Topological structure influences on the gel formation process and mechanical properties of L-lysine based supramolecular gels†
Si Chen,
Huiwen He,
Guodong Tang,
Bozhen Wu,
Meng Ma,
Yanqin Shi and
Xu Wang*
College of Materials Science and Engineering, Zhejiang University of Technology, Hangzhou 310014, China. E-mail: wangxu@zjut.edu.cn; sichen@zjut.edu.cn; Fax: +86-0571-88320855; Tel: +86-0571-88320855
First published on 9th November 2015
Abstract
The influence of a minor modification of the topological structure of a gelator’s core on the mechanism of the gel formation process and the resultant gel properties were researched by comparing the gelation ability of three L-lysine based gelators with the same arm structures and totally different topological core structures, one of which has a cubic topological polyhedral oligomeric silsesquioxane (POSS) core, one has a regular tetrahedron topological pentaerythritol core and the other has an organic linear topological dodecane core (denoted as POSS-Lys, PER-Lys and C12-Lys). Gelation tests, DSC, rheology measurements, SEM and POM investigations indicate that the gel obtained from C12-Lys with an organic linear topological dodecane core in the same solvent has a much greater strength of hydrogen bonding formed between the gelator molecules and much higher mechanical strength. What is more, POSS-Lys with a cubic topological core has a rather strong recovery ability, while PER-Lys cannot form a gel in any of the solvents tested. The key effect of such an obvious difference is in the self-assembly mechanisms which are influenced by the topological structure of the gelators.
Introduction
Soft materials, including gels, formed from supramolecular self-assembly have attracted much attention from researchers due to their potential applications in different fields, including drug delivery,1 biosensors,2 catalysis3 optoelectronics4 and so on. Supramolecules have well defined, three-dimensional branched architectures with different structures of molecular cores, and constitute a unique nanoscale toolkit, which achieves a reversible sol–gel phase transition by means of the non-covalent nature of the interactions including ion–ion, dipole–dipole, hydrogen bonding, π–π stacking, van der Waals force, host–guest, and ion coordination, and in so doing trap solvent molecules in the supramolecular network to form supramolecular gels.5,6 So the resultant material properties are generally determined by such non-covalent interactions, which are effective tools for constructing well-defined supramolecular structures driven by molecular self-assembly, controlled by the interplay of self-complementary and intermolecular supramolecular interactions that are further enforced through the generation of a multidimensional matrix structure.7 This makes the design of new classes of small molecules that can give rise to gelation with targeted properties often highly challenging as minor modifications can greatly affect the macroscopic properties of the resulting material in an unexpected manner.8 That is to say, the mechanisms of how the structures of supramolecules have significant influence on the properties are poorly understood. As previously reported, the aggregation of gelator molecules builds the primary structure. Further assembly to form fibrous objects such as rods, tubes, or sheets is the secondary structure. The interconnection of individual fibrous objects leads to the tertiary structure, the gel network. It has been demonstrated that the primary structures can be morphologically controlled by proper design of the gelator.9 Although a wide variety of research articles trying to understand the mechanism of the self-assembly process from different points of view, including structurally diverse molecules, generations, solvents and so on,10 very few studies have focused on the influence of topological factors on the self-assembly behavior of gelators. Not to mention to research the relationship of the topological change of the gelators and the self-assembly mechanism so as to understand the variation of the resultant gels in detail about how a minor modification of topological structure can greatly affect the macroscopic properties of the resulting materials.In this paper, the influence of minor modifications of a topological structure of a gelator’s core on the gel formation processes and the resultant gel properties were researched by comparing the gelation ability of three L-lysine based gelators with the same arm structures and totally different topological core structures. L-Lysine, one of the amino acids, has biocompatibility, biodegradation and non-toxicity, and is an environmentally friendly material, which is often used as a platform for low-molecular-weight gelators because of simple synthetic procedures and ease of introduction of various functional groups.11 The L-lysine based dendritic supramolecules researched in this paper focus on three different kinds, one of which has a cubic topological polyhedral oligomeric silsesquioxane (POSS) core, one has a regular tetrahedron topological pentaerythritol core and the other has an organic linear topological dodecane core (denoted as POSS-Lys, PER-Lys and C12-Lys, Scheme 1). It turns out that the gel obtained from POSS-Lys with a cubic topological core in the same solvent has a much greater strength of hydrogen bonding formed between the gelator molecules, and much higher mechanical strength, while PER-Lys cannot form a gel in any of the solvents tested. And the key effect of such an obvious difference is on the self-assembly mechanisms which are influenced by the topological structure of the gelators. This research attempts to give a typical example showing that when changing the topological structure of a gelator’s core, the properties of the obtained gel have been changed remarkably. Gaining this type of fundamental understanding is essential if the key effect of this important class of self-assembled soft materials is to be truly understood.
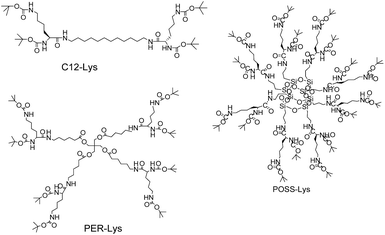 | ||
| Scheme 1 Chemical structures of three L-lysine based dendritic supramolecules C12-Lys, PER-Lys and POSS-Lys. | ||
Experimental
Gelation test
The L-lysine based dendritic supramolecules POSS-Lys, PER-Lys and C12-Lys were synthesised and characterized successfully as shown in the ESI†. An accurately weighed sample of gelator was mixed with 1 mL organic solvent in a sealed vial. Then, the vial was heated in an oil bath until the solution became homogeneous. The vial was cooled to room temperature and allowed to stand for several minutes or transferred to a refrigerating cabinet (2 °C) and left to settle overnight. The tube-inversion method was used to measure the thermal stability of the gel in terms of gel-to-sol transition temperature (Tgel). The minimum gelation concentration (MGC) was determined by measuring the minimum amount of gelator required for the formation of a completely gelled sample. The dynamic gel-to-sol transition process was visually captured by a digital camera. All gelator concentrations are expressed in % as the ratio of weight (mg) to liquid volume (mL).Differential scanning calorimetry (DSC)
Detailed thermal properties of gels were determined using a TA Q-100 differential scanning calorimeter. Heating and cooling rates of 10 °C min−1 were employed over a range of −5 to 140 °C. Sample weights were of the order of 8 mg, and experiments were performed using 40 μL aluminium pans.Rheology measurements
Rheological characterizations of gels were performed on an Anton Paar MCR302 rheometer with plate geometry (PP 25). The gap distance between plate and plate was fixed at 0.5 mm.Rheological measurements were carried out on freshly prepared gels which were scooped onto the plate of the rheometer. The following tests were performed: the samples were submitted to the parallel-plate very quickly to minimize solvent evaporation. Dynamic strain sweep tests were performed with increasing amplitude of oscillation from 0.1% up to 100% apparent strain shear (kept at a frequency ω = 6.28 rad s−1) at 25 °C. Dynamic time sweep tests were first given a large strain (γ = 80%) for 200 s and then the strain removed for 800 s, then kept at a frequency ω = 6.28 rad s−1 for its recovery. Dynamic temperature sweeps tests were from 25–150 °C with a heating rate of 3 °C min−1 (kept at a frequency ω = 6.28 rad s−1, strain γ = 0.1%).
Scanning electron microscopy (SEM)
The gel formed in a glass tube was allowed to dry under a vacuum to a constant weight. Then the resulting dried gel (xerogel) was coated with a thin layer of gold. The morphology was then observed by using a Hitachi S-4700 FE-SEM.Polarized optical microscopy (POM)
The polarized optical microscope morphology was investigated by POM (Ningbo Sunny Instruments Co. XP) equipped with a hot stage (KET-XMT-3100). The solution or gel was placed between two glass slides separated by a 1 mm thick spacer.Results and discussion
Gelation behaviour
The gelation ability of these three kinds of gelators, C12-Lys, PER-Lys and POSS-Lys, were tested in 10 of common organic solvents (Table 1). The results listed in Table 1 indicate that, although the “arm” structures and generations are exactly the same in C12-Lys, PER-Lys and POSS-Lys, their gelation behaviors are quite different. Firstly, POSS-Lys can form a stable gel in more solvents than C12-Lys, while PER-Lys cannot form a gel in any of the shown solvents. Secondly, in every solvent that both of them can gelate, the minimal gel concentration (MGC) of POSS-Lys is much lower than C12-Lys, especially in 1,2-dichlorobenzene (DCB) where the MCG of POSS-Lys is only 0.05% while C12-Lys is 1%. Thirdly, the gel–sol transition temperature (Tgel) of POSS-Lys in every solvent is much higher than C12-Lys.| Solventa | POSS-Lysb | PER-Lys | C12-Lysb |
|---|---|---|---|
| a DCB = 1,2-dichlorobenzene, DME = 1,2-dimethoxyethane.b G, PG and S denote gel, partial gel and solution, respectively. Data in brackets represent minimal gelation concentration (MGC) and gel–sol transition temperature in plateau region (Tgel).c Gel formation at low temperature. | |||
| Toluene | G (0.4%, 85 °C) | S | Gc (1%, 20 °C) |
| Benzene | G (0.6%, 85 °C) | S | Gc (1.5%, 20 °C) |
| Chlorobenzene | G (0.4%, 83 °C) | S | Gc (1.5%, 30 °C) |
| DCB | G (0.05%, 88 °C) | S | Gc (1%, 35 °C) |
| Ethyl acetate | G (0.5%, 68 °C) | S | PG |
| Butyl acetate | G (0.4%, 82 °C) | S | PG |
| DME | G (1%, 30 °C) | S | S |
| THF | S | S | S |
| Chloroform | S | S | S |
| Methanol | S | S | S |
The investigation of gelation ability confirms that POSS-Lys has a much higher gelation ability than C12-Lys, and PER-Lys cannot form a gel in any of the solvents. Details are discussed using DCB as a typical example here. In DCB solvent, it takes C12-Lys several hours to gelate only when the temperature is as low as 2 °C. However POSS-Lys only needs several seconds to gelate at room temperature.
We all know that solute molecules have three kinds of transitions at the supersaturation situation, including highly ordered accumulation to form a crystal, completely disordered accumulation to form precipitation and an intermediate state to form a gel. So essentially, the difference between a crystal and gel is the different order of molecular accumulation. The whole system in a crystal is in a thermally stable condition while in gel it is in a dynamically limited condition. In some situations, gels can change into crystals automatically. As reported in the literature,12 supersaturation solvents with gelators of two components firstly gelate at room temperature and automatically change into crystals after several hours, which completely separated from the solvents. There seems to be a competition relationship between the gelation and crystallization processes. Considering the especially long time that C12-Lys needed in the gelation process, which is quite different from normal gelators with the ability to gelate rapidly at room temperature, the gelation mechanism of C12-Lys is investigated in detail as follows.
POSS-Lys can gelate in the solvents mentioned in Table 1, and the resultant transparent gels could be obtained at room temperature. But for C12-Lys, things become different. The gelation behaviour of C12-Lys in DCB at different temperatures during different time periods is shown in Fig. 1. From Fig. 1 we can see that the transparent solution (a) of C12-Lys/DCB changes into suspension (b) after 10 min at 2 °C, and opaque gel (c) is obtained on being kept at 2 °C for 4 h. It is interesting to note that if the opaque gel (c) is kept at 25 °C for 5 minutes, a transparent gel (d) could be obtained, which could change back to opaque gel (c) after being kept at 2 °C for 10 min. That is to say, simply control of the ambient temperature could control the transition between opaque gel (c) and transparent gel (d).
So why are there such significant differences between the gelation behaviour of C12-Lys and POSS-Lys, and PER-Lys cannot even form a gel? The obvious answer is they have different topological structures. But that does not satisfy us enough, we hope to dig more into the fundamental mechanism. We propose a model for the self-assembly mechanism of the gelators with different topological structures as presented in Scheme 2. POSS-Lys with a cubic topological core (Scheme 2c) has much stronger gelation abilities, and could gelate in multiple solvents, the resultant gels of which have a much higher Tgel. C12-Lys with a linear topological core structure (Scheme 2a) shows weaker gelation abilities with a long-time low temperature gelation process in which a special opaque–transparent gel and crystal–amorphous transition could be observed. While PER-Lys cannot form a gel in any of the solvents, presumably because the pentaerythritol molecule has a regular tetrahedron topological core structure (Scheme 2b) which is harmful for the hierarchical self-assembly of PER-Lys. The low density of hydrogen bond between PER-Lys molecules has difficulty in overcoming the steric-hindrance effect of the pentaerythritol molecule to form a stable network structure, which has been proved by the second generation of dendritic PER-G2 (structure as shown in Scheme S3 in ESI†) as described in part 2 in the ESI.† The gelation behaviours of PER-G2 in various solvents are listed in Table S1 in ESI,† and the SEM observation (Fig. S1 in ESI†) of the xerogel of PER-G2 at a low concentration of gelator in xylene also confirms that the gel network is composed of nanofibers with a diameter of about 100 nm and a length of about 1–2 μm. This could further demonstrate that PER-G2 has the ability to form a stable gel in solvents by overcoming the steric-hindrance effect of the pentaerythritol molecule to form a pyknotic network, which is totally different from PER-Lys which always presents a dissolved state in the mentioned solvents.
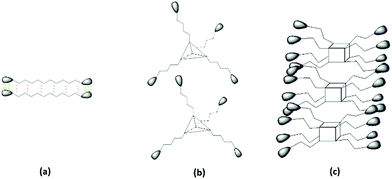 | ||
| Scheme 2 Self-assembly simulation diagram of supramolecules, the pear-shaped parts are the hydrogen bonding part of L-lysine. (a) C12-Lys, (b) PER-Lys, (c) POSS-Lys. | ||
DSC experiments
A DSC experiment has been performed to research the thermal change of such a process. The first heating curve obtained from the DSC of C12-Lys/DCB (3%) transparent gel (D) is shown in Fig. 2a, in which a typical wide gel–sol transition endothermic peak with a large temperature span is seen, indicating an endothermic process starting at 40 °C and achieving a peak temperature at 87 °C. That is to say, the supramolecular aggregates totally “depolymerize” after 87 °C. The first cooling and second heating curves of C12-Lys/DCB (3%) in the DSC experiment are shown in Fig. 2b. The cooling curve keeps level from 120 °C to 7 °C, confirming the fact that C12-Lys could not gel in DCB at room temperature. However, a sharp exothermic peak appears at 7 °C and a corresponding endothermic peak appeared at 14 °C during the second heating process. That is because tiny grains are formed at 7 °C during the cooling process and that is why the solution changes to be cloudy in the macroscale view. And when being heated, the grains melt at 14 °C to show a sharp endothermic peak. Such forming–melting process destroys the formation of gel networks so that probably only some aggregates are obtained and they contribute the small wide endothermic peak at around 46 °C.To further simulate the gel transition process through DSC, we use a different experiment keeping the system at −5 °C for 4 h, which is the least time for the formation of a macroscopic gel. A new clear wide endothermic peak appeared with a peak temperature of 46 °C at the second heating curve (Fig. 2c), which indicates that the gel transforms into a sol, which is formed during the long low temperature storage. But comparing to Fig. 2a we find that the temperature of gel–sol transition (46 °C) in Fig. 2c is much lower than in Fig. 2a (87 °C), and the integration area is also much smaller. That is because the crucibles used in DSC experiment could not totally be sealed to prohibit the volatilization of the solvent during the long experiment time, so the result of DSC experiment could not exactly simulate a realistic sol–gel process in a sealed tube. But the gelation mechanism could still be deduced as follows. Firstly, C12-Lys gathers to form tiny grains at low temperature. When being storage at low temperature, the tiny grains keep growing to form interconnected crystal networks which appear in the macroscopic view as opaque gels. The grains melt at a temperature higher than 14 °C, but instead of a totally disordered status, it changes into gel networks with second order. And the whole system becomes transparent because the density of the gel networks is much lower.
The DSC curve of POSS-Lys/DCB is totally different and reveals the hierarchical self-assembly nature of the POSS-Lys/DCB gel, which is shown in Fig. 2d. Multiple endothermic peaks are clearly observed during the gel–sol transition, including peak temperatures of 92 °C, 95 °C, 107 °C and 111 °C. So it can be figured out that only when the temperature is higher than 111 °C, are the gel networks totally “depolymerizing” into “monomers”, and in the temperature range from 92 °C to 111 °C, the gel networks are hierarchically depolymerized. That is because the POSS-Lys gelator hierarchically self-assembles in DCB and such hierarchical structure provides the gel “viscous flow” state which will discussed later. From the comparison of the DSC curves of C12-Lys/DCB gel and POSS-Lys/DCB gel, we could find out that they have totally different thermal behaviors due to their different self-assembly mechanisms. So the self-assembly processes of these two gels are researched step by step.
Gel network morphology
For the C12-Lys/DCB system, the opaque gel could be obtained after being kept at 2 °C for 4 h. If the opaque gel continues to be kept at low temperature for 5 days, macroscopic crystals could be observed with the naked eye, as shown in the insert picture in Fig. 3a. Different from the report of Smith and Tang,13 the crystal exists as a kind of network in the system instead of homogeneous macrophase separation. From the result of POM (Fig. 3a) we can see that C12-Lys forms spherocrystals which are interconnected to form crystal gel networks to bound solvent molecules. So we call such kinds of gel networks spherocrystal network gels as reported in the literature.14 When the temperature changes to room temperature, the gel becomes transparent, as shown in the insert picture of Fig. 3b. SEM observation shows that the transparent gel network is composed of microfibers with a diameter of about 80 nm and a length of about 4 μm. But the most interesting thing is that, other than the typical gel networks reported in the literature,15 these slim and short fibers have a quite high orientation order, as shown in Fig. 3b, in which most of the fibers have an orientation from top to the bottom, the orientated fibers compose into fiber bundles which have the ability to interlace into networks. | ||
| Fig. 3 (a) The polarizing microscope image (100×) of the C12-Lys/DCB gel after being kept at 2 °C for 5 days, (b) SEM image of C12-Lys/DCB xerogel, 30 mg mL−1. | ||
From Fig. 3 we can see that the transformation of gel fiber networks and spherocrystal networks could be achieved by changing the ambient temperature, furthermore it needs several hours at low temperature to change gel fiber networks into spherocrystal networks while only a couple of minutes are required to change them back at room temperature. An X-ray diffraction (XRD) experiment has been carried out to obtain more information about the molecular packing of self-assembled C12-Lys in the gel state. Fig. S2 in the ESI† shows several major diffraction peaks which demonstrate the microcrystal and orientation order of the gels. Furthermore, a dry sample of C12-Lys at lower concentration (0.5%) in DCB without gelation is also observed through SEM, which shows no clear ordered self-assembly microstructure as shown in Fig. S3 in the ESI,† indicating the ordered self-assembled structures are quite necessary to form gel networks.
Tube-inversion experiments
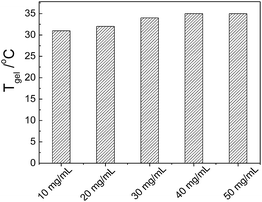
Although the results of SEM and POM confirm that the gel networks in the transparent and opaque C12-Lys/DCB gels are totally different, they still have some relevance to each other, such as even in the transparent gels, the fibers have high orientations. So the morphology influence on the macroscopic properties of the transparent gel is researched by testing the gelation properties. Fig. 4 shows the relationship between Tgel and the concentration of C12-Lys, indicating that when the concentration increases from 10 to 50 mg mL−1, Tgel only increases 4 °C (31–35 °C). That is to say the gel networks are sensitive to temperature instead of concentration.Fig. 5a is a photograph of the gel–sol transition process which clearly shows that the gel remains stable at a temperature lower than 33 °C but totally collapses when the temperature raises from 33 °C to 34 °C. Such temperature is close to the start of the endothermic peak shown on the DSC curve. So we can deduce that the gel network collapses at the beginning of the endothermal process. SEM already shows that the aspect ratio of the fibers is relatively small so that they do not have the ability of interpenetration and intertwining, but can compose into bundles with high orientation. So once the temperature reaches the “depolymerization” temperature, the gel networks will collapse immediately, like the melting of crystals with instantaneous properties.
 | ||
| Fig. 5 The dynamic changes in the process of gel–sol transformation. (a) C12-Lys/DCB gel (30 mg mL−1), (b) POSS-Lys/DCB gel (30 mg mL−1). | ||
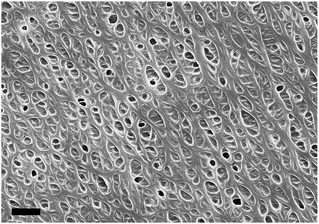
The xerogel of POSS-Lys/DCB has a totally different morphology, which could also be called a “loofah-like” network as shown in Fig. 6, because it looks similar to the reported morphology of POSS-Lys/MMA system by our group,16 indicating a sectional type hexagonal columnar assembly mode can also be obtained in DCB solvents. Such special loofah-like network structure provides the POSS-Lys/DCB gel with an extraordinary melting process that is quite different from nearly all the reported supramolecular gels.17
The morphology of POSS-Lys and C12-Lys xerogels show totally different networks with loofah-like and highly ordered short fibers respectively, demonstrating the totally different self-assembly mechanism of topological structures in these two gelators. As shown in Fig. 5b, when being heated, the POSS-Lys/DCB gel has a special “viscous flow” state and looks like a kind of melting polymer. And when the temperature becomes higher than 85 °C, although the gel could no longer keep in a gel state with a stable shape, it does not drop from the bottom of the tube all at once either, but slowly flows along the inner wall of the tube instead. In this whole process, the solvent has not separated from the gel networks to show a phase separation, which is to say that the gel networks do not “depolymerize” into “monomers” or small molecule aggregates after Tgel. POSS-Lys gels obtained from other solvents including methyl methacrylate, ethyl acetate and toluene are also tested to make sure such “viscous flow” state is not influenced by the solvents. This confirms the POSS-Lys could self-assembly into a kind of hierarchical network.
Rheological properties
The temperature sensitive property of C12-Lys/DCB is also expressed in the rheological properties. The relationship of the G′ of C12-Lys and POSS-Lys DCB gel and temperature is shown in Fig. 7a. We can see that when the temperature is lower than 35 °C, the G′ of C12-Lys gel is as high as 106 Pa, which is due to the crystal like structure of the highly ordered gel network. But when the temperature is higher than 35 °C, the G′ drops rapidly to 1 Pa, indicating the elasticity of the gel is totally lost. The whole rheological curve turns out to be a special Z shape, which is corresponding to the results discussed before. The POSS-Lys gel can show a more clear distinction than C12-Lys. The G′ of POSS-Lys gel shows a gradient drop with increasing temperature instead of the “Z” shape of C12-Lys/DCB, as shown in Fig. 7a. The G′ value of POSS-Lys gel is 103 Pa at 20 °C, 102 Pa at 40 °C and 10 Pa at 80 °C and 1 Pa at 110 °C. That is to say the elasticity of the gel drops gradually, which corresponds to the result of DSC to confirm the hierarchical structure of the POSS-Lys/DCB gel networks. To further research the mechanical properties of the POSS-Lys/DCB gels, a strain sweep test of the dynamic rheological measurement was performed with a frequency of 6.28 rad s−1 at 25 °C to characterize the mechanical properties of the above gels, the G′ and G′′ are shown in Fig. 7b. From Fig. 7b we could find out that when strain <0.5%, the G′ and G′′ of the POSS-Lys/DCB gel remain nearly unchanged which means the gel is at the liner viscoelastic region (LVR) where the elastic and viscous of which are constant. While the C12-Lys/DCB gel could just keep its LVR in 0.2% strain, which is due to the existence of spherocrystal networks. When the strain increases, G′ decreases quickly while G′′ is invariant until intersecting with G′ and then also decreases quickly.The intersection point of G′ and G′′ is the point of gel–sol transition, before which G′ > G′′, which indicates that the gel system formed a stable continuous elastic network. After the intersection point, G′ < G′′ and the gel network is no longer continuous so that the mixture is in a viscous state as a liquid-like state. The intersection point is nearly as large as 1% strain here in both gels, while POSS-Lys shows a larger than 100% strain in MMA16 which is nearly 100 times larger than in DCB, demonstrating the slightly different self-assembly of POSS-Lys in different solvents, furthermore indicating that the loofah-like structure has a stronger ability to resist external stress. The dynamic rheological measurement indicates that a significant strong mechanical property is obtained because of the special self-assembly networks.
Self-recovery abilities
To further research how the topological structures of the gelators affect the macro-mechanical properties of the gels, the self-recovery abilities of the gels were researched through a dynamic viscoelasticity test, as shown in Fig. 7c (C12-Lys gels) and Fig. 7d (POSS-Lys gels), giving a clear comparison from the thixotropic mechanical point of view. Interestingly, we note that the application of a large strain (γ = 80%) for 200 s makes the G′ value of POSS-Lys gel gradually change from the original 130 Pa to 40 Pa, and the gel exhibits a very rapid recovery of its mechanical properties by obtaining 77% of its original G′ value immediately after the strain is removed, and 94% of the original G′ value could be obtained in 600 s, as shown in Fig. 7d. This outstanding self-recovery ability of the POSS-Lys gel is as good as Wei et al. and Aida et al. reported,18 which is much better than the gel reported in the literature19 which recovers 90% strength to be a solid state. Such phenomena definitely indicated that a large strain force could not destroy the network of the gels which also have a rather strong recovery ability, providing the gels with future applications in self-recovery materials. What's more, under the same test condition as POSS-Lys mentioned before, C12-Lys gel also has a self-recovery performance that could not be ignored, which exhibits a rapid recovery of its mechanical properties by obtaining 43% of its original G′ value immediately after the strain is removed (Fig. 7c).Conclusions
In conclusion, we have demonstrated that different self-assembly behaviors could be modulated by the topological structures of the core of L-lysine based dendritic supramolecules and the resultant gels have quite different mechanical properties and gel formation processes in solvents. The results show that POSS-Lys with a cubic topological core has a much stronger gelation ability, which could gelate in multiple solvents and form a special self-assembly network, the resultant gels have a much higher Tgel. C12-Lys with a linear topological core structure shows a weaker gelation ability with a long-time low temperature gelation process in which a special opaque–transparent gel and crystal–amorphous transition could be observed. PER-Lys cannot form a gel in any solvent, which is presumably because the pentaerythritol molecule has a regular tetrahedron topological core structure and it is difficult to overcome the steric-hindrance effect to form a stable network structure, so PER-Lys presents a dissolved state in mentioned solvents. Gaining this type of fundamental understanding is essential if the key effect of this important class of self-assembled soft materials is to be truly understood.Acknowledgements
Financial support from the National Natural Science Foundation of China (Grant No. 51173167, 21004052), Zhejiang Provincial Natural Science Foundation of China (Grant No. LY14E030003, LY14E030004) and 2014 Zhejiang Province technology applied research project for public welfare (Grant No. 2014C31129) are gratefully acknowledged.Notes and references
- X. F. Hou, Y. Chen and Y. Liu, Soft Matter, 2015, 11, 2488 RSC.
- Q. Lin, Q. P. Yang, B. Sun, Y. P. Fu, X. Zhu, T. B. Wei and Y. M. Zhang, Soft Matter, 2014, 10, 8427 RSC.
- L. F. Rodriguez, B. Escuder, W. Hamley, W. M. Hayes and F. Juan, Soft Matter, 2012, 8, 8865 RSC.
- A. A. R. Watt, J. P. Bothma and P. Meredith, Soft Matter, 2009, 5, 3754 RSC.
- V. C. Edelsztein, A. S. Mac Cormack and M. Ciarlantini, et al., Beilstein J. Org. Chem., 2013, 9, 1826 CrossRef PubMed.
- S. Chen, G. D. Tang, B. Z. Wu, M. Ma and X. Wang, RSC Adv., 2015, 5, 35282 RSC.
- P. A. Korevaar, S. J. George, A. J. Markvoort, M. M. Smulders, P. A. J. Hilberts, A. P. H. Schenning, F. A. De Greef and E. W. Meijer, Nature, 2012, 481, 492 CrossRef CAS PubMed.
- M. Martínez-Calvo, O. Kotova and M. E. Möbius, et al., J. Am. Chem. Soc., 2015, 137, 1983 CrossRef PubMed.
- K. Araki, A. Brizard, F. Fages, A. R. Hirst, D. K. Smith, et al., in Low Molecular Mass Gelators: Design, Self-assembly, Function, Springer, Berlin, 2005 CrossRef CAS; O. Gronwald, E. Snip and S. Shinkai, J. Colloid Interface Sci., 2002, 7, 148 CrossRef CAS; B. A. Simmons, C. E. Taylor, F. A. Landis, V. T. John, G. L. McPherson, D. K. Schwartz and R. Moore, J. Am. Chem. Soc., 2001, 123, 2414 CrossRef PubMed; A. Aggeli, I. A. Nyrkova, M. Bell, R. Harding, L. Carrick, T. C. B. McLeish, A. N. Semenov and N. Boden, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 11857 CrossRef PubMed.
- Z. Ding, R. Xing, X. Wang, J. Ding, L. Wang and Y. Han, Soft Matter, 2013, 9, 10404 RSC; J. Lee, J. Kim, M. Yun, C. Park, J. Park, K. H. Lee and C. Kim, Soft Matter, 2011, 7, 9021 RSC; M. M. Su, H. K. Yang, L. J. Ren, P. Zheng and W. Wang, Soft Matter, 2015, 11, 741 RSC.
- M. Suzuki, M. Yumoto, M. Kimura, H. Shirai and K. Hanabusa, Chem.–Eur. J., 2003, 9, 348 CrossRef CAS PubMed.
- H. G. Yang, Synthesis, assembly, and architecture of titanium dioxide based semiconductor nanostructures, Diss., 2005 Search PubMed.
- J. R. Moffat and D. K. Smith, Chem. Commun., 2008, 2248 RSC; Y. Wang, L. Tang and J. Yu, Cryst. Growth Des., 2008, 8, 884 Search PubMed.
- X. Huang, P. Terech and S. R. Raghavan, et al., J. Am. Chem. Soc., 2005, 127, 4336 CrossRef CAS PubMed; X. Y. Liu, P. D. Sawant and W. B. Tan, et al., J. Am. Chem. Soc., 2002, 124, 15055 CrossRef PubMed.
- H. K. Yang, M. M. Su and L. J. Ren, et al., RSC Adv., 2014, 4, 1138 RSC.
- G. D. Tang, S. Chen, F. Ye and X. Wang, Chem.Commun., 2014, 50, 7180 RSC.
- A. R. Hirst, D. K. Smith, M. C. Feiters and H. P. M. Geurts, Langmuir, 2004, 20, 7070 CrossRef CAS PubMed.
- Y. Zhang, B. Yang, X. Zhang, L. Xu, L. Tao, S. Li and Y. Wei, Chem. Commun., 2012, 48, 9305 RSC; Q. Wang, J. L. Mynar, M. Yoshida, E. Lee, M. Lee, K. Okuro, K. Kinbara and T. Aida, Nature, 2010, 463, 339 CrossRef CAS PubMed.
- P. Xing, X. Chu and M. Ma, et al., Phys. Chem. Chem. Phys., 2014, 16, 8346 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17991b |
| This journal is © The Royal Society of Chemistry 2015 |