Elaboration of FAU-type zeolite beads with good mechanical performances for molecular decontamination
G. Riolanda,
L. Bullotab,
T. J. Daou*a,
A. Simon-Masserona,
G. Chaplaisa,
D. Fayec,
E. Fianib and
J. Patarina
aUniversité de Strasbourg (UdS), Université de Haute Alsace (UHA), Equipe Matériaux à Porosité Contrôlée (MPC), Institut de Science des Matériaux de Mulhouse (IS2M), ENSCMu, 3 bis rue Alfred Werner, F-68093 Mulhouse, France. E-mail: jean.daou@uha.fr; Fax: +33 3 89 33 68 85; Tel: +33 3 89 33 67 39
bFrench Agency for Environment and Energy Management (ADEME), 20 avenue du Grésillé, BP 90406, 49004 Angers Cedex 01, France
cService Laboratoires & Expertise, Centre National d'Etudes Spatiales (CNES), 18 avenue Edouard Belin, 31401 Toulouse Cedex 9, France
First published on 22nd December 2015
Abstract
FAU-type zeolite beads were formed through an shearer/mixer using organic binder (carboxymethylcellulose (CMC)) or inorganic binder (anhydrous sodium metasilicate (Na2SiO3)). Mechanical and adsorption properties of these beads represent necessary characteristics for applications in molecular decontamination. The amount of binder and the size of beads were investigated to determine the optimum conditions to elaborate mechanically stable beads with high adsorption capacities. The size of beads varies from 0.25 mm to 2 mm and the amount of binder was tuned from 5 to 15 wt% of the total bead weight. Nitrogen adsorption–desorption measurements reveal no loss of micropore volume when 5 wt% of binder is used. Adsorption of pollutants were successfully carried out using n-hexane and 1,2-dichlorobenzene as probe molecules. Indeed, FAU-type zeolite adsorbs 26 molecules of n-hexane and 29 molecules of 1,2-dichlorobenzene per unit-cell, whereas the beads prepared with 5 wt% of CMC or Na2SiO3 adsorb about 24 molecules of n-hexane and 25 molecules of 1,2-dichlorobenzene per unit-cell, respectively. The mechanical performances are improved with the addition of only 5 wt% of binder in the mixture. Uniaxial compression tests show that the ultimate compressive strength was multiplied by 4 or 7 when only 5 wt% of CMC or sodium silicate are respectively involved for the conception of 1–2 mm zeolite beads.
Introduction
Nowadays, molecular contamination is a common issue highly published. This problem concerns a large number of industries. In order to reduce or eradicate pollution, different techniques are possible such as condensation,1 catalytic oxidation,2,3 membrane separation3 and adsorption.4–8 The latter is generally preferred due to its good cost-effectiveness and ease of use. Adsorbent materials such as zeolites have been successfully used to solve the contamination problem, especially in the space and environmental industries.4–6,9–16Zeolites are aluminosilicate materials made from AlO4 and SiO4 tetrahedra sharing their oxygen vertices. Thanks to their high thermal stability coupled with an intrinsic Brönsted acidity, their interesting sorption and catalytic properties are now well-known.17 Different zeolitic structures have been discovered during the last centuries, but only a few of them are really studied and have found industrial applications.18–21
The FAU-type structure is one of the most used in the industries described above. It displays a 3D porosity with pore opening of 7.4 Å.
One of the applications targeted in this work concerns the molecular decontamination in space field. Indeed, satellites in orbital position can be damaged by the deposition of molecules emanating from cables, glues or paints on the surface of on-boarded equipments. Thanks to their great adsorption capacities and their ability to adsorb the pollutants, zeolites represent the ideal molecular adsorbents candidates.9 The French Space Agency (CNES) has already successfully used them.6,10–13
The other targeted application is the analysis of waste incineration plant emissions in air. Indeed, these smoke emissions contain very toxic molecules, in particularly polychlorinated dibenzo-p-dioxins (PCDDs) and polychlorinated dibenzofurans (PCDFs), usually known as “dioxins”. Zeolites have also been studied as adsorbents of dioxins and have shown high adsorption capacities of these molecules, which is required for the continuous and on-line measurements of waste incineration emissions.4,5,14–16
The synthesis of zeolites generally leads to crystalline powders. For more convenience, the shaping of zeolites is necessary in most applications (transport, handling…). In the case of the two applications mentioned above, it is mainly to avoid particulate contamination in satellites, and to prevent an excessive pressure loss and a possible clogging of columns used for the dioxin measurement process in waste incineration plant output. On the other hand, it is necessary to introduce additives to improve the mechanical performances of shaped objects. In the literature, large amounts of binders, such as clays,22,23 silica based compounds24,25 or alumina26 are used to improve the cohesion of the crystals and thus the mechanical resistance of the shaped objects. However, the excess of binder may generate some drawbacks such as the blocking of micropores and active sites or the incorporation of other atoms in the zeolitic framework by diffusion.22,26 Consequently, it is important to prepare beads with high mechanical resistance but with a minimal amount of binder to avoid the decrease of the adsorption capacities.
Zeolite beads have already been prepared with the use of silica precursor27,28 or ion-exchanger resin,29,30 a method called polymerization-induced colloid aggregation,31–33 by pseudomorphism34 or with a shearer/mixer.35
In this work, a shearer/mixer is also used to prepare FAU-type zeolite beads since it requires no specific preparation. It is easy to use and it enables a more important amount to be obtained compared to the above mentioned methods. Moreover, the final dimensions of beads can be tuned with this procedure. In order to improve the mechanical resistance of beads, two binders have been studied in this work, i.e., carboxymethylcellulose (CMC) and sodium metasilicate (Na2SiO3), which are used in pharmaceutical industry,36 and in the conception of bone cements,37 respectively.
This article deals with the elaboration of zeolitic beads and their characteristics mainly the mechanical resistance and pollutant sorption capacities. The influence of the amount of binder and the size of beads on their properties will be also studied.
In this work, n-hexane is used to simulate pollutants potentially present in satellites. This probe molecule is often chosen for the studies of pollutant sorptions.10,38 As regards the second application objective, which concerns the analyses of dioxins emissions, 1,2-dichlorobenzene is used to mimic 2,3-dichlorodibenzo-p-dioxin and more largely the dioxins. Indeed, polychlorobenzene are often referenced as dioxin models in order to investigate sorption or catalysis characteristics39–43 due to their similar chemical composition but lower toxicity.
Experimental section
Materials
FAU 13X powder with a crystal size of 3–5 μm and a Si/Al molar ratio of 1.1 was purchased from Sigma-Aldrich. Carboxymethylcellulose (CMC) and anhydrous sodium metasilicate (Na2SiO3) were provided by Acros Organics and Fluka Chemicals, respectively. These solids were used as received.1,2-Dichlorobenzene (anhydrous, 99%) was purchased from Sigma-Aldrich. n-Hexane (with less than 0.01% of water, 95%) was purchased from VWR, Prolabo. Both probe molecules were used as received.
Characterization techniques
A PANanalytical X'Pert Pro MPD diffractometer using Cu/Kα radiation (λ = 1.5418 Å) and θ − 2θ mounting (Bragg–Brentano geometry) was used to record XRD powder patterns. The latter were registered in 2θ range from 3 to 50 with a scanning step of 0.017° in 2θ.The FAU-type zeolite powder was investigated with a scanning electron microscope (Philips XL30 FEG).
Nitrogen adsorption–desorption isotherms were performed at 77 K with a Micromeritics ASAP 2420 MP instrument. Before each measurement, the samples were outgassed to a residual pressure of less than 0.8 Pa at 150 °C for 15 h. Surface area was calculated according to the BET method (10−6 < p/p0 ≤ 0.05). Micropore volume was determined using the t-plot method. The pollutant adsorption kinetics of the zeolite beads were studied using a Setaram TGA 92 thermobalance under dynamic conditions. The samples were activated at 200 °C during 4 hours. The sorption of pollutants was then performed at 25 °C with a relative pressure p/p0 = 0.5 (p is the vapor pressure and p0 is the saturation vapor pressure of n-hexane or 1,2-dichlorobenzene at 25 °C (p0 = 151 mm Hg for n-hexane and p0 = 1.36 mm Hg for 1,2-dichlorobenzene)). The relative pressure p/p0 = 0.5 was obtained by setting the pressures of auxiliary gas (N2 + pollutant) and carrier gas (N2) to 1.0 bar at the inlet of the oven and controlled by measuring the gas flow rate at the outlet of the oven. The gas flow rate was stabilized at 70 mL min−1. Due to the sorption phenomenon, the mass variations of the zeolitic samples were registered during the time of adsorbent–adsorbate contact thereby obtaining a kinetic adsorption curve.
The thermal analysis was carried out under air using a Setaram Labsys thermoanalyzer between the room temperature and 800 °C with a heating rate of 5 °C min−1.
The Si/Al molar ratio of FAU-type zeolite was determined by X-ray fluorescence (Philips Magix).
The ultimate compressive strength of consolidated beads was determined by uniaxial compression tests. The stress and displacement were recorded until the beads cracked by applying a displacement rate of 0.5 mm min−1 with an Instron 4505 Zwick dynamometer. To obtain a statistical significant distribution of data, three batches of beads for each formulation or condition were analyzed.
Preparation of FAU-type zeolite beads
An Eirich shearer/mixer EL1 equipped with a 1 L cuve and a rotor with star propeller was used. The rotor speed can go up to 5000 rpm. In our case, the rotor turns in the opposite direction to the tank. A mixture of binder, zeolite and water was used to prepare 13X zeolite beads. First, the binder was mixed with a certain amount of distilled water. Then, 13X zeolite powder was added. The resulting mixture was stirred with a speed of the rotor of 1500 or 1000 rpm (when the binder was CMC or Na2SiO3, respectively) during 5 minutes. Beads were finally dried at 70 °C during 24 hours.Hereafter, the different beads will be defined as CMCx−y and Na2SiO3 x−y with x = 0, 5, 10, 15 representing the amount of binder (wt% in the final bead) and y = 0.25 to 0.4 (noted 0.25/0.4) and 1 to 2 (noted 1/2) for the diameter range of beads (mm).
Results and discussion
Characterizations of the reagents
FAU-type zeolite powder used for the bead formation was characterized by XRD, N2 sorption and scanning electron microscopy. The XRD pattern (see Fig. 1a) presents peaks that are characteristic of pure FAU-type zeolite. Nitrogen adsorption-desorption measurements (see Fig. 1b) confirmed the good crystallinity of the FAU sample with a micropore volume (0.30 cm3 g−1) and surface area (800 m2 g−1) closed to values reported in literature for conventional and well crystallized FAU-type zeolite.44 Scanning electron microscopy shows a bipyramidal shape of 2–5 μm which is characteristic of FAU-type phase (see Fig. 1c). | ||
| Fig. 1 (a) XRD pattern, (b) nitrogen adsorption–desorption isotherms at 77 K and (c) scanning electron micrograph of 13X zeolite powder (FAU). | ||
In space environment, the temperature varies from −110 °C to 150 °C. At the measurement point of dioxin emissions, the temperature may reach 200 °C. In both cases the binder present in beads, such as carboxymethylcellulose, must not decompose or degas. Then, the maximum use temperature of zeolitic beads was determined by thermal analysis.
Curves obtained from CMC (see Fig. 2) shows that this binder can be used for adsorption applications at a temperature below 240 °C. Indeed, its thermal decomposition occurs in two main steps associated with exothermic components on the DTA curve. These two weight losses (40 and 15 wt%) are observed between 240 and 800 °C. Therefore, this organic binder was not affected during the activation procedure (150 °C under vacuum or 200 °C under atmospheric pressure) used before the nitrogen or probe molecule sorption experiments of CMC-containing beads.
 | ||
| Fig. 2 Thermal analysis of pure CMC powder showing the weight loss (TGA) and the heat flow (DTA) curves as a function of the temperature (°C). | ||
Mechanical properties of CMCx−y beads
| Beads | Average ultimate compressive strength (MPa) |
|---|---|
| CMC0−1/2 | 0.1 ± 0 |
| CMC5−1/2 | 0.4 ± 0.1 |
| CMC10−1/2 | 1.0 ± 0.1 |
| CMC15−1/2 | 1.4 ± 0.15 |
For the target applications, the beads have to possess sufficient mechanical performances without affecting their adsorption capacities. The CMC5−1/2 beads seem thereby to be the best candidates since they (i) are mechanically robust, (ii) involve a low amount of CMC and (iii) display a micropore volume close to that of pure FAU-type powder (see below).
Nitrogen adsorption–desorption isotherms of CMCx−y beads
The investigation of adsorption capacities of CMCx−y beads was first carried out by nitrogen adsorption–desorption measurements (see Fig. 4 and Table 2).| Surface area (m2 g−1) | Micropore volume of beads (cm3 g−1) | Micropore volume of zeolitea (cm3 g−1) | |
|---|---|---|---|
| a Taking into account the amount of CMC (5, 10, 15 wt%) which does not adsorb N2, the real micropore volume is 5, 10, 15% more important that the micropore volume of beads. | |||
| FAU powder | 800 | 0.30 | 0.30 |
| CMC0−0.25/0.4 | 800 | 0.30 | 0.30 |
| CMC5−0.25/0.4 | 770 | 0.28 | 0.29 |
| CMC5−1/2 | 730 | 0.27 | 0.28 |
| CMC10−0.25/0.4 | 600 | 0.24 | 0.27 |
| CMC15−0.25/0.4 | 480 | 0.17 | 0.20 |
It can be observed that the size of beads has no influence on their adsorption capacities (see Table 2). Indeed, with 5 wt% of CMC, the micropore volume of the zeolitic beads (expressed for 100% of zeolite) is 0.29 and 0.28 cm3 g−1 for the beads with a size of 0.25/0.4 mm and 1/2 mm, respectively. These values are similar to the micropore volume of FAU powder (0.30 cm3 g−1), proving that CMC does not block the access to the micropores nor affect the zeolitic structure when 5 wt% of CMC is used. However, for higher amounts (10 or 15 wt% of CMC) a slight or important decrease of the micropore volume is observed (0.27 and 0.20 cm3 g−1, respectively). This confirms that the amount of 5 wt% of CMC is optimal, and enables the beads to be both highly robust and porous.
Mechanical properties of Na2SiO3 x−y beads
| Beads | Average ultimate compressive strength (MPa) |
|---|---|
| Na2SiO3 0−1/2 | 0.1 ± 0 |
| Na2SiO3 5−1/2 | 0.7 ± 0.1 |
| Na2SiO3 10−1/2 | 1.2 ± 0.1 |
| Na2SiO3 15−1/2 | 1.7 ± 0.2 |
Nitrogen adsorption–desorption isotherms of Na2SiO3 x−y beads
The investigation of the adsorption capacities of Na2SiO3 x−y beads was first realized by nitrogen adsorption–desorption experiments (see Fig. 6). The textural properties are summarized in Table 4.| Surface area (m2 g−1) | Micropore volume of bead (cm3 g−1) | Micropore volume of zeolitea (cm3 g−1) | |
|---|---|---|---|
| a Taking into account the amount of Na2SiO3 (5, 10, 15 wt%) which does not adsorb N2, the real micropore volume is 5, 10, 15% more important that the micropore volume of beads. | |||
| FAU powder | 800 | 0.30 | 0.30 |
| Na2SiO3 0−0.25/0.4 | 800 | 0.30 | 0.30 |
| Na2SiO3 5−1/2 | 770 | 0.28 | 0.29 |
| Na2SiO3 5−0.25/0.4 | 700 | 0.26 | 0.27 |
| Na2SiO3 10−0.25/0.4 | 500 | 0.19 | 0.21 |
| Na2SiO3 15−0.25/0.4 | 450 | 0.16 | 0.19 |
As previously observed in the case of CMC, the size of beads has no real influence on their adsorption capacities (see Table 4). Moreover, Na2SiO3 does not block the access to the micropores nor affect the zeolitic structure when 5 wt% of Na2SiO3 are used. As it was shown for the organic binder, when 10 wt% or 15 wt% of Na2SiO3 are involved, the porosity is also partially blocked leading to a decrease of the micropore volume (0.21 and 0.19 cm3 g−1, respectively).
Taking into account the accessible porous value, the ideal beads with good mechanical properties and the highest adsorption capacities are Na2SiO3 5−1/2 (0.29 cm3 g−1, see Table 4).
Adsorption kinetics of organic molecules
 | ||
| Fig. 7 Adsorption kinetics of n-hexane at 25 °C and p/p0 = 0.5 on FAU powder and CMC5−0.25/0.4, Na2SiO3 5−1/2, CMC5−1/2, Na2SiO3 5−0.25/0.4 beads. | ||
| Sample | Adsorbed amount at saturation stage (mg ganhydrous zeolite−1) | Molecules of n-hexane per unit-cell (25 °C) | Diffusion times L2/D (s) |
|---|---|---|---|
| FAU powder | 142 | 26.0 | 382 |
| CMC5−0.25/0.4 | 132 | 24.1 | 453 |
| CMC5−1/2 | 129 | 23.4 | 872 |
| Na2SiO3 5−0.25/0.4 | 128 | 23.4 | 487 |
| Na2SiO3 5−1/2 | 135 | 24.7 | 604 |
The maximum rate of adsorption is reached for FAU powder and CMC5−0.25/0.4 beads in less than 10 minutes (tsat = 10 min). This duration is a bit longer for CMC5−1/2, Na2SiO3 5−0.25/0.4 and Na2SiO3 5−1/2 beads, i.e., tsat = 25, 15 and 20 min, respectively. Nevertheless, the saturation stage is quickly reached even if the diffusion of n-hexane is slowed down in the beads by the presence of organic or inorganic binders.
Thanks to the kinetics reported in Fig. 7, the n-hexane sorption capacity of the powder or beads can be estimated.
The sorption capacity of the faujasite powder is 26 molecules per unit-cell. This value is in agreement with other experimental results on FAU-type zeolite (see Table 6).
The n-hexane sorption capacity for CMC5−0.25/0.4, Na2SiO3 5−1/2, CMC5−1/2 and Na2SiO3 5−0.25/0.4 beads is almost the same, namely 24.1, 24.7, 23.4 and 23.4 molecules per unit-cell, respectively. These results are very close to the one observed with FAU powder.
The quantification of the diffusion properties in the FAU powder and beads was achieved using Fick's second law (eqn (1)),45 which describes the change of the molecule concentration inside the zeolite crystals as a function of time.46 In the case of 3-dimensional space, the diffusion equation is:
 | (1) |
The solving of the second Fick law for an adsorbent particle of any arbitrary shape placed in a gas phase of constant concentration leads, for short times (with q(t)/q(m) < 0.3), to the relation:46,47
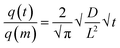 | (2) |
For all samples the plot of the normalized loading as a function of the square root of time leads almost to a straight line, as shown in Fig. 8 (for n-hexane) and 10 (for 1,2-dichlorobenzene).
 | ||
| Fig. 8 Normalized uptake in short time of FAU-type zeolite powder and beads for the n-hexane adsorption (at 25 °C and p/p0 = 0.5). Straight lines correspond to fitted data by eqn (2). | ||
Although the model does not perfectly fit the experimental data, the fitted data were used to analyze the tendencies. Concerning the adsorption of n-hexane, the characteristic diffusion times (L2/D) are 382 s for the FAU powder and 453, 487, 604 and 872 s for CMC5−0.25/0.4, Na2SiO3 5−0.25/0.4, Na2SiO3 5−1/2 and CMC5−1/2 beads, respectively (Table 5).
In zeolite beads, the diffusion of n-hexane through the zeolitic structure might be slowed down by the interaction with the binders present on the surface of zeolites (382 s for the FAU powder and 872 s for the CMC5−1/2 beads for example). The nature of the crystal surface can highly influence the diffusion time of VOCs through zeolites, as mentioned by Gueudré et al.50 This phenomenon is more pronounced for low diffusion time which is our case here, with the n-hexane adsorption over FAU samples.
| Sample | Adsorbed amount at saturation stage (mg ganhydrous zeolite−1) | Molecules of 1,2-dichlorobenzene per unit-cell (25 °C) | Diffusion times L2/D (s) |
|---|---|---|---|
| FAU powder | 316 | 29.0 | 203![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| CMC5−0.25/0.4 | 273 | 25.4 | 141![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| CMC5−1/2 | 265 | 24.6 | 162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Na2SiO3 5−0.25/0.4 | 260 | 24.1 | 141![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Na2SiO3 5−1/2 | 271 | 25.1 | 151![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Fig. 9 shows the adsorption kinetic curves of 1,2-dichlorobenzene using FAU powder and CMC5−0.25/0.4, CMC5−1/2, Na2SiO3 5−0.25/0.4 and Na2SiO3 5−1/2 beads. It appears that the saturation level for FAU powder and CMC5−1/2 beads is reached at 37 hours but this duration is shorter for CMC5−0.25/0.4, Na2SiO3 5−0.25/0.4 and Na2SiO3 5−1/2 beads with tsat = 28, 25 and 26 h, respectively.
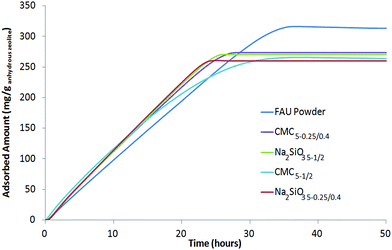 | ||
| Fig. 9 Adsorption kinetics of 1,2-dichlorobenzene at 25 °C and p/p0 = 0.5 on FAU powder and CMC5−0.25/0.4, Na2SiO3 5−1/2, CMC5−1/2, Na2SiO3 5−0.25/0.4 beads. | ||
At these high diffusion times, the effect of the surface on the global diffusion time (interaction with the binder presents at the surface of the crystals) can be neglected, on the contrary to what has been observed above for the n-hexane adsorption. The decrease of the high diffusion times of 1,2-dichlorobenzene through FAU beads compared to FAU powder can be only explained by the shaping of the beads, which reduces the distance between pores and thus make the beads much favorable for the sorption process.
The sorption capacities of 1,2-dichlorobenzene by the powder and the beads have also been investigated. According to the curves reported in Fig. 9, the 1,2-dichlorobenzene sorption capacity of the powder is 29 molecules per unit-cell. This adsorption capacity is higher than the one observed for n-hexane (26 molecules per unit-cell) probably because 1,2-dichlorobenzene has a higher heat of adsorption and it packs more efficiently (higher entropy) in the NaX super cages as already observed by Yu et al.51
The 1,2-dichlorobenzene sorption capacity for CMC5−0.25/0.4, CMC5−1/2, Na2SiO3 5−0.25/0.4, and Na2SiO3 5−1/2 beads are quite similar and close to 25 molecules per unit-cell. The presence of binder slightly affects the adsorption capacity of FAU-type zeolite. Indeed, expressed for 100 wt% of zeolite, the adsorption capacity of beads is close to 26 molecules per unit-cell instead of 29 for the corresponding powder.
The determination of characteristic diffusion times (L2/D) of 1,2-dichlorobenzene through pure FAU-type zeolite and the beads was also performed in the same manner as previously (see Fig. 10). The values are recapitulated in Table 7.
 | ||
| Fig. 10 Normalized uptake in short time of FAU-type zeolite powder and beads for the 1,2-dichlorobenzene adsorption (at 25 °C and p/p0 = 0.5). Straight lines correspond to fitted data by eqn (2). | ||
The diffusion of 1,2-dichlorobenzene through the zeolitic structure is much slower than the n-hexane diffusion (203 × 103 s and 382 s for the adsorption of 1,2-dichlorobenzene and n-hexane by the FAU powder, respectively) because of the lower vapor pressure of this probe molecule as mentioned before.
However, it is worthy to observe that the diffusion of the 1,2-dichlorobenzene is much quicker in beads than that in powder (203 × 103 s for the FAU powder and 162 × 103 s for the CMC5−1/2 beads for example). The decrease of the diffusion times of 1,2-dichlorobenzene through FAU beads compared to FAU powder can be only explained by the shaping of the beads as mentioned above.
It is also remarkable to notice that the diffusion times are higher for 1/2 mm beads than 0.24/0.4 mm beads whatever the probe molecule (n-hexane or 1,2-dichlorobenzene) or the binder (carboxymethylcellulose or sodium metasilicate). This could be explained by the greater diffusion distance that the probe molecules have to cross in order to reach the micropores present in the core of the bigger beads.
Conclusion
FAU-type zeolite beads with tuned size were elaborated thanks to a shearer/mixer in the presence of carboxymethylcellulose or sodium metasilicate as binders.The introduction of 5 wt% of binder is the optimum amount to design beads with good mechanical performances and high adsorption capacities. The addition of binder in shaped object really improves the mechanical performance. The inorganic binder allows a significant gain in mechanical stability. Indeed, the ultimate compressive strength was multiplied by 4 and 7 when only 5 wt% of CMC or sodium silicate are involved respectively.
Optimum beads are able to adsorb and trap pollutants as evidenced by the adsorption of n-hexane and 1,2-dichlorobenzene. Indeed, FAU-type zeolite adsorbs 29 molecules of 1,2-dichlorobenzene and 26 molecules of n-hexane per unit-cell. Whatever the bead size in the range 0.25 to 2 mm, zeolitic beads prepared with 5 wt% of CMC or Na2SiO3 adsorb about 25 molecules of 1,2-dichlorobenzene and 24 molecules of n-hexane per unit-cell. It is worthy to notice that the diffusion times increase by increasing the bead size whatever the probe molecule (n-hexane or 1,2-dichlorobenzene) or the binder (carboxymethylcellulose or sodium metasilicate). This study shows that these beads possess high potentials for molecular decontamination applications.
Acknowledgements
We would like to thank Ludovic Josien, Laure Michelin, Gautier Schrodj and Habiba Nouali for their assistance with the scanning electron microscopy, the X-ray fluorescence, the mechanical tests and adsorption tests, respectively. We acknowledge the financial support from the Centre National d'Etudes Spatiales (CNES), the French Environment and Energy Management Agency (ADEME), the Region Alsace and the ENSCMu Foundation. This study is part of the Meterdiox + CORTEA funding program (Project “METERDIOX+” contract #1281C0038).Notes and references
- V. K. Gupta and N. Verma, Chem. Eng. Sci., 2002, 57, 2679–2696 CrossRef CAS.
- D. Shahidi, R. Roy and A. Azzouz, Appl. Catal., B, 2015, 174–175, 277–292 CrossRef CAS.
- F. Martínez, M. J. López-Muñoz, J. Aguado, J. A. Melero, J. Arsuaga, A. Sotto, R. Molina, Y. Segura, M. I. Pariente, A. Revilla, L. Cerro and G. Carenas, Water Res., 2013, 47, 5647–5658 CrossRef PubMed.
- M. Mercury, R. Denoyel, A. Simon-Masseron, M. Carette, Y. Zerega, J. Patarin, M. Soulard, C. Reynard and A. Janulyte, Adsorption, 2011, 17, 747–758 CrossRef CAS.
- M. Mercury, N. Zouaoui, A. Simon-Masseron, Y. Zerega, C. Reynard-Carette, R. Denoyel, M. Carette, M. Soulard, A. Janulyte and J. Patarin, Microporous Mesoporous Mater., 2013, 177, 25–31 CrossRef CAS.
- T. J. Daou, N. Lauridant, G. Arnold, L. Josien, D. Faye and J. Patarin, Chem. Eng. J., 2013, 234, 66–73 CrossRef CAS.
- L. T. Gibson, Chem. Soc. Rev., 2014, 43, 5163–5172 RSC.
- G. Weber, F. Benoit, J.-P. Bellat, C. Paulin, P. Mougin and M. Thomas, Microporous Mesoporous Mater., 2008, 109, 184–192 CrossRef CAS.
- J. B. Barengoltz, S. Moore, D. Soules and G. Voecks, JPL Publ., 1994, 94, 1–48 Search PubMed.
- H. Kirsch-Rodeschini, PhD Thesis, Haute-Alsace University, 2006.
- N. Lauridant, T. J. Daou, G. Arnold, M. Soulard, H. Nouali, J. Patarin and D. Faye, Microporous Mesoporous Mater., 2012, 152, 1–8 CrossRef CAS.
- N. Lauridant, T. J. Daou, G. Arnold, H. Nouali, J. Patarin and D. Faye, Microporous Mesoporous Mater., 2013, 172, 36–43 CrossRef CAS.
- G. Rioland, T. J. Daou, D. Faye and J. Patarin, Microporous Mesoporous Mater., 2016, 221, 167–174 CrossRef CAS.
- P. Neumann and K. G. Schmidt, Organohalogen Compd., 1999, 40, 543–546 CAS.
- M. Ben Abda, O. Schäf and Y. Zerega, Microporous Mesoporous Mater., 2015, 217, 178–183 CrossRef CAS.
- R. Jäger, A. M. Schneider, P. Behrens, B. Henkelmann, K.-W. Schramm and D. Lenoir, Chem.–Eur. J., 2004, 10, 247–256 CrossRef PubMed.
- M. E. Davis, Nature, 2002, 417, 813–821 CrossRef CAS PubMed.
- C. H. Bartholomew and R. J. Farrauto, in Fundamentals of Industrial Catalytic Processes, J. Wiley and Sons, London, 2006 Search PubMed.
- A. Corma, Chem. Rev., 1995, 95, 559–614 CrossRef CAS.
- J. Čejka, G. Centi, J. Perez-Pariente and W. Roth, Catal. Today, 2012, 79, 2–15 CrossRef.
- Z. Liu, Y. Wang and Z. Xie, Chin. J. Catal., 2012, 33, 22–38 CrossRef CAS.
- P. Sánchez, F. Dorado, A. Fúnez, V. Jiménez, M. J. Ramos and J. L. Valverde, J. Mol. Catal. A: Chem., 2007, 273, 109–113 CrossRef.
- R. Jasra, B. Tyagi, Y. Badheka, V. Choudary and T. Bhat, J. Am. Chem. Soc., 2003, 42, 3263–3272 CAS.
- G. Chandraseka, M. Hartmann and V. Murugesan, J. Porous Mater., 2009, 16, 175–183 CrossRef.
- P. Topka, J. Karban, K. Soukup, K. Jirátová and O. Šolcová, Chem. Eng. J., 2011, 168, 433–440 CrossRef CAS.
- L. Itani, V. Valtchev, J. Patarin, S. Rigolet, F. Gao and G. Baudin, Microporous Mesoporous Mater., 2011, 138, 157–166 CrossRef CAS.
- W. Shan, Y. Zhan, Y. Wang, J. Xia and Y. Tang, Chem. Lett., 2004, 33, 270–271 CrossRef CAS.
- L. Yu, J. Gong, C. Zeng and L. Zhang, Sep. Purif. Technol., 2013, 118, 188–195 CrossRef CAS.
- V. Naydenov, L. Tosheva and J. Sterte, Microporous Mesoporous Mater., 2003, 66, 321–329 CrossRef CAS.
- L. Tosheva, B. Mihailova, V. Valtchev and J. Sterte, Microporous Mesoporous Mater., 2001, 48, 31–37 CrossRef CAS.
- M. J. Annen, R. Kizhappali, P. W. Carr and A. McCormick, J. Mater. Sci., 1994, 29, 6123–6130 CrossRef CAS.
- Z. T. Jiang and Y. M. Zuo, Anal. Chem., 2001, 73, 686–688 CrossRef CAS PubMed.
- Y. Kang, W. Shan, J. Wu, Y. Zhang, X. Wang, W. Yang and Y. Tang, Chem. Mater., 2006, 18, 1861–1866 CrossRef CAS.
- M. Manko, J. Vittenet, J. Rodriguez, D. Cot, J. Mendret, S. Brosillon, W. Makowski and A. Galarneau, Microporous Mesoporous Mater., 2013, 176, 145–154 CrossRef CAS.
- K. Schumann, B. Unger, A. Brandt and F. Scheffler, Microporous Mesoporous Mater., 2012, 154, 119–123 CrossRef CAS.
- A. Franc, J. Muselik, D. Sabadkova and D. Neumann, Eur. J. Pharm. Sci., 2015, 75, 72–80 CrossRef CAS PubMed.
- V. S. Komlev, J. V. Rau, M. Fosca, A. S. Fomin, A. N. Gurin, S. M. Barinov and R. Caminiti, Mater. Lett., 2012, 73, 115–118 CrossRef CAS.
- I. Kabalan, G. Rioland, H. Nouali, B. Lebeau, S. Rigolet, M. B. Fadlallah, J. Toufaily, T. Hamiyeh and T. J. Daou, RSC Adv., 2014, 4, 37353–37358 RSC.
- J. G. Bell, X. Zhao, Y. Uygur and K. M. Thomas, J. Phys. Chem. C, 2011, 115, 2776–2789 CAS.
- C. Gannoun, A. Turki, H. Kochkar, R. Delaigle, P. Eloy, A. Ghorbel and E. M. Gaigneaux, Appl. Catal., B, 2014, 147, 58–64 CrossRef CAS.
- F. Bertinchamps, C. Gregoire and E. M. Gaigneaux, Appl. Catal., B, 2006, 66, 10–22 CrossRef CAS.
- S. P. Verevkin and V. N. Emel'yanenko, J. Chem. Eng. Data, 2007, 52, 499–510 CrossRef CAS.
- D. P. Debecker, F. Bertinchamps, N. Blangenois, P. Eloy and E. M. Gaigneaux, Appl. Catal., B, 2007, 74, 223–232 CrossRef CAS.
- J. Dhainaut, T. J. Daou, A. Chappaz, N. Bats, B. Harbuzaru, G. Lapisardi, H. Chaumeil, A. Defoin, L. Rouleau and J. Patarin, Microporous Mesoporous Mater., 2013, 174, 117–125 CrossRef CAS.
- A. Fick, Poggendorff's Ann. Phys., 1855, 94, 59–86 CrossRef.
- J. Karger and D. M. Ruthven, Diffusion in Zeolites and Other Microporous Solids, J. Wiley & Sons, Inc., New York, 1992 Search PubMed.
- J. C. Groen, W. Zhu, S. Brouwer, S. J. Huynink, F. Kapteijn, J. A. Moulijn and J. Pérez-Ramirez, J. Am. Chem. Soc., 2007, 129, 355–360 CrossRef CAS PubMed.
- R. le van Mao, O. Pilati, A. Marzi, G. Leofanti, A. Villa and V. Ragaini, React. Kinet. Catal. Lett., 1980, 15, 293–302 CrossRef CAS.
- U. D. Joshi, P. N. Joshi, S. S. Tamhankar, V. V. Joshi and V. P. Shiralkar, J. Colloid Interface Sci., 2001, 235, 135–143 CrossRef CAS PubMed.
- L. Gueudré, N. Bats and E. Jolimaître, Microporous Mesoporous Mater., 2012, 147, 310–317 CrossRef.
- M. Yu, J. T. Hunter, J. L. Falconer and R. D. Noble, Microporous Mesoporous Mater., 2006, 96, 376–385 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |




