KOtBu-promoted synthesis of multi-substituted 4-aminopyrimidines from benzonitriles and aliphatic amides†
Jian-Bo Fengab and
Xiao-Feng Wu*ab
aDepartment of Chemistry, Zhejiang Sci-Tech University, Xiasha Campus, Hangzhou, Zhejiang Province 310018, People’s Republic of China. E-mail: xiao-feng.wu@catalysis.de
bLeibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Strasse 29a, 18059 Rostock, Germany
First published on 7th December 2015
Abstract
Multi-substituted 4-aminopyrimidines have been prepared from commercially-available benzonitriles and aliphatic amides under transition-metal-free conditions. With KOtBu as the only promoter, the desired pyrimidines were isolated in moderate to excellent yields.
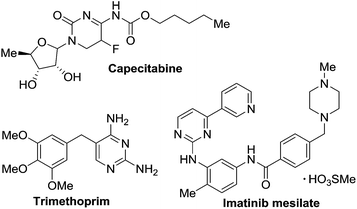
Pyrimidine and its derivatives are ubiquitous in natural products, functional materials, etc.1 Various biological activities, including anticonvulsant,2 antitumor,3 anticancer,4 antiflammatory5 and antimicrobial activities6 have been reported. Representative examples of pharmaceuticals containing a pyrimidine moiety as the core structure, i.e. trimethoprim,7 capecitabine8 and imatinib,9 are shown in Scheme 1.
Considering the importance of pyrimidines, a variety of elegant approaches have been developed.10 Examples of transition metal catalyst-based procedures are gold-catalyzed cycloadditions of ynamides with two nitriles,11 iron-catalyzed construction of 2-aminopyrimidines from alkynenitriles and cyanamides,12 iridium-catalyzed multi-component synthesis of pyrimidines from amidines and alcohols13 and niobium-catalyzed cycloaddition of alkynes and nitriles.14 Transition metal-free procedures have also been developed.15 Among these methodologies, the use of Tf2O as a promoter to prepare pyrimidines from nitriles and methyl ketones has been established and applied. Here, we wish to report a new reaction pathway for the synthesis of pyrimidines from nitriles and amides. In our new procedure, KOtBu has been applied as the promoter. Various 4-aminopyrimidines were isolated in moderate to excellent yields.
Initially, various inorganic bases were examined in 2 mL of DMAc with benzonitrile at 100 °C (Table 1, entries 1–7). To our delight, 68% of the target product was produced with KOtBu as the base (Table 1, entry 1). The other tested bases, which included NaOtBu, K2CO3, KOAc, K3PO4 and KOH, were ineffective here. No improvement in yield could be obtained when the amount of KOtBu was lowered or increased (Table 1, entries 8 and 9). However, 84% of the desired product was isolated when the reaction was conducted at 110 °C (Table 1, entry 11).
| Entry | Base (equiv.) | T (°C) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: benzonitrile (3 equiv.), base, DMAc (2 mL), 16 h.b GC yield, with hexadecane used as the internal standard.c Isolated yield. | |||
| 1 | KOtBu (2) | 100 | 68 |
| 2 | NaOtBu (2) | 100 | 0 |
| 3 | K2CO3 (2) | 100 | 0 |
| 4 | K3PO4 (2) | 100 | 0 |
| 5 | KOH (2) | 100 | 0 |
| 6 | NaOMe (2) | 100 | 5 |
| 7 | KOAc (2) | 100 | 0 |
| 8 | KOtBu (1) | 100 | 27 |
| 9 | KOtBu (3) | 100 | 60 |
| 10 | KOtBu (2) | 80 | 19 |
| 11 | KOtBu (2) | 110 | 86 (84)c |
| 12 | KOtBu (2) | 120 | 73 |
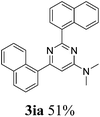
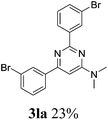
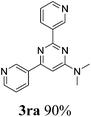
With the best reaction conditions in hand (Table 1, entry 11), we investigated the generality of this reaction. As shown in Table 2, electron-donating and electron-withdrawing substituents and even heterocyclic aromatic nitriles can be applied as the substrates in this new procedure. 71% of the desired pyrimidine (3ba) was produced from 4-methylbenzonitrile and DMAc. However, the yield decreased to 10% when the methyl group was substituted at the ortho position, which can be explained by steric hindrance. Ether and thioether groups can be well tolerated, and gave the corresponding pyrimidines in good to excellent yields (3da–3ga). Naphthonitriles were also tested under our conditions and gave the desired products in good yields (3ha–3ja). In the cases of halogen-substituted benzonitriles, the yields of the target pyrimidines decreased. This phenomenon can be explained by the nucleophilic substitution between the halogen groups and DMAc-decomposed N,N-dimethyl amine to give the corresponding N,N-dimethylaniline derivatives. To our delight, 90% of N,N-dimethyl-2,6-di(pyridin-3-yl)pyrimidin-4-amine (3ra) was isolated when nicotinonitrile was applied as the starting material. However, aliphatic nitriles failed here and no desired products could be obtained.
| a Reaction conditions: 1 (3 equiv.), KtOBu (2 equiv.), DMAc (2 mL), 110 °C, 16 h, isolated yield. | ||
|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
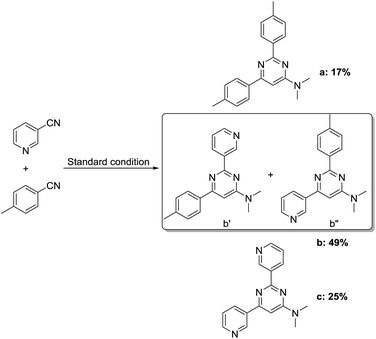
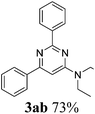
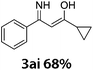
Subsequently, various DMAc derivatives were examined with benzonitrile. As illustrated in Table 3, moderate to good yields were achieved in all cases. A limitation of this procedure is that no primary and secondary amides can be applied. Additionally, besides amides, acetone, acetophenone, 2-phenylacetophenone, 1-cyclopropylethan-1-one, DMSO, etc. were tested as well. Rather than the desired pyrimidines, enaminones were obtained as the main products (3ai). From a synthetic point of view, it’s interesting to prepare cross-cyclized pyrimidines. Hence, we applied 3-cyanopyridine (1.5 equiv.) and p-methylbenzonitrile (1.5 equiv.) as two different starting materials in DMAc under our best reaction conditions (Scheme 2). From the obtained results, we see that no selectivity could be achieved here.
| a Reaction conditions: 1 (3 equiv.), KtOBu (2 equiv.), 2 (2 mL), 110 °C, 16 h, isolated yield. | ||
|---|---|---|
 |
 |
 |
 |
 |
 |
 |
 |
|
Interestingly, when quinoline-3-carbonitrile was tested as substrate under our conditions, only 2-(3-cyanoquinolin-2-yl)-N,N-dimethylacetamide was formed and isolated. After further optimization, the yield was improved to 90% with 3 equiv. of KOtBu. Instead of DMAc, N,N-diethylacetamide and 1-morpholinoethan-1-one are suitable solvent and substrates as well (Scheme 3).
Based on the obtained results, a possible reaction pathway is proposed (Scheme 4). In the presence of KOtBu, the activated α-H of DMAc could be trapped and transformed into a carbon anion, I. Then, nucleophilic addition of the in situ-formed carbon anion to the nitrile group could take place and afford the intermediate enaminone, II. The amidine intermediate, III, could be formed after enaminone II reacts with another molecule of benzonitrile. The nitrogen anion in amidine intermediate III could then go through intramolecular nucleophilic addition to the carbonyl group to give the target molecule, through nucleophilic addition to the enol form of III.
Conclusions
In conclusion, we have presented a new pathway for the synthesis of pyrimidine derivatives. This procedure has advantages which include being highly economical, efficient and convenient. All the reactions were conducted in a one-pot, one-step manner, and without the addition of transition metal catalysts. The desired multi-substituted 4-aminopyrimidines were isolated in moderate to excellent yields from commercially-available benzonitriles and aliphatic amides, with KOtBu as the only promoter.Acknowledgements
The authors thank the state of Mecklenburg-Vorpommern, the Bundesministerium für Bildung und Forschung (BMBF) and the Deutsche Forschungsgemeinschaft for financial support. In addition, the research leading to these results has received funding from the Innovative Medicines Initiative Joint Undertaking (CHEM21) under grant agreement no. 115360, resources of which are composed of a financial contribution from the European Union’s Seventh Framework Programme (FP7/2007–2013) and EFPIA companies in kind contribution. We also thank Dr C. Fischer, S. Schareina, and Dr W. Baumann for their excellent technical and analytical support. We also appreciate the general support from Prof. Matthias Beller.Notes and references
- For reviews, see (a) A. W. Erian, Chem. Rev., 1993, 93, 1991 CrossRef CAS; (b) K. Undheim and T. Benneche, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees, E. F. V. Scriven and A. McKillop, Pergamon, Oxford, 1996, vol. 6, p. 93 Search PubMed; (c) J. A. Joule and K. Mills, in Heterocyclic Chemistry, Blackwell Science Ltd., Cambridge, MA, 4th edn, 2000, p. 194 Search PubMed; (d) J. P. Michael, Nat. Prod. Rep., 2005, 22, 627 RSC; (e) I. M. Lagoja, Chem. Biodiversity, 2005, 2, 1 CrossRef CAS PubMed; (f) M. D. Hill and M. Movassaghi, Chem.–Eur. J., 2008, 14, 6836 CrossRef CAS PubMed.
- (a) J. L. Kelley, R. G. Davis, E. W. McLean, R. C. Glen, F. E. Soroko and B. R. Cooper, J. Med. Chem., 1995, 38, 3884 CrossRef CAS PubMed; (b) A. E. G. E. Amr, H. H. Sayed and M. M. Abdulla, Arch. Pharm., 2005, 338, 433 CrossRef CAS PubMed; (c) O. Alam, P. Mullick, S. Verma, S. J. Gilani, S. A. Khan, N. Siddiqui and W. Ahsan, Eur. J. Med. Chem., 2010, 45, 2467 CrossRef CAS PubMed.
- (a) X.-L. Zhao, Y.-F. Zhao, S.-C. Guo, H.-S. Song, D. Wang and P. Gong, Molecules, 2007, 12, 1136 CrossRef CAS PubMed; (b) S. C. Shivhare, H. K. Kunjwani, A. M. Manikrao and A. V. Bondre, J. Chem. Pharm. Res., 2010, 2, 106 Search PubMed; (c) H.-Y. He, J.-N. Zhao, R. Jia, Y.-L. Zhao, S.-Y. Yang, L.-T. Yu and L. Yang, Molecules, 2011, 16, 10685 CrossRef PubMed; (d) A. Tiwari and R. K. Shukla, J. Chem. Pharm. Res., 2010, 2, 172 CAS; (e) M. G. Badrey and S. M. Gomha, Molecules, 2012, 17, 11538 CrossRef CAS PubMed.
- (a) S. M. Sondhi, M. Johar, S. Rajvanshi, S. G. Dastidar, R. Shukla, R. Raghubir and J. W. Lown, Aust. J. Chem., 2001, 54, 69 CrossRef CAS; (b) F.-C. Xie, H.-B. Zhao, L.-Z. Zhao, L.-G. Lou and Y.-H. Hu, Bioorg. Med. Chem. Lett., 2009, 19, 275 CrossRef CAS PubMed.
- (a) A. Gangjee, A. Vidwans, E. Elzein, J. J. McGuire, S. F. Queener and R. L. Kisliuk, J. Med. Chem., 2001, 44, 1993 CrossRef CAS; (b) S. M. Sondhi, N. Singh, M. Johar and A. Kumar, Bioorg. Med. Chem., 2005, 13, 6158 CrossRef CAS PubMed; (c) M. Amir, S. A. Javed and H. Kumar, Indian J. Pharm. Sci., 2007, 68, 337 CrossRef.
- N. Kumar, G. Singh and A. K. Yadav, Heteroat. Chem., 2001, 12, 52 CrossRef CAS.
- A. M. Joffe, J. D. Farley, D. Linden and G. Goldsand, Am. J. Med, 1989, 87, 332 CrossRef CAS PubMed.
- J. L. Blum, Oncologist, 2001, 6, 56 CrossRef CAS PubMed.
- E. Nadal and E. Olavarria, Int. J. Clin. Pract., 2004, 58, 511 CrossRef CAS PubMed.
- For reviews, see (a) I. M. Lagoja, Chem. Biodiversity, 2005, 2, 1 CrossRef CAS; (b) M. D. Hill and M. Movassaghi, Chem.–Eur. J., 2008, 14, 6836 CrossRef CAS PubMed; (c) A. Sylvain and P. Nelly, Curr. Org. Chem., 2012, 9, 163 Search PubMed.
- S. N. Karad and R.-S. Liu, Angew. Chem., Int. Ed., 2014, 53, 9072 CrossRef CAS.
- T. K. Lane, M. H. Nguyen, B. R. D’Souza, N. A. Spahn and J. Louie, Chem. Commun., 2013, 49, 7735 RSC.
- N. Deibl, K. Ament and R. Kempe, J. Am. Chem. Soc., 2015, 137, 12804 CrossRef CAS PubMed.
- Y. Satoh, K. Yasuda and Y. Obora, Organometallics, 2012, 31, 5235 CrossRef CAS.
- For representative reports, see (a) A. G. Martínez, A. H. Fernández and F. M. Jiménez, J. Org. Chem., 1992, 57, 1627 CrossRef; (b) K. Kobayashi, T. Kitamura, R. Nakahashi, A. Shimizu, K. Yoneda, O. Morikawa and H. Konishi, Heterocycles, 2000, 53, 1021 CrossRef CAS; (c) U. Ghosh and J. A. Katzenellenbogen, J. Heterocycl. Chem., 2002, 39, 1101 CrossRef CAS; (d) M. Movassaghi and M. D. Hill, J. Am. Chem. Soc., 2006, 128, 14254 CrossRef CAS PubMed; (e) T. Sasada, F. Kobayashi, N. Sakai and T. Konakahara, Org. Lett., 2009, 11, 2161 CrossRef CAS PubMed; (f) A. A. Estrada, J. P. Lyssikatos, F. S. Jean and P. Bergeron, Synlett, 2011, 2387 CrossRef CAS; (g) E. Gayon, M. Szymczyk, H. Gérard, E. Vrancken and J. M. Campagne, J. Org. Chem., 2012, 77, 9205 CrossRef CAS PubMed; (h) C.-Y. Liang, C.-Y. Shi, H.-H. Song, H.-L. Jiang and Q.-Z. Yao, J. Chem. Pharm. Res., 2014, 6, 720 Search PubMed; (i) O. K. Ahmad, M. D. Hill and M. Movassaghi, J. Org. Chem., 2009, 74, 8460 CrossRef CAS PubMed; (j) M. Blangetti, A. Deagostino, C. Prandi, C. Zavattaro and P. Venturello, Chem. Commun., 2008, 1689 RSC; (k) M. D. Hill and M. Movassaghi, Synthesis, 2008, 823 CAS; (l) A. Herrera, R. Martínez-Alvarez, R. Chioua and M. Chioua, Tetrahedron Lett., 2003, 44, 2149 CrossRef CAS; (m) A. Herrera, R. Martínez-Alvarez, M. Chioua, R. Chatt, R. Chioua, A. Sánchez and J. Almy, Tetrahedron, 2006, 62, 2799 CrossRef CAS; (n) A. Herrera, R. Martínez-Álvarez, M. Chioua, R. Chioua and Á. Sánchez, Tetrahedron, 2002, 58, 10053 Search PubMed; (o) A. G. Martínez, A. H. Fernández, D. M. Vilchez, M. Hanack and L. R. Subramanian, Synthesis, 1992, 1053 CrossRef; (p) J. Cabaj, J. Doskocz, J. Soloducho and A. Chyla, Heterocycles, 2006, 68, 137 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General procedure, analytic data and NMR spectra. See DOI: 10.1039/c5ra24292d |
| This journal is © The Royal Society of Chemistry 2015 |