass
(B) Condition II has the biggest mass
(C) Condition III has the biggest mass
(D) I and II has the same weight and III is less
(*E) All of them has the same mass
Q6. The following closed container, as shown in picture, contains a mixture of oxygen (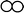 ) and helium (■) gases at 25 °C. Which one of the following situations would lead to the partial pressure of the oxygen gas if the pressure of only oxygen gas was measured?
) and helium (■) gases at 25 °C. Which one of the following situations would lead to the partial pressure of the oxygen gas if the pressure of only oxygen gas was measured?
Appendix II
As a cycling enthusiast, Onur does not miss the annual cycling tournaments. After a long preparation time, he completes his work for this year's tournament. Once doing necessary maintenance of his bike, Onur waits for the tournament day excitedly. Since the tournament will be international, there will be high level of participation and he will have many challenging opponents. The expected day finally comes and the tournament begins but it consists of tough stages (Fig. 3). After a few miles while passing through wooded areas, Onur's bicycle tire deflates. He loses the chance of racing as a result of this misfortune and he inflates the tire before leaving. Although he pumps the air from the same point of the rubber, tire inflation is the same in every point of the tire (Fig. 4). Which feature(s) of the gases do you think is the reason for this situation?What can be said about the motion/state of gas particles in the deflated bicycle tire?
Compare the inside pressure of deflated bicycle tire with the outside pressure.
Compare the inner pressures of the deflated tire exerted to the sides and to the bottom, please explain the reason.
Can using up the energy of the gas particles in time and ceasing their motion be the reasons of deflation of the bicycle tire? Why?
Draw the distribution of the gas particles in the inflated and the deflated bicycle tire.
What exists between the particles of gas in the bicycle tire?
Appendix III
| Name of the cases | Concept |
|---|---|
|
Boiling of water on the mountains
Ali likes to climb on the mountains in his free time. One day, during the camp, he realizes that water boils faster on the mountain compared to the sea level and he decides to investigate the reason for this. Why do you think that water boils faster on the mountains? |
Air pressure |
|
Heating of water in the bottle covered with a balloon
Children put some water in a glass bottle and closes the mouth of the bottle with a balloon. They begin to heat the bottle slowly from its bottom. After a few minutes, they measure the circumference of the balloon. After water in the bottle is heated, what happened to the balloon? What is the reason for this? |
Avagadro's law |
|
Air bag
Ayse experiences an accident while travelling, fortunately air bag in the car saves her life. Do you have any idea about the working principle of air bags? Which properties should the gas filling in the airbag have? Does the gas in the air bag exert the same pressure on all parts of the air bag? |
Properties of gases |
|
Cold and hot air
Mervan is curious about similarities and differences between cold and hot air and he decides to do an experiment in order to find out the similarities and differences between cold and hot air. He closes the glass container tightly, in which there is air. He weighs it before heating this container and he also weighs it after heating. What can be said about the mass of the container after being heated? |
Properties of gases |
|
Soda
When CO2 gas is dissolved in the liquid either by high pressure or low temperature, carbonated drinks occur. When we open the bottle of soda and wait for a while, CO2 gas dissolved in liquid mixes into air. In this case, is there a difference in the mass of soda? Why? |
Properties of gases |
| Bicycle tire (please see Appendix II) | Gas behavior, gas pressure |
|
Lung model
Breathing deeply can be associated with gas laws. When we breathe in, the muscles push the diaphragm downwards and the chest broadens. What is the effect of this on the pressure inside and volume of the lungs? When we take too much air in, how does it affect the elastic texture and the volume of the lungs? At the moment air taken in fills the lungs, if body temperature increases, what will happen to the volume of lungs? |
Boyle's law, Avagadro's law and Charles's law |
|
Book
As seen from the figure, Ozge placed half of a pipette in the plastic food storage bag with a zipper. Mouth of the bag is sealed tight with a zipper. In order to prevent the air escaping from the plastic bag, fingers were placed at each side of the pipette. Afterwards, a book was placed on the bag and air is blowed into the storage bag by pipette. After a while, it is seen that the book rises. What causes the book rise? |
Partial pressure, gas pressure |
|
Bottle covered with balloon
Children closes the mouth of a glass bottle, in which there is air, with a balloon that does not leak air in and out. First, they put the bottle in a cup that has hot water. After a while, what will be the shape of the balloon and why? Afterwards, they put the bottle in a cup full of ice in it. What will be the shape of the balloon after a while? |
Charles's law |
|
Air bubbles
As it is known, divers use scuba tanks that have compressed air (nitrogen–oxygen) in them. When we examine the actions of a diver swimming in the deep sea, we can observe that bubbles come out the mouth of the diver and these bubbles rise upwards. While these bubbles are rising up, it is seen that the volume of these bubbles increase gradually and they become several times bigger than they were at the beginning, Matter inside bubbles as well as chemical structure of matter do not change during the raise of bubbles. What may cause the change in the volume of the bubbles? (Assume that temperature of sea water is constant at each point of the sea) |
Boyle's law |
|
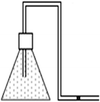
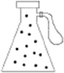
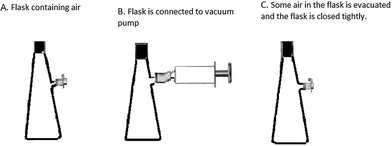
Vacuum pump
Dilek wants to remove some of the air in the flask by using vacuum pump in the laboratory. Figure A shows the flask without the vacuum pump being attached. In figure B flask is connected to the vacuum pump and some of the air in the flask is trapped in the vacuum pump. In figure C, process of evacuation of some air finishes and the mouth of the flask is closed tightly. Dilek could not observe the evacuation process since she cannot see gas particles. She wants to show the distribution of gases in the flasks by using dots. Could you please show these distributions in the flasks? |
Properties of gases |
|
Hot air balloon
Balloon can fly in the atmosphere by using heated air or light gases such as helium, hydrogen. How does heated air cause the balloon fly? What is the working principles of hot air balloons? If air in the balloon is cooled, what is the direction of the balloon? Is heated air in the flying balloon accumulated at the upwards of the balloon? Why? |
Gas behavior, gas pressure |
|
Competition of gases
In the competition where gases of He, H2, CH4, SO2 and SO3 race at the same conditions (same temperature and pressure), He and H2 gases finish the competition earlier, then CH4, SO2 and SO3 gases follow them respectively. What may be the reason for this? What type of information do we need to know in order to decide the order of gases in the competition? |
Diffusion of gases |
|
Formation of white ring
Two friends, Tugba and Aysel like to measure the diffusion rates of two different gases, NH3 and HCl by the help of apparatus below. They put cotton that is soaked into concentrated NH3 solution at one end of the apparatus and the other cotton was put at the other end of the tube and this cotton was soaked in the concentrated HCl solution (note that solutions are at the same environment) after a while, NH3 and HCl gases meet somewhere in the tube (temperature is constant during the experiment). Near to which end of the tube do these gases meet? Why? (HCl = 36.5 g mol−1, NH3 = 17 g mol−1) |
Diffusion of gases |
|
Boiling teapot
Ozlem is watching her mother cook. Her mother wants Ozlem take care of the food for a while, but when food begins to boil, Ozlem realizes that lid of the saucepan bounces up and she closes the cooker in panic. In another day, during the boiling of water in the teapot, Ozlem sees that the lid of the teapot moves similarly. She gets curious about this and wants to investigate the reason for this. What may be the reason for this? |
Gay-Lussac's law |
References
- Airasian P. W. and Walsh M. E., (1997), Constructivist cautions, Phi Delta Kappan, 78(6), 444–449.
- Anderson C. W. and Smith E. L., (1987), Teaching science, in Koehler V. (ed.), The Educators' Handbook, A research perspective, New York: Longman, pp. 84–111.
- Ayyıldız Y. and Tarhan L., (2013), Case study applications in chemistry lesson: gases, liquids, and solids, Chem. Educ. Res. Pract., 14(4), 408–420.
- Azizoğlu N., (2004), Conceptual change oriented instruction and students' misconceptions in gases, Unpublished doctoral dissertation, Ankara: Middle East Technical University.
- Benson D. L., Wittrock M. C. and Baur M. E., (1993), Students' preconceptions of the nature of gases, J. Res. Sci. Teach., 30(6), 587– 597.
- Ben-Zvi R., Eylon B. and Silberstein J., (1982), Students vs. chemistry: A study of student conceptions of structure and process (Unpublished technical report), Rehovot, Israel: Weizmann Institute, Dept. of Science Teaching.
- Bilgin İ., Şenocak E. and Sözbilir M., (2009), The effects of problem-based learning instruction on university students' performance of conceptual and quantitative problems in gas concepts, Eurasia Journal of Mathematics, Science & Technology Education, 5(2), 153–164.
- Brook A., Briggs H. and Driver R., (1984), Aspects of secondary students' understanding of the particulate nature of matter, Leeds: University Leeds, centre for Studies in Science and Mathematics Education.
- Brook A., Briggs H. and Driver R., (2003), Study of the evolution of students' initial knowledge during a teaching sequence on gases at the upper secondary school level, J. Res. Sci. Teach., 30(6), 587–597.
- Brotherton P. N. and Preece F. W. P., (1995), Science process skills: their nature and interrelationships, Research in Science & Technological Education, 13(1), 5–11.
- Champagne A. B., Gunstone R. F. and Klopfer L. E., (1985), Cognitive structure and conceptual change, New York: Academic Press.
- Cho I. Y., Park H. J. and Choi B. S., (2000), Conceptual types of Korean high school students and their influences on learning style, Paper presented at the Annual Meeting of the National Association for Research in Science Teaching, New Orleans, LA.
- Cobern W. W., Schuster D., Adams B., Applegate B., Skjold B., Undreiu A., Loving C. C. and Gobert J. D., (2010), Experimental comparison of inquiry and direct instruction in science, Research in Science & Technological Education, 28(1), 81–96.
- Çakır Ö. S., (2002), The development, implementation, and evaluation of a case method in science education, Unpublished doctoral dissertation, Ankara: Middle East Technical University.
- Çam A., (2009), Effectiveness of case-based learning instruction on students' understanding of solubility equilibrium concepts, Unpublished doctoral dissertation, Ankara: Middle East Technical University.
- De Berg K. C., (1995), Student understanding of the volume, mass, and pressure of air within a sealed syringe in different states of compression, J. Res. Sci. Teach., 32(8), 871–884.
- DeYoung S., (2003), Teaching strategies for nurse educators, Upper Saddle River, NJ: Prentice Hall.
- Driscoll M.P., (2005), Psychology of learning for instruction, Toronto: Allyn and Bacon.
- Frerichs V. A., (2012), ConfChem Conference on Case-Based Studies in Chemical Education: Use of Case Study for the Introductory Chemistry Laboratory Environment, J. Chem. Educ., 90(2), 268–270.
- Gabel C., (1999), Using case studies to teach science, National Association for Research in Science Teaching National Conference, Boston, Massachusetts.
- Gabel D. L., Samuel K. V. and Hunn D., (1987), Understanding the particulate nature of matter, J. Chem. Educ., 64, 695–697.
- Gallucci K., (2006), Learning concepts with cases, J. Coll. Sci. Teach., 36 (2), 16–20.
- Gallucci K., (2007), The case method of instruction, conceptual change, and student attitude, Unpublished doctoral dissertation, Raleigh: North Carolina State University.
- Gay L. R. and Airasian P., (2000), Educational Research: Competencies for analysis and application, New Jersey: Prentice-Hall Inc.
- Geban Ö., Aşkar P. and Özkan İ., (1992), Effects of computer simulated experiments and problem solving approaches on high school students, J. Educ. Res., 86, 5–10.
- Geban Ö., Ertepınar H., Yılmaz G., Altın A. and Şahbaz F., (1994), Bilgisayar destekli eğitimin öğrencilerin fen bilgisi başarılarına ve fen bilgisi ilgilerine etkisi. I.Ulusal Fen Bilimleri Eğitimi Sempozyumu: Bildiri Özetleri Kitabı, 1–2, 9 Eylül Üniversitesi, zmir.
- Gilbert J. K., Osborne R. J. and Fensham P. J., (1982), Children's science and its consequences for teaching, Sci. Educ., 66, 623–633.
- Givry D., (2003) Study of the evolution of students' initial knowledge during a teaching sequence on gases at the upper secondary school level (15 years old, grade 10), in the Proceedings of ESERA Summer-school, Radovljica (Slovénie).
- Griffiths A. and Preston K., (1992), Grade-12 students' misconceptions relating to fundamental characteristics of atoms and molecules, J. Res. Sci. Teach., 29, 611–628.
- Haidar A. H. and Abraham M. R., (1991), A comparision of applied and theoretical knowledge of concepts based on the particulate neture of matter, J. Res. Sci. Teach., 28(10), 919–938.
- Harlen W., (1999), Purpose and procedures for assessing science process skills, Assessment in Education, 6, 129–144.
- Hwang B. T., (1995), Students' conceptual representations of gas volume in relation to particulate model of matter, Paper Presented at the Annual Meeting of the National Association for research in Science Teaching, San Francisco, CA.
- İpek İ., (2007), Implementation of conceptual change oriented instruction using hands on activities on tenth grade students' understanding of gases concept, Unpublished master's thesis, Ankara: The Middle East Technical University.
- Jonassen D. H., (1994), Thinking technology, Educ. Technol., 34(4), 34–37.
- Kautz C. H., Heron P. R. L., Loverude M. E. and McDermott L. C., (2005a), Student understanding of the ideal gas law, Part I: a macroscopic perspective, Am. J. Phys., 73(11) 1055–1063.
- Kautz C. H., Heron P. R. L., Shaffer P. S. and McDermott L. C., (2005b), Student understanding of the ideal gas law, Part II: a microscopic perspective, Am. J. Phys., 73(11) 1064–1071.
- Koballa T. B. and Glynn S. M., (2007), Attitudinal and Motivational constructs in science learning, in Abell S. K. and Lederman N. G. (ed.), Handbook of Research on Science Education. Part 1, Mahwah, New Jersey, London: Lawrence Erlbaum Associates, Publishers, pp. 75–102.
- Lee O., Eichinger D. C., Anderson C. W., Berkheimer G. D. and Blakeslee T. D., (1993), Changing middle school students' conceptions of matter and molecules, J. Res. Sci. Teach., 30(3), 249–270.
- Lin H. S., Cheng H. J. and Lawrenz F., (2000), The assessment of students and teachers' understanding of gas laws, J. Chem. Educ., 77(2), 235–238.
- Lonning R. A., (1993), Effects of cooperative learning strategies on students verbal interactions and achievement during conceptual change instruction in 10th grade general science, J. Res. Sci. Teach., 30(9), 1087–1101.
- Mas C. J. F., Perez J. H. and Harris H. H., (1987), Parallels between adolescents' conception of gases and history of chemistry, J. Chem. Educ., 64(7), 616–618.
- Mayer R., (1999), Designing instruction for constructivist learning, in Reigeluth C. M. (ed.), Instructional-design theories and models: Vol. 2. A new paradigm of instructional theory, Mahwah, NJ: Lawrence Erlbaum Associates, pp. 141–160.
- Mayo J. A., (2002), Case-based instruction: a technique for increasing conceptual application in introductory psychology, J. Constr. Psychol., 15, 65–74.
- Mayo J. A., (2004), Using case-based instruction to bridge the gap between theory and practice in psychology of adjustment, J. Constr. Psychol., 17, 137–146.
- Niaz M., (2000), Gases as idealized lattices: a rational reconstruction of students' understanding of the behavior of gases, Sci. Educ., 9, 279–287.
- Novick S. and Nussbaum J., (1978), Junior high school pupils' understanding of the particulate nature of matter: an interview study, Sci. Educ., 62(3), 273–281.
- Novick S. and Nussbaum J., (1981), Pupils' understanding of the particulate nature of matter: a cross age study, Science EducationSci. Educ., 65(2), 187- 196.
- Nussbaum J., (1985), The particulate nature of matter in the gaseous phase, in Driver R., Guesne E. and Thiberghien, A. (ed.), Children's Ideas in Science, Philadelphia: Open University Press, pp. 124–144.
- Okey J. R., Wise K. C. and Burns J. C., (1982), Test of Integrated Process Skills (TIPS II). Athens: University of Georgia, Department of Science Education.
- Peterson P. L., (1979), Direct instruction: effective for what and for whom, Educ. Leadership, 37(1), 46–48.
- Pintrich P. R., Marx R. W. and Boyle R. A., (1993), Beyond cold conceptual change: the role of motivational beliefs and classroom contextual factors in the process of conceptual change, Rev. Educ. Res., 63(2), 167–169.
- Pintrich P. R., Smith D. A. F., Garcia T. and McKeachie W. J., (1991), A Manual for the use of the Motivated Strategies for Learning Questionnaire (MSLQ), Ann Arbor, MI: National Center for Research to Improve Postsecondary Teaching and Learning, The University of Michigan.
- Posner G. J., Strike K. A., Hewson P. W. and Gertzog W. A., (1982), Accommodation of a scientific conception: toward a theory of conceptual change, Sci. Educ., 66(2), 211–227.
- Richmond G. and Neureither B., (1998), Making case for cases, Am. Biol. Teach., 60(5), 335–340.
- Rollnick M. and Rutherford M., (1990), African primary school teachers: What ideas do they hold on air and air pressure?, Int. J. Sci. Educ., 12(1), 101–113.
- Rybarczyk B., Baines A. T., McVey M., Thompson J. T. and Wilkins H., (2007), A case-based approach increases student learning outcomes and comprehension of cellular respiration concepts, Biochem. Mol. Biol. Educ., 35(3), 181–186.
- Sanger M. J., Phelps A. J. and Fienhold J., (2000), Using a computer animation to improve students' conceptual understanding of a can-crushing demonstration, J. Chem. Educ., 77(11), 1517–1520.
- Saral S., (2008), The effect of case-based learning on tenth grade students' understanding of human reproductive system and their perceived motivation, Unpublished master's thesis, Middle East Technical University, Ankara.
- Séré M. G., (1986), Children's conception of the gaseous state prior to teaching, Eur. J. Sci. Educ., 8(4), 413–425.
- She H. C., (2002), Concepts of a higher hierarchical level require more dual situated learning events for conceptual change: a study of air pressure and buoyancy, Int. J. Sci. Educ., 24(9), 981–996.
- Stavy R., (1988), Children's conception of gas, Int. J. Sci. Educ., 10(5), 553–560.
- Stavy R., (1990), Children's conceptions of changes in the state of matter: from liquid (or solid) to gas, J. Res. Sci. Teach., 27(3), 247–266.
- Sungur, S (2004), The implementation of problem based learning in high school biology courses, Unpublished PhD thesis, Turkey: Middle East Technical University.
- Taber K. S., (2008), Exploring student learning from a constructivist perspective in diverse educational contexts, Journal of Turkish Science Education, 5(1), 2–21.
- Thompson J. and Soyibo K., (2002), Effects of lecture, teacher demonstrations, discussion and practical work on 10th graders' attitudes to chemistry and understanding of electrolysis, Research in Science & Technology Education, 20(1), 25–37.
- Wandersee J. H., Mintzes J. J. and Novak J. D., (1994), Research on alternative conceptions in science, in Gabel D. L. (ed.), Handbook of Research on Science Teaching and Learning, New York: Macmillan, pp. 177–210.
- Wassermann S., (1994), Introduction to case method teaching: A quide to the galaxy, New York: Teachers College Press.
- Woods D., (1994), Problem-based Learning: How to Gain the Most from PBL, Hamilton: W. L. Griffin Printing Limited.
| This journal is © The Royal Society of Chemistry 2015 |