Promotional effect of copper(II) on an activated carbon supported low content bimetallic gold–cesium(I) catalyst in acetylene hydrochlorination
Jia Zhaoa,
Shanchuan Gua,
Xiaolong Xua,
Tongtong Zhanga,
Xiaoxia Dia,
Zhiyan Panb and
Xiaonian Li*a
aIndustrial Catalysis Institute of Zhejiang University of Technology, State Key Laboratory Breeding Base of Green Chemistry-Synthesis Technology, Hangzhou, 310014, P.R. China. E-mail: xnli@zjut.edu.cn; Tel: +86 571 88320002
bDepartment of Environmental Engineering, Zhejiang University of Technology, Hangzhou, 310014, P.R. China
First published on 18th November 2015
Abstract
The synthesis of a vinyl chloride monomer (VCM) from acetylene hydrochlorination is a highly attractive coal-based route using mercury chloride (HgCl2) as the catalyst. On reducing the use of mercury and with increasing concerns about environmental issues, searching for alternative catalysts has gained interest in recent years. However, to achieve high yield and stability using a mercury-free catalyst in this reaction is a substantial challenge. We approach this question by probing a Cu-added AuCs/AC catalyst working as a highly active, stable and cost-effective catalyst for this reaction. Introducing Cu into the catalyst significantly increased the activity and stability compared to a bicomponent AuCs/AC catalyst, underscoring a remarkable synergistic effect of the three metals. The particularly remarkable enhancement of activity was observed for the catalyst with a Au/Cu/Cs weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (Au = 0.25 wt%), which provided a high turnover frequency of 73.8 min−1 based on Au. Further experiments showed that the AuCuCs/AC catalyst delivered a stable performance during a 600 h test with the conversion of acetylene maintaining more than 98.8% at a C2H2 gas hourly space velocity of 50 h−1 and the estimated lifetime exceeding 6540 h. After a careful characterization of the AuCuCs/AC catalyst and additional catalytic tests, we concluded that the observed enhanced catalytic performance could be associated with the enhanced dispersion of Au particles, the stabilization of Au in the state of Au3+ and facile substrate C2H2 molecule desorption. Compared with the commercial high content HgCl2 catalyst (Hg = 12 wt%), this low content AuCuCs/AC catalyst (Au = 0.25 wt%) has similar activity, higher stability, relative low cost and environmental friendliness, meaning it has potential as an alternative to the HgCl2 catalyst for commercial production of VCM.
4 (Au = 0.25 wt%), which provided a high turnover frequency of 73.8 min−1 based on Au. Further experiments showed that the AuCuCs/AC catalyst delivered a stable performance during a 600 h test with the conversion of acetylene maintaining more than 98.8% at a C2H2 gas hourly space velocity of 50 h−1 and the estimated lifetime exceeding 6540 h. After a careful characterization of the AuCuCs/AC catalyst and additional catalytic tests, we concluded that the observed enhanced catalytic performance could be associated with the enhanced dispersion of Au particles, the stabilization of Au in the state of Au3+ and facile substrate C2H2 molecule desorption. Compared with the commercial high content HgCl2 catalyst (Hg = 12 wt%), this low content AuCuCs/AC catalyst (Au = 0.25 wt%) has similar activity, higher stability, relative low cost and environmental friendliness, meaning it has potential as an alternative to the HgCl2 catalyst for commercial production of VCM.
1. Introduction
The acetylene hydrochlorination reaction (C2H2 + HCl → CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCl, ΔH = −124.8 kJ mol−1) has received significant attention as an alternative route to the manufacture of vinyl chloride monomers (VCM).1,2 Industrially, the hydrochlorination of acetylene is performed at temperatures between 130 and 180 °C over an activated carbon supported mercuric chloride (HgCl2) catalyst.3 However, it suffers from high toxicity and sublimation under the reaction conditions in spite of the high conversion. This not only leads to deactivation of the catalyst, but also contaminates the product, and may cause severe environmental problems and threats to human health. From a sustainable point of view, the development of efficient non-mercury catalysts with high activity and stability for the hydrochlorination of acetylene is highly desirable.
CHCl, ΔH = −124.8 kJ mol−1) has received significant attention as an alternative route to the manufacture of vinyl chloride monomers (VCM).1,2 Industrially, the hydrochlorination of acetylene is performed at temperatures between 130 and 180 °C over an activated carbon supported mercuric chloride (HgCl2) catalyst.3 However, it suffers from high toxicity and sublimation under the reaction conditions in spite of the high conversion. This not only leads to deactivation of the catalyst, but also contaminates the product, and may cause severe environmental problems and threats to human health. From a sustainable point of view, the development of efficient non-mercury catalysts with high activity and stability for the hydrochlorination of acetylene is highly desirable.
Among the numerous metal chlorides including Au,4–10 Pt,11,12 Pd,13,14 Ru,15–17 Cu,18,19 Bi20 etc., AuCl3 is widely used as catalyst because of its high activity, but Au3+ is readily reduced to Au0 under reaction conditions and consequently loses its activity.21,22 Although its catalytic stability can be improved by alloying with a second metal component such as LaCl3,23 CoCl3,24 NiCl2,25 BaCl2,26 CsCl27,28 or TiO2,29 this type of Au-based catalysts still deactivates continuously with increasing time on stream. Additionally, current state-of-the-art hydrochlorination catalysts employ Au or Au-based catalysts with total Au loading levels of 1.0 wt%, making the catalysts too expensive to use for large scale production. Ru-based catalyst is a good candidate due to its environmental friendship, cheapness, and high activity.17 However, the abundance of Ru is about 0.001 ppm, which is much smaller than that of other precious metals (Fig. 1). It is worthwhile to note that we should keep in mind the difference in their abundance and annual output when we attempt to design such noble metal industrial catalysts. For another, non-precious metals catalysts (e.g., BiCl3 (ref. 20) or SnCl2 (ref. 30)) with low cost also suffer the twin disadvantages of poor activity and fast deactivation, leading to limited production capacity and difficult purification. In addition, N-doped carbon materials are also known to catalyse this reaction.31–35 However, low conversion efficiency and high energy demand preclude the wider commercial applicability of these catalysts. As reported previously, with N-doped carbon materials no complete conversion could be achieved, which are far from the satisfactory level. Thus, Au-catalyzed hydrochlorination reaction is still recognized as the most promising acetylene hydrochlorination technology currently, provided that the Au-based catalysts used are sufficiently stable and cheap.
 | ||
| Fig. 1 Crustal abundance and annual output of the metals that are used for constructing acetylene hydrochlorination catalysts. | ||
Extensive efforts have been made to reduce the Au loading and further improve the activity and stability of Au-based catalysts. For example, it demonstrated that the addition of CuCl2 (ref. 36) or BiCl3 (ref. 37) to Au can reduce the Au loading down to 0.5 wt% and 0.3 wt%, respectively. Although significant improvement has been achieved, it is still a significant challenge to maintain or improve catalyst activity and stability when the Au catalyst loading is reduced or eliminated. In our recent study, 1Au–4Cs/AC catalyst demonstrates considerably higher catalytic activity and stability than monometallic Au-based catalyst.27 The issue facing Au-based hydrochlorination catalysts is typically to create and maintain the oxidation state of the AuCl3 species during preparation and reaction. Most importantly, the ability of CuCl2 to stabilize the cationic Au(III) in many gold(III)–CuCl2 catalyst systems (e.g., AuCl3–CuCl2) has been reported before.38–43 Inspired by the findings in literatures mentioned above, we proceeded to modify this catalyst herein by the addition of controlled amounts of Cu (0.25 wt%) to a low content AuCs/AC catalyst (Au = 0.25 wt%) and evaluate the properties of AuCuCs/AC material by several characterisation techniques (TEM, XRD, H2-TPR, ICP-MS, TPD, TPO, TGA and FTIR) comparing to monometallic Au/AC and bimetallic AuCs/AC catalysts as reference. We originally demonstrate that the trimetallic AuCuCs/AC catalyst preserves a high turnover frequency of 73.8 min−1 based on Au, and an estimated lifetime exceeding 6540 h with the conversion of acetylene and the selectivity of vinyl chloride reaching more than 98.1% and 99.9%, respectively. This catalyst has the advantages of low cost of preparation and use, high catalytic activity and excellent stability which make it possible to be widely applied in industrial hydrochlorination of acetylene.
2. Experimental
2.1. Catalyst preparation
A commercially activated carbon (AC) NORIT ROX 0.8 (pellets of 0.8 mm diameter and 5 mm length) was selected for the preparation of supports. The activated carbon was first pretreated with HNO3 (65 wt%) at room temperature for 1 h to remove Na, Fe, and Al contaminants.6 The pretreated activated carbon was filtered, washed with deionized water until pH = 7 and then dried at 110 °C for 12 h.Trimetallic AuCuCs/AC catalysts were prepared using incipient wetness impregnation (IWI) technique. The catalyst precursors containing HAuCl4·4H2O, CuCl2·2H2O and CsCl were weighted separately ca. 0.25, 0.25 wt% and 1.00 wt% to the mass of AC and then dissolved in aqua regia solution. After the solution was homogeneously mixed, the pretreated AC support was then added into the solution with continuous stirring. Then the system was aged at 40 °C for 4 h, followed by drying at 110 °C for 12 h for use. The same procedure was also followed to prepare the corresponding Au/AC (0.25 wt% Au), AuCu/AC (0.25 wt% Au and 0.25 wt% Cu), AuCs/AC (0.25 wt% Au and 1.00 wt% Cs), Cu/AC (1.00 wt% Cu), and Cs/AC (1.00 wt% Cs) catalysts for comparison.
2.2. Catalyst characterization
The textural properties of the prepared Au-based catalysts were obtained from the N2 adsorption–desorption isotherms at −196 °C measured with a Micromeritics ASAP 2020 apparatus. Prior to the measurements, samples were outgassed at 100 °C and 10−4 mbar overnight. Surface areas were determined by using the Brunauer–Emmet–Teller (BET) equation and a nitrogen molecule cross section of 16.2 Å. The total pore volume was calculated from the adsorption isotherm at P/P0 = 0.95.XRD measurements of the catalyst samples were performed on a PANalytical-X′Pert PRO generator with Cu Kα radiation (λ = 0.1541 nm) that was operated at 60 kV and 55 mA. Diffraction patterns were recorded at a scanning rate of 2° min−1 and at a step of 0.02°.
TEM analysis was conducted using a transmission electron microscope (TEM, Tecnai G2 F30 S-Twin), operating at an acceleration voltage of 300 keV. The solid samples were finely ground. The resultant fine powders were dispersed ultrasonically in the ethanol and then two drops of the solution were transferred to a carbon/Ni grid (Beijing Zhongjingkeyi Technology Co., Ltd). Grids were allowed to dry before TEM characterization. The sizes of particles on samples were also observed by a transmission electron microscope. The number weighted average Au diameter (dTEM) was determined from a count of 300 nanoparticles (NPs).
TPD experiments were performed in a tubular quartz reactor. The samples (100 mg) were first treated in situ at 180 °C for 0.5 h using pure C2H2 and then the sample was swept with pure Ar at a flow rate of 30 mL min−1 for 1 h to remove physisorbed and/or weakly bound species. TPD was performed by heating the sample from room temperature to 850 °C at a ramp rate of 10 °C min−1 in pure Ar, and the TPD spectra were recorded by a quadrupole mass spectrometer (QMS 200 Omnistar).
H2-TPR experiments were performed in the same apparatus as the TPD experiments. The weight of the tested samples was 75 mg. The temperature was linearly increased from 30 to 850 °C at a rate of 10 °C min−1. The hydrogen consumption was measured using a thermal conductivity detector (TCD).
TPO experiments were also performed in the same apparatus as the TPR experiments. Each sample (100 mg) was previously heated in Ar flow at 200 °C for 1 h to remove the volatile compounds and cooled to room temperature in the same flow of Ar. Then the gas flow was switched to 5% O2/Ar and the temperature was raised to 850 °C at a rate of 10 °C min−1.
A thermogravimetric analysis (TGA) of the samples was performed using a NETZSCH STA 449 F3 Jupiter thermogravimetric-differential scanning calorimetry (TG-DSC) simultaneous thermal analyzer in an air atmosphere at a flow rate of 30 mL min−1. The temperature was increased from 30 to 850 °C at a rate of 10 °C min−1.
2.3. Gas phase hydrochlorination of acetylene
Catalysts were tested for acetylene hydrochlorination in a fixed-bed glass micro-reactor (i.d. 10 mm). Acetylene (5.0 mL min−1, 1 bar) and hydrogen chloride (6.0 mL min−1, 1 bar) were fed though a mixing vessel via calibrated mass flow controllers to a heated glass reactor containing catalyst (200 mg) with a total GHSV (C2H2) of 740 h−1. The long-term stability experiment was performed in the same apparatus with acetylene (24.3 mL min−1, 1 bar) and hydrogen chloride (29.2 mL min−1, 1 bar) fed though a mixing vessel via calibrated mass flow controllers to a heated glass reactor containing the catalyst (1.0 g) with a total GHSV (C2H2) of 720 h−1. A reaction temperature of 180 °C was chosen. Blank tests using an empty reactor filled with quartz wool did not reveal any catalytic activity, and quartz sand was used to extend the bed length, above and below the catalyst itself, separated by quartz wool. The gas phase products were passed through an absorption bottle containing NaOH solution to remove excess HCl first and then analyzed on-line by GC equipped with a flame ionization detector (FID). Chromatographic separation and identification of the products was carried out using a Porapak N packed column.The composition of the reactor outflow (dissolve in the N-methylpyrrolidone solution) was determined using a Waters GCT Premier chromatograph equipped with a HP-5 capillary column.44 Product mass fractions were obtained using an internal standard. The main product of acetylene hydrochlorination was vinyl chloride, a small amount of the by-products 1,1-dichloroethane and 1,2-dichlorethane were also produced. The gas-phase products of acetylene hydrochlorination were quantified using the peak area normalization method. Given that hydrogen chloride is absorbed by the absorption liquid after the reaction, the volume of the reaction system can be considered constant during the calculations, and the carbon balance values based on these products are above 95%.The conversion of acetylene and the selectivity to VCM were calculated by eqn (1) and (2), as follows:
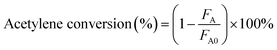 | (1) |
 | (2) |
3. Results and discussion
3.1. Catalytic activity of trimetallic AuCuCs/AC catalyst
Table 1 compared the catalytic performances of the different Au-based catalysts as well as Cu/AC and Cs/AC for acetylene hydrochlorination under the same reaction conditions. The Cu/AC catalyst gave rather low but stable activity with only 8.6% C2H2 conversion and more than 99.9% VCM selectivity in 24 h. Controlled tests of the Cs/AC catalyst showed that only a small amount of VCM is observed in the gas phase (<6.0% based on C2H2). Hydrochlorination of acetylene over 1Au/AC catalyst provided high activity with an initial conversion of 72.4%. It should be note that the C2H2 conversion on pure AC support was below 5%. The activity of the activated carbon can arise from the presence of trace amounts of K+ and Al3+ in the carbon matrix, as these metals can display some activity to the hydrochlorination reaction of acetylene.8| Catalyst | Chemical composition | C2H2 conversion (%) | VCM selectivity (%) | Deactivation rateb (% h−1) |
|---|---|---|---|---|
a Reaction conditions: T = 180 °C, V(HCl)/V(C2H2) = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, GHSV = 740 h−1, t = 24 h.b Deactivation rate was defined as (the initial maximum C2H2 conversion – the final C2H2 conversion)/(deactivation period, h), deactivation period = 24 h. 1, GHSV = 740 h−1, t = 24 h.b Deactivation rate was defined as (the initial maximum C2H2 conversion – the final C2H2 conversion)/(deactivation period, h), deactivation period = 24 h. |
||||
| 1Au/AC | 1.00 wt% Au | 72.4 | >99.9 | 0.61 |
| Au/AC | 0.25 wt% Au | 13.9 | >99.9 | 0.24 |
| 1Cu/AC | 1.00 wt% Cu | 8.6 | >99.9 | — |
| 1Cs/AC | 1.00 wt% Cs | 5.2 | 99.7 | — |
| AuCu/AC | 0.25 wt% Au | 57.9 | 99.8 | 1.6 |
| 0.25 wt% Cu | ||||
| AuCs/AC | 0.25 wt% Au | 42.2 | >99.9 | 0.19 |
| 1.00 wt% Cs | ||||
| AuCuCs/AC | 0.25 wt% Au | 84.1 | >99.9 | 0.17 |
| 0.25 wt% Cu | ||||
| 1.00 wt% Cs | ||||
It has been reported that 1.00 wt% Au loading was an appropriated compromise for Au/Carbon catalyst.5–7,36 However, with such an amount of Au loading, Au/AC still deactivated continuously from 72.4% to 57.7% with about 14.7% C2H2 conversion loss after 24 h. The observation was in line with previous studies.23–29,36,37 However, when Au loading decreased from 1.00 wt% to 0.25 wt%, the conversion shapely decreased from 72.4% to less than 14.0%, indicating that high conversions required high Au loading, as reported before.37 For the bimetallic AuCs/AC catalyst, the C2H2 conversion increases to approximately 42.2%, which represents a significant improvement compared with the Au/AC catalyst.
Table 1 showed that the activity can be further enhanced by adding Cu species to the bimetallic AuCs/AC catalyst. Indeed, AuCuCs/AC exhibited a much higher activity (84.1%) compared to monometallic Au/AC and bimetallic AuCs/AC and also delivered a fairly stable catalytic performance for more than 24 h. A comparable experiment with AuCu/AC catalyst revealed high catalytic activity (57.9%) and selectivity (>99.9%), but this enhancement was lost rapidly, demonstrating that the coexistence of Cu and Cs species is essential for the observed catalyst modification effects for an active low content Au-based catalyst.
To prove the outstanding performance of this catalyst system, the AuCuCs/AC catalyst was compared with a wide range of other catalyst materials reported in literature (note that the experimental conditions are not necessarily identical), including ones based on different monometallic catalysts as well as bimetallic and trimetallic Au-based catalysts, and the result is shown in Fig. 2. It is clear the AuCuCs/AC catalyst is one of the best catalysts reported in terms of offering a high TOF (73.8 min−1) at a relatively high GHSV. To the best of our knowledge, this value is much higher than those reported previously for the state-of-the-art Au catalyst (max TOF = 49.3 min−1, all TOF values were calculated with respect to the total Au content of the catalyst), further highlighting the excellent catalytic performance of the AuCuCs/AC catalyst.
 | ||
| Fig. 2 (a) The TOF value for the different catalysts: Au/AC,5 Pt/AC,45 Pd/AC,5 Ru/AC,15 Rh/AC,5 Ir/AC,45 Bi/AC,20 Ag/AC,5 Cu/AC,18 Cd/AC,5 Zn/AC,5 and Hg/AC.5 (b) The TOF value for the Au-based catalysts: AuBa/AC,26 AuLa/AC,23 AuCo/AC,24 AuCuCo/AC,44 Au/Ni,25 AuCs/AC,28 AuTiO2/AC,29 AuInCs/AC,46 AuCuK/AC,47 AuBi/AC37 and AuCuCs/AC. | ||
In order to determine the optimized ratio of Au and Cu, the ratio of Au/Cu was varied from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 to 1
0.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (weight ratio), whilst maintaining a constant Au (0.25 wt%) and Cs (1.00 wt%) loadings of the catalysts, as shown in Fig. 3. As the ratio of Au/Cu increased from 1
5 (weight ratio), whilst maintaining a constant Au (0.25 wt%) and Cs (1.00 wt%) loadings of the catalysts, as shown in Fig. 3. As the ratio of Au/Cu increased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 to 1
0.5 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, the initial conversion increased; as the ratio was further increased, there was little increase in the conversion. Besides that, the catalysts having higher Cu content show a significant rate of deactivation. The results of the present study demonstrated that the activity and stability of Au-based catalyst are highly sensitive to their formulation. Thus, based on the results of our catalysis experiments, it is clear that a Au/Cu ratio of 1
3, the initial conversion increased; as the ratio was further increased, there was little increase in the conversion. Besides that, the catalysts having higher Cu content show a significant rate of deactivation. The results of the present study demonstrated that the activity and stability of Au-based catalyst are highly sensitive to their formulation. Thus, based on the results of our catalysis experiments, it is clear that a Au/Cu ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was considered optimized in terms of catalytic activity, selectivity and stability.
1 was considered optimized in terms of catalytic activity, selectivity and stability.
 | ||
Fig. 3 Catalytic performance of AuCuCs/AC with different Au/Cu weight ratio. Reaction conditions: T = 180 °C, V(HCl)/V(C2H2) = 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, GHSV = 740 h−1. 1, GHSV = 740 h−1. | ||
To evaluate whether there was also a chemical effect of Cu and Cs on the structure of Au which may responsible for the improved catalyst performance, four different synthesis routes of Au–Cu–Cs series catalysts were investigated (Fig. 4a). All four catalysts contained equal amounts of Au (0.25 wt%), Cu (0.25 wt%) and Cs (1.00 wt%) and evaluated under identical reaction conditions. For the bimetallic AuCs/AC, the maximum acetylene conversion is 42.2% during a reaction time of 24 h. However, all trimetallic catalysts with the different synthesis method showed higher catalytic activity than the bimetallic AuCs/AC catalyst. These results show that the presence of CuCl2 species promoted the initial activity of the AuCs/AC catalyst. The highest activity was obtained on the catalyst named as AuCuCs/AC synthesized via route 4 by co-impregnation of an aqueous solution of HAuCl4, CuCl2 and CsCl onto AC, with the initial conversions being 84.1%. Besides that, the AuCuCs/AC catalyst also displayed optimal catalytic stability for the hydrochlorination reaction of acetylene with only 4.0% C2H2 conversion loss after 24 h, as shown in Fig. 4b. These results show that the different method can largely affect the catalytic performance of the trimetallic Au–Cu–Cs series catalysts. We have observed that the optimal catalytic performance using the AuCuCs/AC catalyst only occurs when the metal precursor is co-impregnated onto AC. We originally considered that the formation of trimetallic Au–Cu–Cs complex structured during the preparation is essentially responsible for the special catalytic behaviour of the sample. However, we cannot provide direct evidence of this phenomenon currently, and further efforts for accurately characterising the catalyst surface structure should be carried out.
To further prove the outstanding performance of this catalyst system, the AuCuCs/AC catalyst was compared with the most active 1Au/AC catalyst (1.00 wt% Au), as well as a commercial high content 12Hg/AC catalyst (12.00 wt% Hg). Compared to the commercial 12Hg/AC catalyst, AuCuCs/AC catalyst has a slightly lower initial conversion, but the value is significantly higher than the 1Au/AC, as shown in Fig. 5. More importantly, the AuCuCs/AC catalyst is also highly stable catalyst, in the gas phase hydrochlorination of acetylene. For the 24 h running, the values of acetylene conversion have dropped for all three catalysts. However, the deactivation is the least severe for this trimetallic AuCuCs/AC catalyst. The high activity together with the extraordinary stability, support the possible applicability of this catalyst in industry.
3.2. Characterization of AuCuCs/AC catalyst
It is known that Au-based hydrochlorination catalysts deactivate due to sintering of the metal NPs, valence change of the active component during the reaction and carbon deposition. To gain an insight into the cause of the performance improvement observed we have analyzed the catalysts using STEM, XRD, ICP, H2-TPR, BET, TPO and TPD analysis.The TEM of fresh and used Au/AC, AuCs/AC and AuCuCs/AC catalysts was performed to evaluate possible sintering of NPs after the reaction. Fig. 6 shows exemplary TEM images of selected Au-base catalysts. The small bright dots represent the metal NPs. As can be seen, the fresh Au/AC (Fig. 6a), AuCs/AC (Fig. 6c) and AuCuCs/AC (Fig. 6e) catalysts are made up of small metal NPs ranging from 1 to 8 nm in diameter, indicating the particles are highly dispersed. However, monometallic Au/AC catalysts are not resistant to sintering and show a large particle size distribution ranging from 2 to 12 nm in diameter after reaction for 50 h (Fig. 6b). This indicates that the size distribution for Au NPs in this sample is quite heterogeneous and that the agglomeration of Au occurs easily. In contrast, particles below 5 nm are dominant in used AuCs/AC catalyst, though some are found up to 6 nm in diameter (Fig. 6d). For the sample using used AuCuCs/AC catalyst, most of the particles had particle sizes below 4 nm, with a small fraction between 4 and 6 nm (Fig. 6f). The order of average particle was AuCuCs/AC (4.0 nm) < AuCs/AC (4.6 nm) < Au/AC (6.8 nm). This is indicative of the addition of Cs and Cu to the catalysts, so it can effectively inhibit the catalyst sintering during the hydrochlorination reaction. It is well known that the particle size of the active metal component of the catalyst has an important influence on the catalytic performance. For the hydrochlorination reaction of acetylene, catalytic activity was correlated with Au content and the number of active sites. This is because the activity was ascribed to Au3+ species at the perimeter of the Au NPs in contact with the support.48 AuCuCs/AC had a stable conversion without significant Au agglomeration formation after 24 h test, revealing the valuable improvement of Au dispersion after the introduction of Cu and Cs in Au/AC catalyst.
 | ||
| Fig. 6 Representative STEM images of (a) fresh Au/AC, (b) used Au/AC, (c) fresh AuCs/AC, (d) used AuCs/AC, (e) fresh AuCuCs/AC, and (f) used AuCuCs/AC, respectively. | ||
Fig. 7 displays the XRD patterns of the fresh and used Au/AC, AuCs/AC and AuCuCs/AC catalyst. The peak around 2θ of 26° corresponds to (002) planes of graphitized carbon from AC. Apart from the amorphous diffraction peaks of the AC, no discernible Au0 reflection is detected in fresh Au-base catalysts. This is indicative of extremely small Au-containing NPs on the AC surface below the detection limit of this technique (smaller than 4 nm) or most of the Au exists as non-crystalline Au3+ species.48 In the case of used Au/AC and AuCs/AC catalysts, it shows diffraction peaks located at 2θ values of 38.12° which are assigned to Au (111) planes, indicating the big fraction of Au3+ reduction to Au0 and/or a certain amount of catalyst sintering. However, for used AuCuCs/AC catalyst, no sharp peaks for Au0 can be observed, indicating that Au3+ NPs are still well uniformly dispersed and their crystal size are very small, as observed in the TEM images (Fig. 6f).
To check for exact loading amounts and thermal stability of Au-based catalysts, ICP analysis was conducted and the results are summarized in Table 2. As can be seen, the actual content of Au, Cu and Cs element is almost identical to that of the precursors. Due to the catalyst preparation procedure, no filtrating of the carbon or catalyst washing was carried out. It is reasonable that the metal loading is nearly equal to the nominal amount of metal impregnated onto the support. For Hg-based catalysts, it is apt to be reduced by acetylene and sublimates during reaction, leading to deactivation of the catalyst. However, Au-based catalysts are not particularly volatile under the reaction conditions. And we did not observe markedly loss of Au in this study, as previous studies reported,47 proving their good thermal stability.
| Catalysts | Fresh | Used | ||||
|---|---|---|---|---|---|---|
| Au (wt%) | Cu (wt%) | Cs (wt%) | Au (wt%) | Cu (wt%) | Cs (wt%) | |
| Au/AC | 0.28 | — | — | 0.25 | — | — |
| AuCs/AC | 0.26 | — | 0.97 | 0.24 | — | 0.96 |
| AuCuCs/AC | 0.26 | 0.30 | 1.01 | 0.25 | 0.25 | 1.00 |
Previous literature studies ascribed the activity of the Au-based catalyst to the presence of Au3+ species, postulating them to be active sites.5,7,8 In order to obtain a correlation on the activity of VCM production with the amount of Au3+ species clusters made on the catalyst surface, samples were carefully analyzed by H2-TPR. Fig. 8 presents the H2-TPR profiles of fresh (Fig. 8a) and used (Fig. 8b) Au/AC, AuCu/AC, AuCu/AC, and AuCuCs/AC catalysts. For all the catalysts, a distinguishable hydrogen consumption peak in the range of 450–800 °C can be observed. This peak is a consequence of the reduction of surface groups of activated carbon support.49,50 For all the Cu-containing Au-based catalysts, the TPR profile shows a broad band around 480 °C, which corresponds to the reduction peaks of the Cu2+ species. The analysis of Au/AC sample led to assign the reduction band around 336 °C to Au3+ to Au0. TPR profiles of the fresh AuCu/AC and AuCs/AC catalysts disclosed that the CuCl2 and CsCl additives greatly affect the reducibility of Au3+ catalysts, a straightforward decrease of temperature in the reduction band of Au3+ to 319 °C and a straightforward increase of temperature in the band of Au3+ to 350 °C are observed, respectively. These shifts indicate strong interactions between Au species and Cu2+ or Cs+ exist in AuCu/AC or AuCs/AC catalysts, respectively. More specifically, the reduction temperature of Au3+ may shift to lower or higher temperatures due to the presence of a strong interaction between Au3+ and Cu2+ or Cs+ via the electron transfer which in turn, facilitates or inhibits the reduction. In fact, the Cu2+ species act as electron donors and transfer electrons from Cu atom to the Au3+ species to facilitate electron accumulation has been reported in many Au–Cu catalytic systems before.43,44 This led to a easier reduction of active Au3+ species. In contrast, the presence of Cs makes reducing Au3+ species the reduction temperature of Au3+ shows a slightly shift to a higher temperature (356 °C) as compared to the Au/AC sample. Such a positive shift suggests that there are strong interactions between the three components within the catalyst, and it is reasonable to change the electronic structure of Au. However, the presence of the Au–Cu–Cs complex or alloy cannot be excluded for the AuCuCs/AC catalyst, which may inhibit the reduction of Au3+ and increase the stability of the catalytically active Au3+ species. Through comparing the TCD signals with standard signals, the fractions of different Au species in the fresh catalysts can be estimated (Fig. 8c). This allows estimations of ca. 12.1%, 32.0%, 33.9% and 47.1% for the Au/AC, AuCu/AC, AuCs/AC and AuCuCs/AC catalysts, respectively. As in the case of used Au-based catalysts, also through comparing the TCD signals with a standard, this allows an estimation of ca. 2.3%, 13.4%, 28.3% and 38.1% for the used Au/AC, AuCu/AC, AuCs/AC and AuCuCs/AC catalysts, respectively (Fig. 8c). This result showed that the presence of Cu and Cs in the AuCuCs/AC catalyst efficiently inhibited the reduction of Au3+, which can further stabilize the catalytic active Au3+ species both in the preparation and reaction process of AuCl3 catalysts. This result is consistent with the higher activity and slower deactivation of the AuCuCs/AC catalyst.
 | ||
| Fig. 8 H2-TPR patterns of fresh (a) and used (b) Au-based catalysts. (c) The proportional content of Au3+ species for fresh and used Au-based catalysts. | ||
Previous studies have shown that the reduction of Au3+ to Au0 is the main deactivation pathway at temperatures higher than 120 °C, while the deposition of coke/oligomers materials on the catalyst is the predominant deactivation process occurring at temperatures lower than 100 °C.21 Recently, work by Dai et al. indicated that coke/oligomers deposition is another non-negligible reason for catalyst deactivation for the gas phase hydrochlorination of acetylene even under the relative high reaction temperatures (150–180 °C).23–26,44 In general, the coke/oligomers formation in hydrochlorination reaction includes the adsorption of the hydrocarbons on the catalyst surface and the spillovers of coke/oligomers species from the metal active site to the support, which may result in covering some parts of the active sites pore and clogging of the support and further deactivation.
To provide direct evidence of coke/oligomers deposition, N2 adsorption–desorption, TPO and TGA analysis were performed. Table 3 shows the textural parameters of the supports and the prepared Au-based catalysts. As expected, no drastic changes in the textural properties of the materials were observed. The slight decrease in AC surface area may be caused by the phenomenon called the dilution effect, in which the loading of the active component decreases the ratio of the carriers in the catalysts. After the reaction, BET analysis showed that the three catalysts' surface areas all decreased, which may be caused by the coke/oligomers deposition. The variation amplitude of the catalysts' SBET follows the order: Au/AC (ΔSBET% = 19.8%) > AuCs/AC (ΔSBET% = 9.8%) > AuCuCs/AC (ΔSBET% = 3.0%). Thus, the optimal AuCuCs/AC catalyst may have the least coke/oligomers deposition and pore blocking compared to the AuCs/AC and Au/AC catalysts. Therefore, AuCuCs/AC had a better anti-coke ability.
| Sample | SBET (m2 g−1) | Total pore volume (cm3 g−1) | ||
|---|---|---|---|---|
| Fresh | Used | Fresh | Used | |
| AC | 1162 | — | 0.63 | — |
| Au/AC | 1139 | 913 | 0.64 | 0.49 |
| AuCs/AC | 1113 | 1004 | 0.62 | 0.57 |
| AuCuCs/AC | 1016 | 986 | 0.58 | 0.56 |
TPO spectra of the used Au/AC, AuCs/AC and AuCuCs/AC catalysts are presented in Fig. 9. It is not possible to apply TPO analysis to identify the chemical nature of the coke/oligomers species; however, we may use it to indicate where the resulting coke/oligomers formed.51 According to Fig. 9, no discernible oxygen consumption peak is detected in the temperature region below 400 °C in AC support. However, after the reaction, a distinct peak at around 200–350 °C appeared for the Au/AC, AuCs/AC and AuCuCs/AC catalysts, which could confirm the presence of coke/oligomers species. In line with the literature,52–54 this low temperature oxygen consumption peak may associate with the coke/oligomers located on the active metal sites, because the active metal could facilitate the coke/oligomers decomposition. According to Fig. 9, the intensity of oxygen consumption peak resulting from the AuCuCs/AC catalyst is less significant, when it is compared with the Au/AC and AuCs/AC catalysts. The amount of coke/oligomers deposited on the used catalysts was determined by TGA measurements (figure not shown here). From the TGA measurements, the amounts of coke/oligomers formed were 1.1 wt% (AuCuCs/AC), 2.5 wt% (AuCs/AC) and 4.7 wt% (Au/AC). Based on this result, it is clear that the amount of carbon coke species for the Au-based catalyst with addition of Cu and Cs decreases, in good agreement with the results in Table 3.
In the acetylene hydrochlorination mechanism, previous studies have shown that the reaction occurs via the formation of a C2H2/Au due to C2H2 is easier to be adsorbed on the AuCl3 catalyst prior to HCl.6 However, it is a reverse order between catalyst stability and C2H2 adsorption capacity, because C2H2 leads to deactivation.6 TPD is an effective technique providing direct comparison of the adsorption and activation of reactants on different catalysts. Specifically, the desorption temperature in the TPD profiles reflect the binding strength of the adsorbed species with the catalyst surface and the peak area correlates with the amount of active species.31 Fig. 10 presents the C2H2-TPD profiles for Au/AC, AuCs/AC and AuCuCs/AC catalysts. The first weak desorption peak covers the temperature range of 50–180 °C and corresponds to the acetylene desorption from the support, which is consistent with our previous report. For Au/AC, there is a obvious desorption peaks can be observed higher than 180 °C, suggesting that Au3+ species favor the adsorption of C2H2. The desorption area of acetylene of the bimetallic catalysts AuCs/AC is larger than that of Au/AC catalysts. Furthermore, the desorption area of acetylene was further increased when both Cu and Cs was added to the Au/AC catalysts, which is significantly larger than that of either of the monometallic and bimetallic catalysts, indicating that addition of Cs and/or Cu improves the amounts of active sites of the Au-based catalyst, which agrees well with the activity sequence displayed in Table 1. Besides that, by adding Cs and Cu species, the desorption temperature of C2H2 shifts to a lower temperature for AuCs/AC sample (201 °C) and AuCuCs/AC sample (179 °C), respectively, compared to the Au/AC sample (249 °C). The shifts in the Au3+ peak to lower temperatures may be attributed to the electronic modifications on Au by Cs and Cu in trimetallic catalysts. The presence of strong interactions between Au, Cu and Cs is thus confirmed. Hence, the promotion of the C2H2 activation should be one reason for the outstanding catalytic performance of AuCuCs/AC catalyst for acetylene hydrochlorination.
3.3. Catalytic stability and lifetime estimation of the trimetallic AuCuCs/AC catalyst
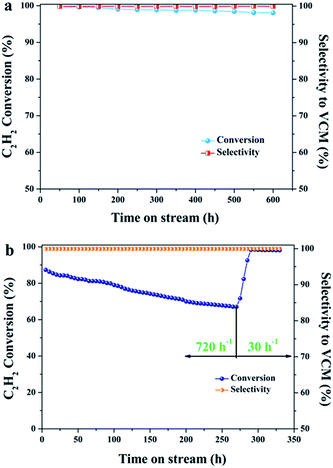
The important limiting factor for industrial acetylene hydrochlorination with supported Au-based catalysts is the long-term stability under reaction conditions. To demonstrate the superior performance of AuCuCs/AC, we further investigated the stability of the catalysts for acetylene hydrochlorination under the conditions of 180 °C and 50 h−1 of gas hourly space velocity (GHSV, C2H2 based) over 600 h. The AuCuCs/AC catalyst showed more than 98.8% conversion of C2H2 and more than 99.9% selectivity to VCM during a 600 h test under the upper limit of 50 h−1 of industrial hydrochlorination space velocity, as demonstrated in Fig. 11a. The results indicated that the low content AuCuCs/AC catalyst is stable under typical acetylene hydrochlorination reaction conditions.Furthermore, to preliminary estimate the lifetime of AuCuCs/AC catalyst, high GHSV (720 h−1) test was carried out to accelerate the deactivate process of AuCuCs/AC catalyst. This reaction under GHSV 720 h−1 for 1 h is equivalent to a reaction under GHSV 30 h−1 for 24 h. The catalytic results are shown in Fig. 11b. As shown, the conversion declines only by about 32% from the initial 99% after running for 270 h under conditions of 180 °C, GHSV(C2H2) = 720 h−1 and a feed HCl/C2H2 volume ratio = 1.2. This indicates that AuCuCs/AC is deactivated under reaction conditions. At this point, the GHSV was adjusted to 30 h−1. This change increases the acetylene conversion to 98.5% within 20 h; at this GHSV, a high conversion is maintained, even after 40 h. This result suggests that the AuCuCs/AC catalyst can maintain high catalytic activity at C2H2 hourly space velocity 30 h−1 for at least 6540 h. Evidently, the high activity combined with remarkable stability of this catalyst illustrates the potential of low content trimetallic AuCuCs/AC catalyst for industrial hydrochlorination of acetylene.
4. Conclusions
In summary, we have shown that the addition of Cu as a third metallic component to AC supported AuCs catalyst led to a reduction in the Au loading to about 0.25 wt% and enhanced the activity and stability of Au-based catalyst in the hydrochlorination of acetylene. A maximum TOF of 73.8 min−1 (based on total Au content) was obtained on the AuCuCs/AC catalyst. Furthermore, the AuCuCs/AC catalyst remained highly active and stable after a 600 h test at C2H2 hourly space velocity of 50 h−1 and an estimated lifetime exceeding 6540 h. The synergic catalysis derived from Au–Cu–Cs interaction found in this work may be associated with the enhanced dispersion of Au particles, the stabilization of Au in the state of Au3+ and facile substrate C2H2 molecule desorption. The optimized AuCuCs/AC catalyst featured in low cost, high catalytic activity, good long-term stability and low toxicity, demonstrating the potential for the replacement of Hg-based catalysts for acetylene hydrochlorination.Acknowledgements
The financial support by the National Natural Science Foundation of China (NSFC-21476207) is gratefully acknowledged.References
- M. W. Allsopp and G. Vianello, Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, Germany, 2000 Search PubMed.
- J. Bing and C. Li, Polyvinyl Chloride, 2011, 39, 1–8 Search PubMed.
- G. J. Hutchings and D. T. Grady, Appl. Catal., 1985, 17, 155–160 CrossRef CAS.
- G. J. Hutchings, J. Catal., 1985, 96, 292–295 CrossRef CAS.
- B. Nkosi, N. J. Coville and G. J. Hutchings, J. Chem. Soc., Chem. Commun., 1988, 1, 71–72 RSC.
- M. Conte, A. F. Carley, C. Heirene, D. J. Willock, P. Johnston, A. A. Herzing, C. J. Kiely and G. J. Hutchings, J. Catal., 2007, 250, 231–239 CrossRef CAS.
- M. Conte, C. J. Davies, D. J. Morgan, A. F. Carley, P. Johnston and G. J. Hutchings, Catal. Lett., 2014, 144, 1–8 CrossRef CAS.
- M. Conte, C. J. Davies, D. J. Morgan, T. E. Davies, A. F. Carley, P. Johnston and G. J. Hutchings, Catal. Sci. Technol., 2013, 3, 128–134 CAS.
- M. Conte, A. F. Carley, G. Attard, A. A. Herzing, C. J. Kiely and G. J. Hutchings, J. Catal., 2008, 257, 190–198 CrossRef CAS.
- W. Wittanadecha, N. Laosiripojana, A. Ketcong, N. Ningnuek, P. Praserthdam and J. R. Monnier, Appl. Catal., A, 2014, 475, 292–296 CrossRef CAS.
- S. A. Mitchenko, T. V. Krasnyakova, R. S. Mitchenko and A. N. Korduban, J. Mol. Catal. A: Chem., 2007, 275, 101–108 CrossRef CAS.
- S. A. Mitchenko, E. V. Khomutov, A. A. Shubin and Y. M. Shul'ga, J. Mol. Catal. A: Chem., 2004, 212, 345–352 CrossRef CAS.
- S. A. Mitchenko, T. V. Krasnyakova and I. V. Zhikharev, Theor. Exp. Chem., 2010, 46, 32–38 CrossRef CAS.
- T. V. Krasnyakova, I. V. Zhikharev, R. S. Mitchenko, V. I. Burkhovetski, A. M. Korduban, T. V. Kryshchuk and S. A. Mitchenko, J. Catal., 2012, 288, 33–43 CrossRef CAS.
- Y. Pu, J. Zhang, L. Yu, Y. Jin and W. Li, Appl. Catal., A, 2014, 488, 28–36 CrossRef CAS.
- J. Zhang, W. Sheng, C. Guo and W. Li, RSC Adv., 2013, 3, 21062–21068 RSC.
- G. Li, W. Li, H. Zhang, Y. Pu, M. Sun and J. Zhang, RSC Adv., 2015, 5, 9002–9008 RSC.
- K. Zhou, J. Si, J. Jia, J. Huang, J. Zhou, G. Luo and F. Wei, RSC Adv., 2014, 4, 7766–7769 RSC.
- J. Xu, J. Zhao, T. Zhang, X. Di, S. Gu, J. Ni and X. Li, RSC Adv., 2015, 5, 38159–38163 RSC.
- K. Zhou, J. Jia, X. Li, X. Pang, C. Li, J. Zhou, G. Luo and F. Wei, Fuel Process. Technol., 2013, 108, 12–18 CrossRef CAS.
- B. Nkosi, N. J. Coville, G. J. Hutchings, M. D. Adams, J. Friedl and F. E. Wagner, J. Catal., 1991, 128, 366–377 CrossRef CAS.
- J. Zhang, Z. He, W. Li and Y. Han, RSC Adv., 2012, 2, 4814–4821 RSC.
- H. Zhang, B. Dai, X. Wang, L. Xu and M. Zhu, J. Ind. Eng. Chem., 2012, 18, 49–54 CrossRef CAS.
- H. Zhang, B. Dai, X. Wang, W. Li, Y. Han, J. Gu and J. Zhang, Green Chem., 2013, 15, 829–836 RSC.
- Y. Pu, J. Zhang, X. Wang, H. Zhang, L. Yu, Y. Dong and W. Li, Catal. Sci. Technol., 2014, 4, 4426–4432 CAS.
- H. Zhang, W. Li, X. Li, W. Zhao, J. Gu, X. Qi, Y. Dong, B. Dai and J. Zhang, Catal. Sci. Technol., 2015, 5, 1870–1877 CAS.
- J. Zhao, J. Xu, J. Xu, J. Ni, T. Zhang, X. Xu and X. Li, ChemPlusChem, 2015, 80, 196–201 CrossRef CAS.
- J. Zhao, J. Xu, J. Xu, T. Zhang, X. Di, X. Li, J. Ni and X. Li, RSC Adv., 2015, 5, 6925–6931 RSC.
- C. Huang, M. Zhu, L. Kang, X. Li and B. Dai, Chem. Eng. J., 2014, 242, 69–75 CrossRef CAS.
- G. Deng, B. Wu and T. Li, Polyvinyl Chloride, 1994, 6, 5–9 Search PubMed.
- X. Li, X. Pan, L. Yu, P. Ren, X. Wu, L. Sun, F. Jiao and X. Bao, Nat. Commun., 2014, 5, 3688–3694 CAS.
- X. Li, Y. Wang, L. Kang, M. Zhu and B. Dai, J. Catal., 2014, 311, 28–294 CrossRef.
- X. Wang, B. Dai, Y. Wang and F. Yu, ChemCatChem, 2014, 6, 2339–2344 CrossRef CAS.
- K. Zhou, B. Li, Q. Zhang, J. Huang, G. Tian, J. Jia, M. Zhao, G. Luo, D. Su and F. Wei, ChemSusChem, 2014, 7, 723–728 CrossRef CAS PubMed.
- C. Zhang, L. Kang, M. Zhu and B. Dai, RSC Adv., 2015, 5, 7461–7468 RSC.
- S. Wang, B. Shen and Q. Song, Catal. Lett., 2010, 134, 102–109 CrossRef CAS.
- K. Zhou, W. Wang, Z. Zhao, G. Luo, J. T. Miller, M. S. Wong and F. Wei, ACS Catal., 2014, 4, 3112–3116 CrossRef CAS.
- A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896–7936 CrossRef PubMed.
- A. S. K. Hashmi, Angew. Chem., Int. Ed., 2005, 44, 6990–6993 CrossRef CAS PubMed.
- A. Hoffmann-Röder and N. Krause, Org. Biomol. Chem., 2005, 3, 387–391 Search PubMed.
- E. Jiménez-Núñeza and A. M. Echavarren, Chem. Commun., 2007, 333–346 RSC.
- D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395–403 CrossRef CAS PubMed.
- X. Zhang and A. Corma, Chem. Commun., 2007,(29), 3080–3082 RSC.
- H. Zhang, B. Dai, W. Li, X. Wang, J. Zhang, M. Zhu and J. Gu, J. Catal., 2014, 316, 141–148 CrossRef CAS.
- B. Nkosi, M. D. Adams and G. J. Hutchings, J. Catal., 1991, 128, 378–386 CrossRef CAS.
- J. Zhao, T. Zhang, X. Di, J. Xu, S. Gu, Q. Zhang, J. Ni and X. Li, Catal. Sci. Technol., 2015, 5, 4973–4984 CAS.
- K. Zhou, J. Jia, C. Li, H. Xu, J. Zhou, G. Luo and F. Wei, Green Chem., 2015, 17, 356–364 RSC.
- M. Conte, C. J. Davies, D. J. Morgan, T. E. Davies, D. J. Elias, A. F. Carley, P. Johnston and G. J. Hutchings, J. Catal., 2013, 297, 128–136 CrossRef CAS.
- S. De Miguel, O. Scelza, M. Román-Martınez, C. Salinas-Martinez de Lecea, D. Cazorla-Amorós and A. Linares-Solano, Appl. Catal., A, 1998, 170, 93–103 CrossRef CAS.
- J. Figueiredo, M. Pereira, M. Freitas and J. Orfao, Carbon, 1999, 37, 1379–1389 CrossRef CAS.
- E. Esmaeili, A. M. Rashidi, A. A. Khodadadi, Y. Mortazavi and M. Rashidzadeh, Fuel Process. Technol., 2014, 120, 113–122 CrossRef CAS.
- R. Arasteh, M. Masoumi, A. M. Rashidi, L. Moradi, V. Samimi and S. T. Mostafavi, Appl. Surf. Sci., 2010, 256, 4447–4455 CrossRef CAS.
- H. S. Oh, J. G. Oh, Y. G. Hong and H. Kim, Electrochim. Acta, 2007, 52, 7278–7285 CrossRef CAS.
- J. Jae, G. Tompsett, A. Foster, K. Hammond, S. Auerbach, R. Lobo and G. Huber, J. Catal., 2011, 279, 257–268 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |