Stability and controlled antibiotic release from thin films embedded with antibiotic loaded mesoporous silica nanoparticles
Abstract
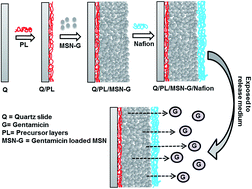
Herein we report the preparation of antibiotic thin film coatings with excellent stability and well-regulated drug release profile. These films were prepared by pre-loading of the antibiotic gentamicin into mesoporous silica nanoparticles (MSN–G) to form drug nanoreservoirs, which were then coated onto glass substrates followed by adding a polymer (Nafion) protecting layer. Fourier transform infrared (FTIR) spectroscopy and scanning electronic microscopy (SEM) confirmed the successful coating of MSN–G particles on slides. Factors such as the thickness and number of Nafion layers, the thickness of the MSN–G layer, as well as the pH of the surrounding medium were found to affect the stability of the films in solution. A typical film with 7.5 mg MSN–G particles and a thin layer of Nafion coating remained intact more than 80 days in a pH 7.4 simulated body fluid at 37 °C, which is highly promising for further biomedical application. The loaded gentamicin was found to be slowly released from thin films and the release profile could be tuned by varying the pH of the release media. For example, a steady and sustained release of gentamicin (total 95% of the loading amount) was achieved over up to 38 days in a mildly acidic solution (pH 5.5) or 56 days at the physiologic condition (pH 7.4). These films with outstanding stability and controlled release profile are expected to find promising applications in many fields, such as antibiotic coatings for biomedical devices.

 Please wait while we load your content...
Please wait while we load your content...