The positive effect of anatase and rutile on the brookite-photocatalyzed degradation of phenol†
Bangde Luo,
Zhen Li and
Yiming Xu*
State Key Laboratory of Silicon Materials and Department of Chemistry, Zhejiang University, Hangzhou, Zhejiang 310027, China. E-mail: xuym@zju.edu.cn; Fax: +86-571-87951895; Tel: +86-571-87952410 Tel: +86-571-87951934
First published on 9th December 2015
Abstract
In this study, the biphase effect of brookite with anatase or rutile has been reinvestigated by using phenol degradation in aqueous solution as a model reaction. Prior to use, each oxide was sintered at 500 °C. Then two oxides were mixed in alcohol, followed by drying and sintering at 450 °C. Solid characterization showed that each oxide remained intact, in terms of the average crystallite size and surface area. As the weight percent of anatase or rutile in the mixture increased, the apparent activity of TiO2 for phenol degradation under O2 increased, and then decreased. A maximum apparent activity of TiO2 was observed at 50% of anatase and rutile, respectively. However, with the same amount of silver ions on the oxide surface for phenol degradation under N2, the intrinsic activity of TiO2 became independent of the content of either anatase or rutile. These observations indicate that the thermodynamically possible charge transfer between two phases does not occur in practice. Moreover, for the photocatalytic reduction of O2 to H2O2 in an aqueous suspension containing excess phenol, anatase was more active than brookite, followed by rutile. Accordingly, it is proposed that there is an interfacial O2 transfer from anatase to brookite, and/or from brookite to rutile. This would improve the efficiency of the charge separation and consequently increase the rate of phenol degradation at interfaces.
Introduction
It is known that a variety of toxic and recalcitrant organic pollutants, in the presence of TiO2 and under UV light, can degrade into CO2 and small fragments at ambient temperature and pressure when only using O2 as an oxidant.1–6 Because of that, TiO2 photocatalysis has been widely studied as a potential technology for environmental remediation. It is generally recognized that TiO2 after excitation with UV light will generate electrons (ecb−) and holes (hvb+) in the conduction and valence bands, respectively. Then these charge carriers migrate into the surface, and eventually react with suitable substrates. However, the quantum yield of TiO2 photocatalysis for organic degradation is usually very low,7 mainly due to fast recombination of ecb− and hvb+. In terms of the kinetics, the rate of the interfacial reaction is not only proportional to the number of ecb− and hvb+ available on the oxide surface, but also proportional to the surface concentration of O2 and organic pollutant. Therefore, to improve the quantum yield of organic degradation, both the carrier mobility of TiO2, and its affinity to the dissolved target substrates in aqueous solution are equivalently important.In nature, there are three crystal forms of TiO2 (anatase, rutile and brookite). As a photocatalyst, anatase and rutile have been intensively investigated, but brookite is the least studied, because of the difficulty in synthesis.8 It is often observed that the apparent photocatalytic activity of TiO2 for organic degradation is determined by many factors, including the physical parameters of TiO2 itself,2 and the model reactions used.9 In general, anatase is much more active than rutile, whereas their mixture is more active than single phase oxide. A typical sample is the outstanding performance of P25 TiO2, which composes of about 80% anatase and 20% rutile. The observed biphase effect is ascribed to the interfacial charge transfer from anatase to rutile,10–12 and/or from rutile to anatase.13–15 As a result, the efficiency of the charge separation is improved, and the rate of organic degradation is increased. Recently, it has been also claimed that a mixture of brookite with anatase,16–19 or with rutile,20,21 is more active than single phase anatase, brookite, and rutile, for the photocatalytic degradation of chlorophenol and organic dyes in aqueous solution. Interestingly, the observed biphase effect is also ascribed to the interfacial charge transfer from brookite to anatase or to rutile. However, these charge transfer models are proposed only on the basis of thermodynamics, without direct evidence. The conduction band edge potentials for anatase, rutile and brookite are different, which are about −0.12, 0.10, and −0.24 V versus normal hydrogen electrode (NHE) in aqueous solution at pH 0, respectively.2,20 Moreover, in those studies, the biphase oxide was prepared from the hydrolysis of a Ti compound, whereas the phase ratio was regulated by changing the content of a specific acid and base reagent. Then, it is disputable that one component in the biphase oxide is not the same as that in pure phase obtained under different conditions. In other words, the observed biphase effect may result from changes in the physical properties of TiO2 itself. Thus, the exact mechanism responsible for the observed biphase effect needs to be further verified.
The relative apparent photocatalytic activity of TiO2 among the samples is usually evaluated from the rate of organic degradation in aqueous solution, without consideration of a possible difference in O2 adsorption. This may result in a wrong judgment about the catalyst activity. According to the principle, the electrons and holes of TiO2 are photogenerated and consumed in a pair. Then a catalyst that has a high intrinsic activity, but a low sorption capacity toward O2 in aqueous solution, would show a low apparent activity. In other words, the activity assessment should be made at the same amount of O2 adsorption. Unfortunately, this is difficultly achieved in practice. Recently, we have used Ag+ and Cr(VI) as the electron scavengers of TiO2 for phenol degradation in water under N2. Surprisingly, with the same amount of Ag(I) or Cr(VI) on the oxide, the intrinsic photocatalytic activity of TiO2 only increases with its synthesis temperature (Ts), regardless of the solid structures in the forms of anatase, rutile, anatase/rutile, and brookite.22–25 At given Ts, anatase and rutile have similar intrinsic photocatalytic activities. Then, the observed higher apparent photocatalytic activity of anatase than that of rutile is due to its larger uptake of O2 from aqueous solution, whereas the observed biphase effect is ascribed to the O2 transfer from anatase to rutile. Increasing the surface concentration of O2 would not only accelerate the O2 reduction, but also facilitate the charge separation. As a result, the apparent photocatalytic activity of TiO2 for organic degradation is enhanced.
Here, the brookite-related biphase effect has been reinvestigated, in terms of both the apparent and intrinsic photocatalytic activities. Pure brookite was mixed with pure anatase or pure rutile at different ratios in isopropanol, followed by drying and sintering at 450 °C. To ensure intact the physical changes of individual phase, each oxide before use was pretreated at 500 °C. To avoid the effect of organic adsorption, direct photolysis, and dye sensitization, phenol was used as a model substrate. This substrate in aqueous solution was stable against UV light at wavelengths longer than 320 nm, and its dark adsorption on TiO2 was also negligible. The solid was characterized with several techniques, and the rate of phenol degradation was measured in the presence of O2 and AgNO3, respectively. In terms of the apparent photocatalytic activity of TiO2, the brookite-related biphase effect was observed. However, in terms of the intrinsic photocatalytic activity of TiO2, the brookite-related biphase effect was not observed at all. Furthermore, to understand the possible mechanism, the photocatalytic generation of H2O2 from the electron reduction of O2 was also measured.
Experimental section
Materials
Titanium bis(ammonium lactate) dihydroxide (TALH), TiCl4, phenol, peroxidase (POD), N,N-diethyl-1,4-phenylenediamine (DPD), p-dimethylaminobenzalrhodanine (DMABR), and anatase TiO2 (cAT) were purchased from Sigma-Aldrich, and other chemicals from Shanghai Chemicals Inc., China. Pure brookite (sBT) and pure rutile (sRT) TiO2 were made by following the literature methods.26,27 For sBT, an aqueous solution (100 mL) containing 10 mL TALH and 6.0 M urea was heated in an autoclave at 160 °C for 24 h. For sRT, the hydrolysis of 0.36 M TiCl4 in 1.2 M HCl was carried out in an iced bath, followed by heating at 60 °C for 2 h. After the suspension cooled down, the precipitates were collected, and washed thoroughly with water and ethanol. The solid was then dried in a vacuum oven at 60 °C. Finally, each oxide (cAT, sRT and sBT) was thermally treated in air at 500 °C for 3 h.The biphase oxide was prepared as follows. The powders of sBT were mixed with 0–100 wt% of cAT or sRT in 50 mL of isopropanol under magnetic stirring. Then, the suspension was shaken in a microwave cleaner for 1.5 h, and dried in a fume hood at 90 °C. Finally, the sample was sintered in air at 450 °C for 3 h.
Characterization
X-ray diffraction (XRD) pattern was recorded on a D/max-2550/PC diffractometer (Rigaku), using a Cu Kα as the X-ray irradiation source. According to the full-widths at half-maximum of the (121) brookite at 2θ = 30.8°, the (101) anatase at 2θ = 25.3°, and the (110) rutile at 2θ = 27.4°, the average crystallite diameters (ds) for brookite, anatase, and rutile were calculated, respectively, by using the Scherrer equation. Note that except the (121) peak, all other diffractions of brookite seriously overlap those of anatase. Raman spectra were obtained on a Jobin Yvon LabRam 1B with a He–Ne laser excitation at 632.8 nm. Adsorption–desorption isotherms of N2 on solid were measured at 77 K on a Micromeritics ASAP2020 apparatus. The Brunauer–Emmett–Teller (BET) specific surface area (Asp), and total pore volume (Vp) of the solid were calculated from the adsorption and desorption branches of the isotherms, respectively. Diffuse reflectance spectrum was recorded on a Shimadzu UV-2550 with BaSO4 as a reference. The reflectance (R) was transferred into the Kubelka–Munk (K–M) absorbance, according to the equation of FR = (1 − R)2/(2R). The band gap energy (Eg) of TiO2 was estimated by using a derivative method.28Photocatalysis
The reactor was made of a Pyrex-glass with a water jacket. The light source was a 375 W high pressure mercury lamp (Shanghai Mengya, China). The distance between the reactor and lamp was fixed at 10 cm. During the experiments, the reactor was thermostated at 25 °C, and stirred magnetically under a constant rate. Except stated otherwise, the experiments were carried out at a fixed initial condition (1.00 g L−1 catalyst, 0.43 mM phenol, 1.0 mM AgNO3, and pH 6.5). Before light irradiation, the aqueous suspension (50.0 mL) was stirred in the dark for 2 h. At given intervals, 2.0 mL of the suspension was withdrawn by a micro-syringe, filtered through a membrane (0.22 μm in pore size), and immediately analyzed. When AgNO3 was used, the suspension was first purged with N2 (99.99%) for 0.5 h, and then sealed off for the subsequent experiments. Organic substrate was analyzed by HPLC (high performance liquid chromatography) on a Dionex P680 (Apollo C18 reverse column, and 50% CH3OH/H2O as an eluent). Silver ion was analyzed at 468 nm on an Agilent 8451 spectrometer, through its complex with DMABR.29 Hydrogen peroxide was measured at 551 nm through the POD catalyzed-oxidation of DPD.30Results and discussion
Characterization
The solid structure was confirmed by XRD and Raman spectroscopy (Fig. 1A and B). The diffraction patterns of cAT, sRT and sBT were in agreement with those for anatase (PDF no. 12-1272), rutile (PDF no. 21-1276), and brookite (PDF no. 29-1360), respectively. However, most of the peaks for sBT overlapped those for anatase. To examine the phase purity, the Raman spectra were recorded. For sBT, there were 14 peaks at 100−700 cm−1, which can be assigned to the vibration modes, A1g (126, 148, 196, 244, 408, 540 and 634 cm−1), B1g (211 and 284 cm−1), B2g (363, 454 and 582 cm−1), and B3g (318 and 498 cm−1) of brookite, respectively.31 The Raman spectra of cAT and sRT were similar to those for anatase (141, 390, 512 and 634 cm−1), and for rutile (141, 235, 442 and 607 cm−1), respectively.32,33 These observations indicate that all of cAT, sRT and sBT are in the pure forms of anatase, rutile, and brookite, respectively. | ||
| Fig. 1 (A) XRD patterns, (B) Raman spectra, (C) absorption spectra, and (D) isotherms of N2 adsorption–desorption (open symbols), measured with the samples (a) cAT, (b) sRT, and (c) sBT. | ||
In the absorption spectrum of each oxide (Fig. 1C), there was a strong and broad band, assigned to the ligand-to-metal charge transfer (O2− → Ti4+). By using a derivative method, the band gap energies (Eg) for sBT, cAT, and sRT were estimated to be 3.37, 3.25, and 3.04 eV, respectively. In the adsorption–desorption isotherms of N2 (Fig. 1D), there was a hysteresis loop, due to the presence of mesopores. From the adsorption branch, the total pore volume (Vp) at a relative pressure of 0.99 was calculated, which increased in the order of cAT (0.311 cm3 g−1) > sBT (0.273 cm3 g−1) > sRT (0.151 cm3 g−1). However, the average pore size (dp), calculated from the desorption branch, increased in another order of sRT (35.7 nm) > sBT (22.2 nm) > cAT (14.8 nm). Through a t-plot, the micropore volume (Vm) was estimated, which was 4.34, 0.59, and 0.44 in unit of 10−3 cm3 g−1 for sBT, cAT, and sRT, respectively. These observations indicate that these single-phase oxides have different amounts of micropores and mesopores. Moreover, the BET surface area was calculated, which was 48.3, 73.4, and 25.3 m2 g−1 for sBT, cAT, and sRT, respectively. Among the samples, sRT has the largest value in average pore size, but the lowest values in surface area, micropore volume, and total pore volume.
In biphase oxide, each phase remains nearly intact, in terms of the crystallite size and surface area. As the weight percent of cAT (sRT) in the biphase oxide increased, the diffraction peaks due to brookite and anatase (rutile) decreased and increased, respectively (Fig. S1†). The average crystallite size (dXRD) of brookite in sBT was 20.4 ± 0.05 nm, whereas the values of dXRD for brookite in cAT/sBT and sRT/sBT were 21.2 ± 0.7 nm, and 22.0 ± 0.6 nm, respectively. Similarly, the values of dXRD for anatase in cAT/sBT (14.8 ± 2.0 nm), and rutile in sRT/sBT (17.4 ± 1.0 nm) were also very close to those of anatase in cAT (18.0 ± 0.02 nm), and rutile in sRT (18.3 ± 0.03 nm), respectively. A large error in dXRD for anatase in cAT/sBT is due to the peak overlapping of anatase with those of brookite. On the other hand, the BET surface area of cAT/sBT and sRT/sBT increased and decreased with the weight percent of cAT and sRT, respectively (Fig. 2A). Interestingly, the measured surface area was nearly the same as the calculated one from individual components in the mixed oxide. Since N2 mainly adsorbs onto the external surface of the solid, it follows that the average particle size of TiO2 in the biphase oxide is the same as that in the single phase oxide.
 | ||
| Fig. 2 (A) BET specific surface area, and (B) total pore volume of (a) cAT/sBT, and (b) sRT/sBT. The solid and open symbols represent the measured and calculated values, respectively. | ||
However, the measured total pore volume for sRT/sBT was obviously lower than the calculated one (Fig. 2B). Since the measured and calculated micropore volumes were consistent (Table S1†), it follows that the mesopores of sRT are partially damaged, with the formation of a new mesopore in sRT/sBT. This can occur when the mesopores of sRT are filled up with the fine particles of sBT. In fact, as the content of sRT increased, the hysteresis loop originally from sBT did not shift in the whole toward the right side of the isotherm (Fig. S2†). Instead, there appeared a new type of hysteresis loop at a high relative pressure. Furthermore, the absorption spectrum of biphase oxide was approximately equal to the sum of the absorption spectra of individual oxides (Fig. S3†). These observations indicate that the biphase oxide is a simple mixture of sBT with cAT or sRT. In the following, these biphase oxides will be used as photocatalysts. If a biphase effect is observed, it would surely originate from the synergism between two phases, rather than from changes in the physical parameters of TiO2 itself.
Photoactivity in the presence of O2
In this study, phenol was used as a model substrate. Under UV light, the concentration of phenol in an aerated aqueous suspension decreased with the irradiation time, the kinetics well fitting the first-order rate equation. At the same time, there were produced several intermediates, including hydroquinone. However, when Ag+ was used as electron scavenger, phenol degradation completely eased at a certain time, due to the concurrent reduction of Ag+ ions to silver particles. To minimize the effect of the intermediates, the initial rate of phenol degradation at the first 10 min was measured. Fig. 3A shows the results of phenol degradation in an aerated aqueous solution. Among single phase oxides, the rate of phenol degradation increased in the order of cAT > SBT > sRT. As the weight percent of cAT or sRT in biphase oxide increased, the rate of phenol degradation increased, and then decreased after reaching a maximum at 50% of cAT or sRT. At this optimal loading, the rates of phenol degradation over cAT/sBT and sRT/sBT were about 1.64 and 1.41 times that over sBT, respectively. When the catalyst surface area was taken into account, a maximum specific rate of phenol degradation was still observed at 50% of cAT or sRT in the sBT-mixed oxides (Fig. 3B). Recall that each phase in the mixed oxide remains intact. Then, the observed rate enhancement of phenol degradation is surely due to the biphase effect of brookite with anatase, or with rutile. Such biphase effect results in great improvement in the apparent photocatalytic activity of TiO2 for phenol degradation in an aerated aqueous solution. | ||
| Fig. 3 (A) Initial rates of phenol degradation over (a) cAT/sBT, and (b) sRT/sBT, measured in an aerated aqueous suspension. (B) The normalized rates with the solid BET surface area (Asp). | ||
Photoactivity in the presence of AgNO3
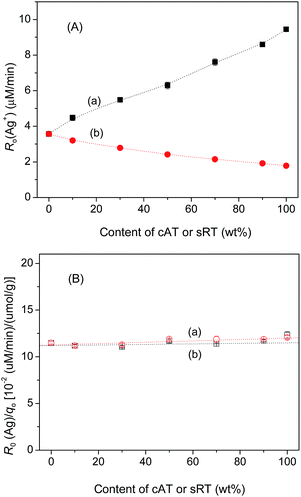
Fig. 4A shows the result of phenol degradation over TiO2, measured in a N2-purged aqueous suspension containing 1.0 mM AgNO3. As the weight percent of cAT and sRT in biphase oxide increased, the initial rate of phenol degradation [R0(Ag)] increased, and decreased, respectively. In this case, no maximum rate of phenol degradation was observed any more. Control experiments in the homogenous aqueous solution of AgNO3 showed negligible degradation of phenol either in the dark or under UV light. Moreover, the initial amount of Ag+ adsorption [q0(Ag)], measured in the dark and before light irradiation, also changed with the content of cAT or sRT in biphase oxide (Fig. 4B). Since phenol degradation is the outcome of Ag(I) reduction, R0(Ag) was then normalized with q0(Ag). Strikingly, this specific rate of phenol degradation [R0(Ag)/q0(Ag)] was nearly independent of the weight percent of cAT or sRT in the biphase oxide. This observation is in agreement with our previous result that all of anatase, rutile, anatase/rutile, and brookite at given Ts have similar intrinsic photocatalytic activities.22–25 In the present work, all the catalysts were prepared at the same temperature (see the Experimental section). Therefore, they showed similar intrinsic photocatalytic activities for phenol degradation. If there is an interfacial charge transfer between two phases, the rate of Ag+ reduction, and then the rate of phenol degradation under N2 should be enhanced at a certain content of cAT or sRT in biphase oxide. In fact, no biphase effect was observed either from cAT/sBT or from sRT/sBT for phenol degradation under N2 and in the presence of AgNO3. These observations indicate that the previously proposed charge transfer between brookite and anatase or rutile is less likely.Photocatalytic reduction of O2 to H2O2
The observed difference in the apparent and intrinsic photocatalytic activities of TiO2 among the above samples may result from that difference in O2 adsorption. To practise this hypothesis, the formation of H2O2 from the photocatalytic reduction of O2 was examined. In this case, the holes of TiO2 were consumed by excess phenol. Fig. 5 shows the time profiles of H2O2 production, measured in an aerated aqueous suspension containing 0.43 mM phenol. As the irradiation time increased, the concentration of H2O2 in aqueous solution increased, and then decreased. According to the formation rate of H2O2, the apparent photocatalytic activity of TiO2 increased in the order of cAT > sBT > sRT. Since these oxides have similar intrinsic photocatalytic activities (Fig. 4B), it follows that the sorption capacity of TiO2 toward O2 in aqueous solution would increase in the order of cAT > sBT > sRT. In the previous study, the observed biphase effect between anatase and rutile is ascribed to the O2 transfer from anatase to rutile, which explores the intrinsic photocatalytic activity of rutile.25 In the present work, we propose that there is also an interfacial transfer of O2 from anatase to brookite, and/or from brookite to rutile. Such O2 transfer would increase the rate of O2 reduction, and improve the efficiency of the charge separation. As a result, the apparent photocatalytic activity of TiO2 for phenol degradation under air is increased (Fig. 3). | ||
| Fig. 5 Formation of H2O2 in the aerated aqueous suspensions of (a) cAT, (b) sRT, and (c) sBT, in the presence of 0.43 mM phenol. | ||
Conclusions
In this work, a mixture of brookite with 10–90 wt% of anatase or rutile have been prepared, without noticeable changes in the physical parameters of each phase. For phenol degradation in aqueous suspension, the biphase effect was observed under air, but not under N2 by using Ag+ as electron scavenger. According to the latter observation, the possible charge transfer from brookite to anatase, and/or from brookite to rutile is not observed here for phenol degradation in aqueous solution. Instead, there is an interfacial O2 transfer from anatase to brookite, and/or from brookite to rutile. This result would be important to the design and development of a highly active TiO2-based photocatalyst for water treatment.Acknowledgements
This work was supported by NSFC (No. 21377110), and National Basic Research Program of China (No. 2011CB936003).References
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- O. Carp, C. L. Huisman and A. Reller, Solid State Chem., 2004, 32, 33 CrossRef CAS.
- S. G. Kumar and L. G. Devi, J. Phys. Chem. A, 2011, 115, 13211 CrossRef CAS PubMed.
- N. Zhang, M. Q. Yang, S. Q. Liu, Y. G. Sun and Y. J. Xu, Chem. Rev., 2015, 115, 10307 CrossRef CAS PubMed.
- S. Q. Liu, Z. R. Tang, Y. G. Sun, J. C. Colmenares and Y. J. Xu, Chem. Soc. Rev., 2015, 44, 5053 RSC.
- X. Y. Pan, M. Q. Yang, X. Z. Fu, N. Zhang and Y. J. Xu, Nanoscale, 2013, 5, 3601 RSC.
- A. V. Emeline, X. Zhang, M. Jin, T. Murakami and A. Fujishima, J. Phys. Chem. B, 2006, 110, 7409 CrossRef CAS.
- D. Di Paola, M. Bellardita and L. Palmisano, Catalysts, 2013, 3, 36 CrossRef.
- J. Ryu and W. Choi, Environ. Sci. Technol., 2008, 42, 294 CrossRef CAS PubMed.
- T. Kawahara, Y. Konishi, H. Tada, N. Tohge, J. Nishii and S. Ito, Angew. Chem., Int. Ed., 2002, 41, 2811–2813 CrossRef CAS.
- H. Nakajima, T. Mori, S. Q. Hen and T. Toyoda, Chem. Phys. Lett., 2005, 409, 81 CrossRef CAS.
- T. A. Kandiel, R. Dillert, A. Feldhoff and D. W. Bahnemann, J. Phys. Chem. C, 2010, 114, 4909 CAS.
- D. C. Hurum, A. G. Agrios, K. A. Gray and T. Rajh, J. Phys. Chem. B, 2003, 107, 4545 CrossRef CAS.
- T. Ohno, K. Tokieda, S. Higashida and M. Matsumura, Appl. Catal., A, 2003, 244, 383 CrossRef CAS.
- B. Sun, A. V. Vorontsov and P. G. Smirniotis, Langmuir, 2003, 19, 3151 CrossRef CAS.
- S. Ardizzone, C. L. Bianchi, G. Cappelletti, S. Gialanella, C. Pirola and V. Ragaini, J. Phys. Chem. C, 2007, 111, 13222 CAS.
- Y. Jiao, F. Chen, B. Zhao, H. Yang and J. Zhang, Colloids Surf., A, 2012, 402, 66 CrossRef CAS.
- X. Shen, J. Zhang, B. Tian and M. Anpo, J. Mater. Sci., 2012, 47, 5743 CrossRef CAS.
- X. Lü, D. Mao, X. Wei, H. Zhang, J. Xie and W. Wei, J. Mater. Res., 2013, 28, 400 CrossRef.
- H. Xu and L. Zhang, J. Phys. Chem. C, 2009, 113, 1785 CAS.
- R. Boppella, P. Basak and S. V. Manorama, ACS Appl. Mater. Interfaces, 2012, 4, 1239 CAS.
- Q. Sun and Y. Xu, J. Phys. Chem. C, 2010, 114, 18911 CAS.
- Z. Li, R. Liu and Y. Xu, J. Phys. Chem. C, 2013, 117, 24360 CAS.
- Z. Li, S. Cong and Y. Xu, ACS Catal., 2014, 4, 3273 CrossRef CAS.
- S. Cong and Y. Xu, J. Phys. Chem. C, 2011, 115, 21161 CAS.
- M. Anpo, T. Shima, S. Kodama and Y. Kubokawa, J. Phys. Chem., 1987, 91, 4305 CrossRef CAS.
- T. A. Kandiel, A. Feldhoff, L. Robben, R. Dillert and D. W. Bahnemann, Chem. Mater., 2010, 22, 2050 CrossRef CAS.
- S. Chakrabarti, D. Ganguli and S. Chaudhuri, Phys. E, 2004, 24, 333 CrossRef CAS.
- G. C. B. Cave and D. N. Hume, Anal. Chem., 1952, 24, 1503 CrossRef CAS.
- H. Bader, V. Sturzenegger and J. Hoigné, Water Res., 1988, 22, 1109–1115 CrossRef CAS.
- M. N. IIiev, V. G. Hadjiev and A. P. Litvinchuk, Vib. Spectrosc., 2013, 64, 148 CrossRef.
- A. Mattsson and L. Österlund, J. Phys. Chem. C, 2010, 114, 14121 CAS.
- M. Gotić, M. Ivanda, S. Popović, S. Musić, A. Sekulić, A. Turković and K. Furić, J. Raman Spectrosc., 1997, 28, 555 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: XRD pattern, N2 adsorption isotherm, diffuse reflectance spectrum, physical parameters of samples, and their correlation with Ag+ adsorption. See DOI: 10.1039/c5ra24312b |
| This journal is © The Royal Society of Chemistry 2015 |