Photo-cross-linked poly(ether amine) micelles for controlled drug release†
Haozhe Hea,
Yanrong Ren*a,
Yuge Doua,
Tao Dinga,
Xiaomin Fanga,
Yuanqing Xua,
Hao Xua,
Wenkai Zhanga and
Zhigang Xie*b
aCollege of Chemistry and Chemical Engineering, Henan Universi, Kaifeng, 475004, P. R. China. E-mail: renyr@henu.edu.cn; Fax: +86-371-23881586; Tel: +86-371-23881586
bState Key Laboratory of Polymer Physics and Chemistry, Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun, 130022, P. R. China. E-mail: xiez@ciac.ac.cn; Fax: +86-431-85262775; Tel: +86-431-85262775
First published on 1st December 2015
Abstract
In order to improve the stability of micelles and decrease the burst release of loaded drugs, photo-cross-linked micelles were prepared via photodimerization of the coumarin moiety on amphiphilic poly(ether amine) (PEAC). The structures of the obtained monomer and polymers were confirmed by Fourier transform infrared spectroscopy (FTIR), ultraviolet-visible spectroscopy (UV-vis), 1H NMR and 13C NMR (Nuclear Magnetic Resonance, NMR). PEAC could self-assemble into micelles by directly dispersing in water with a hydrophobic coumarin core and a hydrophilic poly(ethylene glycol) (PEG) shell. The photo-cross-linking process of the PEAC micelles was monitored by UV-vis spectroscopy. The morphology and size distribution of the micelles was characterized by transmission electron microscopy (TEM) and dynamic light scattering (DLS). Anticancer drug doxorubicin (DOX) was loaded into the micelles during the process of micelle formation. Photo-cross-linked micelles showed slower drug release and cellular uptake in comparison with the uncross-linked micelles. And both DOX-loaded micelles displayed pH-sensitive release behaviours. Moreover, the DOX-loaded photo-cross-linked micelles exhibit comparative anticancer efficacy as free DOX. These results indicated that photo-cross-linked PEAC micelles can be used as potential drug carriers for intelligent drug delivery.
Introduction
Polymeric nanocarriers have been extensively investigated and used in drug delivery. In order to obtain high efficacy of delivered drugs and enhanced therapeutic effect, a nanocarrier should meet the following requirements: (i) maintaining high structural stability in blood after intravenous injection and eliminating undesirable drug release before reaching the target site, (ii) releasing drugs specifically and completely within targeted cells, (iii) as a nanocarrier itself being non-toxic, and not accumulate in the body.1–6 Among many potent nanocarriers, polymer micelles assembled from amphiphilic copolymers have attracted great attention in the past few years and have been one of the important candidates for intracellular drug delivery.7–12 However, the micelles often disintegrate after dilution in fluid blood vessel upon intravenous administration and the nanocarriers generally have issues of premature burst drug release within the first several hours.13–17 If so, micelles can neither hold entrapped drugs in blood stream nor release drugs specifically in the interior of targeted cells in a controlled manner.1,2,15–18In order to improve the stability of micelles and decrease the burst release of loaded drugs, chemical and physical cross-linking have been recognized as the powerful approaches and widely used in previous work.19,20 Comparing with other ways of cross-linking, photo-initiated cross-linking21–23 has attracted considerable interest due to rapid, effective, well-controlled unique advantages in practical applications.21–28 Among various photo-crosslinkable molecules, the coumarin analogues have been utilized in medicine, biology, and material sciences.22,29–31 More recently, the reversible photodimerization of coumarin has also been explored for designing photocontrollable micelles.29–34 Coumarin has the potential to photodimerize via a photo-induced [2 + 2] cycloaddition under irradiation of UV light at λ > 310 nm, and the formed dimer can be reversibly dissociate into two coumarin molecules under irradiation of UV light at λ < 260 nm.22,29–32 For example, Luo and coworkers31 prepared coumarin-containing telodendrimers micelles cross-linked at 310 nm, which showed superior drug loading efficiency, capacity and stability. Ji and coworkers22 prepared a novel coumarin-based pH-responsive polymer, indicating cross-linked micelles could avoid the unfavourable premature release of loaded drugs during circulation.
However, toxic organic solvents or catalysts are often used during the process of synthesizing polymers. Avoiding toxic reagents, or using low toxicity and non-toxic solvents is important for ideal drug release systems.22,31,32,35 In addition, polymeric micelles are usually prepared by first dissolving the copolymers in organic solvents for both blocks and following by the addition of water.21,22 Herein, we made the amphiphilic copolymers by nucleophilic addition and obtained their micelles by directly dispersing of PEAC in aqueous solution. It is worthy to mention that Yin and coworkers36,37 previously reported the synthesis of coumarin-containing poly(ether amine) (PEAC) and investigated its temperature-responsive behaviour. In this work, we further modified the synthetic method, investigated the pH-responsive and photo-crossing behaviour of PEAC, and evaluated the potential of PEAC as intelligent drug carriers.
Experimental details
Materials
Phloroglucinol, sodium bisulfate monohydrate and piperazine (Pip) were purchased from Aladdin Industrial Inc., Shanghai, China. Ethyl diacetoacetate, epichlorohydrin, methylbenzene, triethylamine (TEA) were purchased from China National Pharmaceutical Group Corporation, Beijing, China. Doxorubicin (DOX) in the form of a hydrochloride salt was purchased from Hisun Pharmaceutical Co., Ltd. Zhejiang, China. Poly(ethylene glycol) diglycidyl ether (PEGDGE, Mn = 500 g mol−1), 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co, Shanghai, China. Lyso-Tracker Red was purchased from Beyotime Biotechnology, China.Measurements
1H NMR spectra were characterized on a Bruker AVANCE 400 M spectrometer in CDCl3 and DMSO-d6 at 25 °C. Chemical shifts were given in parts per million with respect to tetramethylsilane (TMS) as an internal reference. Gel permeation chromatography (GPC) measurements were conducted with a Waters 410 GPC equipped with Waters Styragel column (HT4 + HT3) using CDCl3 as the eluent, the molecular weights were calibrated with polystyrene standards, and the flow rate was set at 1.0 mL min−1 at 35 °C. FT-IR spectra were recorded by the US Nicolet company's AVATAR360 type collector, collection range: 4000–400 cm−1. DLS measurements were performed by Malvern Zetasizer Nano ZS90, and the scattering angle was fixed at 90°. TEM studies were performed on a JEM-1011 electron microscope operating at an acceleration voltage of 100 kV. Samples were prepared by drop-casting onto Formvar Support Films and then air-dried at room temperature before measurement. The amount of the released DOX was measured using UV-vis spectroscopy (UV-2450PC, Shimadzu) at 480 nm wavelength and calculated on the basis of following calibration curve 1 (pH 7.4), calibration curve 2 (pH 5.0) and calibration curve 3 (in DI water) using different concentrations of free DOX (2.5–100 μg mL−1) in the same buffer solution:| y = 0.0289x + 0.0259, R2 = 0.9995 | (1) |
| y = 0.0169x + 0.0185, R2 = 0.9992 | (2) |
| y = 0.0185x + 0.0175, R2 = 0.9993 | (3) |
Synthesis of 5,7-dihydroxy-4-methyl coumarin (DHMC)
The mixture of phloroglucinol (0.1 mol), ethyl diacetoacetate (0.12 mol) and sodium bisulfate monohydrate (3 g) were reacted at 110 °C for 40 min. The resulting mixture was dissolved by anhydrous ethanol under refluxing. Then the solution was filtered to remove the catalyst. The filtrate was poured into ice-cold water to yield a light yellow precipitate. The product was dried in vacuum to get DHMC with a yield about 94%. 1H NMR (400 MHz, DMSO): δ 6.24 (t, J = 9.9 Hz, 1H), 6.16 (d, J = 2.2 Hz, 1H), 5.84 (s, 1H), 2.49 (d, J = 6.6 Hz, 2H); FTIR (neat, cm−1) 3200, 1732, 1616, 1110.Synthesis of 4-methyl-5,7-bi(2,3-epoxypropoxy) coumarin (DEMC)
The mixture of DHMC (0.01 mol), epichlorohydrin (0.1 mol) and potassium carbonate (0.0125 mol) were dissolved in toluene and reacted at 75 °C for 8 hours. Then, sodium hydroxide was added in and reacted for another 6 hours. The resulting mixture was dissolved by anhydrous ethanol under refluxing. Then the solution was filtered and poured into ice-cold water. The resulting precipitate was collected by filtration and washed with water to get DEMC with a yield about 70%. 1H NMR (400 MHz, DMSO): δ 6.61 (s, 1H), 6.55 (s, 1H), 6.04 (s, 1H), 4.46 (d, J = 11.4 Hz, 2H), 3.98–3.85 (m, 2H), 3.39 (d, J = 29.6 Hz, 4H), 2.87 (dd, J = 10.0, 5.1 Hz, 2H), 2.76–2.66 (m, 2H); FTIR (neat, cm−1) 1732, 1616, 1110.Synthesis of PEAC
The mixture of PEGDGE (0.005 mol), DEMC (0.005 mol) and piperazine (0.01 mol) were refluxed in anhydrous ethanol (10 mL) for 24 h under nitrogen. After the reaction, the mixture was poured into n-hexane and the precipitation was dried in vacuum to get PEAC. 1H NMR (400 MHz, DMSO): δ 6.24 (t, J = 9.9 Hz, 1H), 6.16 (d, J = 2.2 Hz, 1H), 5.84 (s, 1H); FTIR (neat, cm−1) 3400, 1732, 1616, 1110.Preparation of PEAC/diPEAC micelles
PEAC is an amphiphilic polymer, and can be dispersed directly in water to form micelles. 100 mg PEAC was added into 20 mL DI water and stirred for 30 minutes. Then micellar aqueous solution were obtained (5 mg mL−1) with coumarin (hydrophobic segment) as the core and PEG (hydrophilic segment) as the shell.Photo-dimerization crosslinked micelles (diPEAC) were prepared by the following method. PEAC (50 mg) was dissolved in DI water (50 mL) at room temperature. The solution was subjected to UV-irradiation using a UV light (UV LED Glue Curing Machine, UPUL008, Japan) at an intensity of 75 mW cm−2 at 365 nm. To study the cross-linking kinetics of the micelle, a certain amount of sample solution was made from the irradiated solution at predetermined intervals and subjected to UV-vis observation. UV-vis absorption spectra were recorded on a Shimadzu UV-2401PC UV-vis spectrophotometer with a 1.0 cm path length quartz cell.
The photo-dimerization degree (PD) was calculated from the UV-vis spectra by comparing the peak absorption at 320 nm assigned to the coumarin by the following equation:
| PD (%) = [A0 − At]/A0 |
Critical micelle concentration (CMC) measurements
Steady state fluorescence spectra were obtained by a Perkin-Elmer LS50B luminescence spectrometer. The PEAC solutions with various concentrations from 0.0006 to 1 g L−1 were added to a series of volumetric flasks. The emission wavelength was set at 320 nm for fluorescence excitation spectra. The spectra were recorded at a scan rate of 500 nm min−1. Excitation and emission slit widths are 0.75 nm, emission spectra recorded between 350 and 600 nm generated.Evaluation of PEAC/diPEAC stability in the fetal bovine serum
The PEAC/diPEAC solutions were mixed with equal volumes of PBS solution (pH 7.4, 0.01 M) containing 10% fetal bovine serum (FBS, GIBCO) and incubated at 37 °C. At various time points, 5 mL aliquots of the solutions were removed and analyzed by DLS.Preparation of DOX-loaded micelles (PEAC-DOX)
DOX-loaded PEAC was prepared by a straightforward method. Briefly, 20 mg DOX·HCl and 20 μL TEA were dissolved in DI water (5 mL), stir for 30 minutes, then 200 mg PEAC was added to the solution. After being stirred for an additional 2 h, 15 mL water was added slowly to the mixture, dropwise, under magnetic stirring at the same time. After stirred for another 2 h, the mixture was transferred to a 3500 Da molecular weight cutoff dialysis bag and dialyzed for 48 h to get rid of the free DOX. The water was replaced every 8 h, and finally, the mixture in the dialysis bag was freeze-dried to give red sponge-like micelles. To determine the drug loading content, the DOX-loaded PEAC was dissolved in DI water. The UV absorbance at 480 nm was measured to determine the DOX concentration based on the standard calibration curve obtained from free DOX in DI water.Drug loading content (DLC) and drug loading efficiency (DLE) were calculated according to the following formula:2
| DLC (wt%) = (weight of loaded drug/weight of drug loaded micelles) × 100% | (4) |
| DLE (wt%) = (weight of loaded drug/weight of drug in feed) × 100% | (5) |
Photo-cross-linking of the DOX-loaded micelles (diPEAC-DOX)
The cross-linked DOX-loaded micelles were prepared by the same protocol. Freeze-dried PEAC micelle powder (50 mg) was dissolved in 50 mL of water at room temperature. And then the solution was subjected to UV-irradiation using a UV light. The photo-cross-linking process was tracked by UV-vis.In vitro DOX release
The freeze-dried DOX-loaded PEAC was dissolved in phosphate saline buffer (pH 7.4) and acetate buffer (pH 5.0) at a concentration of 1 mg mL−1. The above mixture was transferred into a dialysis bag with a molecular weight cutoff of 3500 Da. The bag was then immersed into a container with 20 mL of buffer solution at the same pH value as that inside the bag. The outer phase of the buffer solution was oscillated at 37 °C (50 rpm). At selected time intervals, 3 mL of the external buffer was withdrawn for UV-vis analysis and replaced with the same amount of fresh buffer solution. The released amount of DOX was determined from the absorbance at 480 nm with the help of a calibration curve of DOX in the same buffer. Then the accumulative weight and relative percentage of the released DOX were calculated as a function of incubation time.Cell lines
Two cell lines, HeLa (cervical cancer cells, human) and HepG2 (Hepatocellular carcinoma cells, human), supplied by the Medical Department of Jilin University, China, were chosen for cell tests. HeLa and HepG2 cells were cultured in Dulbecco's modified Eagle's medium (DMEM, GIBCO) supplied with 10% heat-inactivated FBS 2 mM L-glutamine, 100 U mL−1 penicillin, and 100 μg mL−1 streptomycin (Sigma), at 37 °C in a humidified atmosphere containing 5% CO2.Cytotoxicity
The cytotoxicity of micelles was examined by MTT assay. All sample solutions were diluted with DMEM to obtain preset concentrations. HeLa and HepG2 cells were seeded into 96-well plates with a density of 104 cells per well and incubated in DMEM (100 mL) for 24 h. Then six concentrations (1.0, 0.5, 0.25, 0.12, 0.06 and 0.03 μg mL−1) of PEAC and diPEAC micelles were added to the wells, and three parallel wells for each sample were used at a specific concentration. After co-incubation with cells for 48 h, 20 μL of MTT solution in PBS (5 mg mL−1) was added to each well and the plate was incubated for another 4 h at 37 °C. After that, the medium containing MTT was removed, and 150 μL of DMSO was added to each well to dissolve the MTT formazan crystals. Finally, the plates were shaken for 5 min, and the absorbance of formazan product was measured at 490 nm by a Bio-Rad 680 microplate reader.For anticancer activity analyses, we also use MTT assay to evaluate the cytotoxicity of DOX-loaded PEAC/diPEAC micelles and free DOX against HepG2 cells with different DOX dosages from 0.001 to 100 μg mL−1.
Cellular uptake studies
Cellular uptakes by HepG2 cells were examined using confocal laser scanning microscope (CLSM). HepG2 cells were seeded in 6-well culture plates (a sterile coverslip was put in each well) at a density of 1 × 105 cells per well and allowed to adhere for 24 h. Then the cells were treated with free DOX (5 μg mL−1) or DOX-loaded PEAC/diPEAC (5 μg mL−1, equivalent DOX concentration). After incubation for 0.5 h, 4 h and 24 h at 37 °C, the supernatant was carefully removed and the cells were washed three times with ice-cold PBS and fixed with 4% formaldehyde. After the nucleus was stained with DAPI, the slides were mounted. CLSM images were captured via confocal microscope (Carl Zeiss LSM 710) under the same conditions.Results and discussion
Synthesis of PEAC
Coumarin monomer with two epoxy groups (DEMC) was obtained via two steps reaction. Its structure was confirmed by 1H NMR, 13C NMR and FTIR as show in Fig. S1 and S2† and Fig. 2, respectively. In Fig. S1 and S2A,† all proton atoms corresponding to their characteristic chemical shifts can be found. The chemical shifts at 6.0, 6.4 and 6.5 ppm (Ha–c) were the typical peaks of coumarin. And in Fig. S2B,† the five signals at 161.9, 160.0, 158.4, 156.6 and 154.5 ppm can be unambiguously assigned to C1–5 and the four signals at 111.5, 104.7, 97.3 and 95.0 ppm were explicitly assigned to C6–9, nine signals were the characteristic peak of the benzo pyran ring in coumarin. Two signals at 49.8 and 44.3 ppm are the characteristic peaks of the epoxide group.PEAC was synthesized from DEMC, PEGDGE and Pip by nucleophilic addition/ring-opening reactions as shown in Scheme 1. The polymerization reaction is simple, mild and no small molecules were generated during the polymerization with the feature of “click-chemistry”.39 GPC was utilized to determine the molecular weights Mn and polydispersity (Mw/Mn) which were 2.84 × 104 and 1.96, respectively. The structure of PEAC was characterized by 1H NMR spectra (Fig. 1). The typical peaks of PEG and coumarin presented clearly in Fig. 1A. The chemical shifts at 6.0, 6.4 and 6.5 ppm in CDCl3 were pointed to the proton of coumarin (H1–3).
 | ||
| Scheme 1 Synthetic route for PEAC.36,37 | ||
FTIR spectra of DHMC, DEMC and PEAC were shown in Fig. 2. A strong stretching vibration of hydroxyl peaks at 3400 cm−1 can be seen in PEAC and DHMC, but no corresponding absorption peak in DEMC, because PEAC and DHMC contains hydroxyl structure and none in DEMC. In addition, compared with PEGDGE, new peaks at 1722 and 1616 cm−1 assigned to stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the aromatic ring of the coumarin moieties appear in the FTIR spectrum of PEAC, showing the successful introduction of coumarin into PEAC.
O, and the aromatic ring of the coumarin moieties appear in the FTIR spectrum of PEAC, showing the successful introduction of coumarin into PEAC.
Coumarin contain a strongest ultraviolet absorption peak around 320 nm, as shown in Fig. 3, both DEMC and PEAC has a maximum absorption peak at about 320 nm demonstrated that PEAC contains coumarin moieties.
PEAC could form the micelles in aqueous solution by dispersing in water without any organic solvent. Then concentration of PEAC could reach to 10 mg mL−1 in water. In Fig. 1B, these peaks for coumarin almost disappeared in D2O, revealing that PEAC formed micelles with almost all the coumarin moiety transferring to the inner cores. Coumarin has a benzo pyran ring, which is a very good fluorescence emission group. So this type of polymer does not require additional fluorescence probe such as pyrene to detect the critical micelle concentration. The CMC of PEAC was calculated directly by using the fluorescence of coumarin according to the ref. 38 and 40. As shown in Fig. 4, the CMC value was calculated to be 4.0 × 10−3 g L−1 for PEAC.
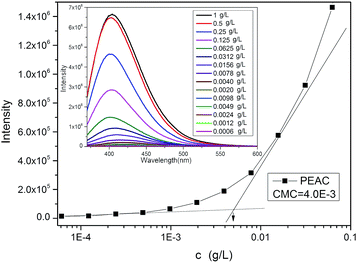 | ||
| Fig. 4 Intensity from the fluorescence excitation spectra with PEAC in different concentration. Inset: fluorescence emission spectra with different PEAC concentration. | ||
Coumarin is a light-sensitive materials, photo-dimerization occur under irradiation at 365 nm. As illustrated in Scheme 2. PEAC was dispersed in DI water with the concentration of 1 g L−1. Under illumination at 365 nm for different time, nanoparticles with different degree of cross-linking could be obtained. The process of cross-linking is monitored by the UV-vis spectrophotometer. As shown in Fig. 5, with the increase of exposure time, maximum absorption intensity at 320 nm decreased gradually, indicating the occurrence of the light dimerization. The photo-dimerization degree of PEAC increased to 50% rapidly in the first 5 minutes and increased to 70% in the next 10 minutes.
 | ||
| Scheme 2 Schematic illustration of micellization of PEAC, the formation of cross-linked micelles under UV-irradiation and DOX-loaded micelles. | ||
DLS was utilized to study the size distribution of the micelles. The average diameter of PEAC micelle was 110 nm (Fig. 6A). After photo-cross-linking, the size changed to 85 nm (Fig. 6B). The diameter of PEAC-DOX micelle was 140 nm (Fig. 6C) and changed to 120 nm (Fig. 6D) after photo-cross-linking. The shrinkage of the micelles was ascribed to the cross-linking. This shrinkage was confirmed by TEM, the mean diameter of spherical micelles before and after photo-cross-linking was about 50 nm (Fig. 6E) and 40 nm (Fig. 6F), respectively. For DOX-loaded PEAC micelles, the diameter is about 70 nm (Fig. 6G) and it changed to 60 nm (Fig. 6H) after cross-linking. Moreover, all particles presented good dispersibility, even the cross-linked particles, implying that there was no micelle aggregation during the process of cross-linking. Particle size measured by TEM is smaller than the DLS, mainly due to the volume shrinkage in the drying process in the preparation of the TEM sample.41,42
 | ||
| Fig. 6 DLS results of PEAC micelles (A) and diPEAC (B); PEAC-DOX (C) and diPEAC-DOX (D); TEM images of PEAC micelles (E) and diPEAC (F); PEAC-DOX (G) and diPEAC-DOX (H) (bar = 500 nm). | ||
PEAC micelles, the diameter are about 70 nm (Fig. 6G) and changed to 60 nm (Fig. 6H) after cross-linking. Moreover, all particles presented good dispersibility, even the cross-linked particles, implying that there was no micelle aggregation during the process of cross-linking. Particle size measured by TEM is smaller than the DLS, mainly due to the volume shrinkage in the drying process in the preparation of the TEM sample.41,42
Stability of PEAC/diPEAC micelles
The stability of the PEAC/diPEAC micelles was studied in PBS (pH 7.4) containing 10% FBS at 37 °C by monitoring the particle size change as a function of time. As it was shown in Fig. 7, there was no significant change in size during 24 h, suggesting that neither aggregation nor destabilization was induced by serum proteins. | ||
| Fig. 7 Particle size and PdI of micelles in PBS (pH 7.4) containing 10% FBS at 37 °C for different time periods determined by DLS. | ||
Drug loading and release in vitro
To assess the influence of tertiary amino groups and photo-dimerization degree (%) on drug incorporation, DOX was used as a model anticancer drug to evaluate the drug loading and release properties. Drug-loading was done by directly dispersing drug and PEAC together into water without any organic solvents or auxiliaries. The drug loading contents (DLC) and the drug loading efficiency (DLE) of PEAC-DOX were measured to be about 6.78 wt% and 67.8% (the theoretical DLC was designed to be 10 wt%). It is worthy to mention that photo-dimerization of micelles under UV irradiation at 365 nm did not affect both DLC and DLE.The in vitro DOX release from PEAC and diPEAC was conducted in two different buffered solutions (pH 7.4 and 5.0) at 37 °C. As shown in Fig. 8, dramatic burst release of DOX was not observed for both PEAC and diPEAC micelles at pH 7.4 and pH 5.0, demonstrating DOX was well entrapped in the inner core of both micelles. Meanwhile, the release of DOX from diPEAC micelles was significantly slower than that from PEAC micelles. At pH 7.4 (Fig. 8A), only approximately 11% of DOX was released during 60 h from diPEAC micelles (photo-dimerization degree was 80%), while only 20% of DOX was released from the PEAC micelles under the same conditions. The same phenomenon can be seen at pH 5.0 (Fig. 8B). The release amount was about 20% from diPEAC micelles for 60 hours, compared with a 50% release of PEAC-DOX micelles. These data also indicated that the both micelles have pH-sensitivity (lower drug release at pH 7.4 and higher drug release at pH 5.0). This character would be useful for drug delivery to obtain a long-term circulation in blood and an effective drug release at the tumor site, since the microenvironment around the tumor cells and inside the endosome is acidic.
 | ||
| Fig. 8 In vitro release profiles of DOX from PEAC and diPEAC micelles at pH 7.4 (A) and pH 5.0 (B) at 37 °C (50%, 60%, 70%, 80% point to different photo-dimerization degree (%)). | ||
At the same time, we noticed that the degree of cross-linking showed a certain influence on the release rate. At pH 7.4, when the photo-dimerization degree is 50%, the release drug was about 15%. It was obvious the release rate decreases with the increase of photo-dimerization degree, because the cross-linking made the micellar core more compact, and suppressed the release of DOX from the micelles. The sustained release of DOX from the diPEAC micelles is conducive to the extension of drug circulation time and protects the drug from enzyme degradation, rapid renal clearance and interactions with serum proteins and thus would significantly enhance drug delivery to tumors.2
Cell internalization
The cellular uptake and intracellular release behaviors of free DOX and DOX-loaded PEAC/diPEAC were investigated by CLSM. Free DOX and DOX-loaded PEAC/diPEAC were incubated with HepG2 cells at 37 °C for 0.5 h, 4 h and 24 h. As shown in Fig. 9, intracellular distribution of DOX-loaded PEAC/diPEAC was different from that of free DOX. Red fluorescence was observed mainly in the cellular nuclei after 0.5 h of incubation with free DOX (Fig. 9A). However, when the cells were incubated with DOX-loaded micelles, the fluorescence was observed mainly in the cytoplasm rather than the cell nuclei (Fig. 9D and G). After 4 h incubation, the fluorescence in the nuclei of the cells became stronger for free DOX (Fig. 9B), while the cells incubated with DOX-loaded micelles for 4 h emitted increased fluorescence in the cytoplasm as well as the very weak fluorescence in nuclei (Fig. 9E and H). When the incubation period increased to 24 h, the fluorescence in the nuclei of the cells became weaker for free DOX (Fig. 9C). For DOX-loaded micelles, the red fluorescence nuclei increased with time (Fig. 9F and I). These data suggest that the internalization mechanism of DOX-loaded micelles is different from that of free DOX. On the other hand, the fluorescence intensity of the diPEAC micelles (Fig. 9G–I) was much stronger than the PEAC micelles (Fig. 9D–F).The smaller particle size and the more stable structure of the photo-cross-linked micelles should take some responsibility for this result.43,44 DOX-loaded micelles will eventually enter the nuclei where DOX interacts with topoisomerase II to cause DNA cleavage and cytotoxicity,2 as the following cytotoxicity results demonstrate that the growth of HepG2 cells can be effectively inhibited (Fig. 8).
In order to check whether the micelles go (even partly) to the lysosomes or not, HepG2 cells incubated with Calcein loaded-micelles for 1 h at 37 °C at a concentration of 10 μM. The calcein fluorescence was encapsulated into micelles instead of DOX. As shown in Fig. S3,† lysosomes stained with Lyso-Tracker Red (red fluorescence) was mostly overlapped with Calcein fluorescence (green), indicating that the micelles go mostly to the lysosomes.
Cytotoxicity
MTT assay was applied to evaluate the cytotoxicity of the blank polymer micelles by using HeLa and HepG2 cells. After incubation with PEAC and diPEAC micelles at gradient concentrations from 1.0 to 0.03 μg mL−1 for 24 h, cells showed high viability (80% and above) even the concentrations of micelle up to 1 mg mL−1, displaying that both micelles have good biocompatibility and safe to be used as drug carriers (Fig. 10). | ||
| Fig. 10 Cell viability of HeLa and HepG2 cells incubated with PEAC and diPEAC micelles. Data were presented as mean ± standard deviation (n = 3). | ||
The in vitro cytotoxicities of DOX-loaded micelles and the free DOX were evaluated by MTT assay against HepG2 cells with different DOX dosages from 0.001 to 100 μg mL−1. As shown in Fig. 11, the half maximal inhibitory concentration (IC50) values for free DOX, PEAC-DOX and diPEAC-DOX against HepG2 cell lines were 0.56, 2.84 and 4.43 μg mL−1, respectively. These results demonstrated that DOX-loaded micelles were able to enter the cells and exhibited a suitable pharmacological effect on cancer cells. Meanwhile, free DOX showed higher inhibition for cancer cell proliferation than that of the DOX-loaded PEAC/diPEAC micelles, which had been seened in the previous literature.45,46 The lower cytotoxicity of DOX-loaded micelles can be attributed to the slow release of DOX from micelles as evidenced by the in vitro release in Fig. 8 and delayed nuclear uptake in HepG2 cells which has been proved by internalization studies by CLSM (Fig. 9).
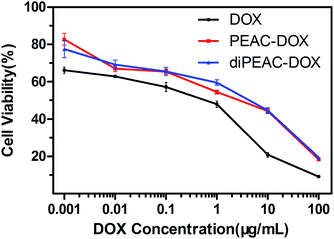 | ||
| Fig. 11 Cell viability of HepG2 cells against DOX, DOX-loaded PEAC/diPEAC micelles after cultured for 48 h with different DOX dosages. | ||
Conclusions
In summary, a coumarin-based pH-responsive copolymer was successfully synthesized. The core of the polymeric micelles was cross-linked by UV-irradiation at 365 nm via photo-dimerization of the coumarin moiety to form the cross-linked micelles. Drugs can be loading easily during the self-assembly of copolymers. The PEAC and diPEAC micelles both have pH-sensitivity, low drug release at pH 7.4 and high drug release at pH 5.0. In addition, the cross-linked micelles had slower drug release in comparison with the uncross-linked micelles. The cross-linked DOX-loaded micelles could be successfully internalized by cancer cells, showing high cellular uptake efficiency. All in all, we are convinced that the cross-linked DOX-loaded micelles would have a great potential in cancer therapy applications.Acknowledgements
The authors would like to thank the financial support from National Natural Science Foundation of China (No. 51203043, No. 51403051, No. 21502042) and Developing Program of Natural Science of Henan province (No. 142300410122).Notes and references
- L. S. Yan, L. X. Yang, H. Y. He, X. L. Hu, Z. G. Xie, Y. B. Huang and X. B. Jing, Polym. Chem., 2013, 3, 1301 Search PubMed.
- H. H. Kuang, H. Y. He, J. Hou, Z. G. Xie, X. B. Jing and Y. B. Huang, Macromol. Biosci., 2013, 13, 1593 CrossRef CAS.
- C. C. Lee, J. A. Mackay, J. M. Fréchet and F. C. Szoka, Nat. Biotechnol., 2006, 23, 1517 CrossRef.
- W. B. Liechty and N. A. Peppas, Eur. J. Pharm. Biopharm., 2012, 80, 241 CrossRef CAS PubMed.
- H. S. Han, K. Y. Choi, H. Ko, J. Jeon, G. Saravanakumar, Y. D. Suh, D. S. Lee and J. H. Park, J. Controlled Release, 2014, 200, 158 CrossRef.
- P. H. Edgar and F. M. Alberto, Eur. J. Pharm. Biopharm., 2015, 93, 52 CrossRef.
- G. Gaucher, M. H. Dufresne, V. P. Sant, N. Kang, D. Maysinger and J. C. Leroux, J. Controlled Release, 2006, 109, 169 CrossRef.
- Z. S. Ge and S. Y. Liu, Chem. Soc. Rev., 2013, 42, 7289 RSC.
- C. Wang, L. Cheng and Z. Liu, Biomaterials, 2011, 32, 1110 CrossRef CAS PubMed.
- V. Shrinivas, J. L. Hedrick, Y. O. Zhan, C. Yang, P. L. R. Ee, P. T. Hammond and Y. Y. Yi, Adv. Drug Delivery Rev., 2011, 63, 1228 CrossRef PubMed.
- H. Cabral and K. Kataoka, J. Controlled Release, 2014, 190, 465 CrossRef CAS PubMed.
- W. T. Lu, X. X. Wang, R. Cheng, C. Deng, F. H. Meng and Z. Y. Zhong, Polym. Chem., 2015, 6, 6001 RSC.
- Y. Q. Shen, J. Erlei, Z. Bo, M. Caitlin, J. M. H. Sui, J. Zhao, J. Q. Wang, J. B. M. H. Fan and V. K. Edward, J. Am. Chem. Soc., 2010, 132, 4259 CrossRef CAS PubMed.
- Y. Shen, Y. Zhan, J. Tang, P. Xu, P. A. Johnson, M. Radosz, E. A. Van Kirk and W. J. Murdoch, AIChE J., 2008, 54, 2979 CrossRef CAS.
- R. F. Pagels and R. K. Prud'homme, J. Controlled Release, 2015, 219, 519 CrossRef CAS PubMed.
- A. R. Bilia, M. C. Bergonzi, C. Guccione, M. Manconi, A. M. Fadda and C. Sinico, J. Drug Delivery Sci. Technol., 2015 DOI:10.1016.
- M. L. Wang, J. Sun, Y. L. Zhai, H. Lian, C. Luo, L. Li, Y. Q. Du, D. Zhang, W. Ding and S. H. Qiu, Biomacromolecules, 2015, 16, 1179 CrossRef CAS.
- W. W. Du, Y. C. Fan, B. He, N. Zheng, L. Yuan, W. B. Dai, H. Zhang, X. Q. Wang, J. C. Wang and X. Zhang, Mol. Pharm., 2015, 12, 1467 CrossRef CAS PubMed.
- R. Ja-Hyoung, R. T. Chacko, J. Siriporn, B. Sean, B. R. Prakash and S. Thayumanavan, J. Am. Chem. Soc., 2010, 132, 17227 CrossRef PubMed.
- K. Alexander and V. Serguei, Angew. Chem., Int. Ed., 2009, 48, 5418 CrossRef PubMed.
- F. Ercole, T. P. Davis and R. A. Evans, Polym. Chem., 2010, 1, 37 RSC.
- F. Jia, Y. Wang, H. B. Wang, Q. Jin, T. G. Cai, Y. J. Chen and J. Ji, Polym. Chem., 2015, 6, 2069 RSC.
- R. K. O'Reilly, C. J. Hawker and K. L. Wooley, Chem. Soc. Rev., 2007, 38, 1068 Search PubMed.
- R. Yang, F. H. Meng, S. B. Ma, F. S. Huang, H. Y. Liu and Z. Y. Zhong, Biomacromolecules, 2011, 12, 3047 CrossRef CAS PubMed.
- A. Elena, M. M. Dolores, M. M. E. Ramón, S. Félix, S. Juan, A. Pedro and G. Carmen, J. Am. Chem. Soc., 2009, 131, 6833 CrossRef PubMed.
- J. Li, S. Guo, M. Wang, L. Ye and F. Yao, RSC Adv., 2015, 5, 19484 RSC.
- M. Nagata and Y. Yu, React. Funct. Polym., 2008, 68, 915 CrossRef CAS.
- S. R. Trenor, A. R. Shultz, B. J. Love and T. E. Long, Chem. Rev., 2004, 104, 3059 CrossRef CAS PubMed.
- H. B. Sun, H. L. Fan and X. H. Peng, J. Org. Chem., 2014, 79, 11359 CrossRef CAS PubMed.
- F. Borges, F. Roleira, N. Milhazes, L. Santana and E. Uriarte, Curr. Med. Chem., 2005, 12, 887 CrossRef CAS.
- G. Xu, C. Shi, D. Guo, L. Wang, Y. Ling, X. Han and J. Luo, Acta Biomater., 2015, 21, 85 CrossRef CAS PubMed.
- J. Qiao, M. J. Samarendra and A. Seema, Polym. Chem., 2012, 3, 2785 RSC.
- C. Chen, X. Liu, J. Ji, G. Liu, S. Pang, C. Zhu and L. Lv, Polym. Chem., 2011, 2, 1389 RSC.
- M. J. Robb, L. A. Connal, B. F. Lee, N. A. Lynd and C. J. Hawker, Polym. Chem., 2012, 3, 1618 RSC.
- N. M. Khan, H. Kapahi, S. Kumar, T. Bhardwaj, S. Arora and N. Mishra, J. Drug Targeting, 2015, 23, 387 CrossRef.
- X. S. Jiang, R. Wang, Y. R. Ren and J. Yin, Langmuir, 2009, 25, 9629 CrossRef CAS PubMed.
- G. X. Fu, Z. L. Su, X. S. Jiang and J. Yin, Polym. Chem., 2014, 5, 2027 RSC.
- J. Q. Jiang, Q. Z. Shu, X. Chen, Y. Q. Yang, C. L. Yi, X. Q. Song, X. Y. Liu and M. Q. Chen, Langmuir, 2010, 26, 14247 CrossRef CAS PubMed.
- H. Nandivada, X. Jiang and J. Lahann, Adv. Mater., 2007, 19, 2197 CrossRef CAS.
- T. J. V. Prazeres, M. Beija, F. V. Fernandes, P. G. A. Marcelino, J. P. S. Farinha and J. M. G. Martinho, Inorg. Chim. Acta, 2012, 381, 181 CrossRef CAS.
- K. Hayakawa, T. Yoshimura and K. Esumi, Langmuir, 2003, 19, 5517 CrossRef CAS.
- C. Feng, Z. Shen, Y. G. Li, L. N. Gu, Y. Q. Zhang, G. L. Lu and X. Y. Huang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1811 CrossRef CAS.
- S. H. Wu, H. H. Kuang, F. B. Meng, Y. G. Wu, X. Y. Li, X. B. Jing and Y. B. Huang, J. Mater. Chem., 2012, 22, 15348 RSC.
- Y. Kim, M. H. Pourgholami, D. L. Morris and M. H. Stenzel, Biomacromolecules, 2012, 13, 814 CrossRef CAS PubMed.
- X. T. Shuai, A. Hua, N. Nasongkla, S. Kim and J. M. Gao, J. Controlled Release, 2004, 98, 415 CrossRef CAS PubMed.
- B. Younsoo, F. Shigeto, H. Atsushi and K. Kazunori, Angew. Chem., 2003, 42, 4640 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra22679a |
| This journal is © The Royal Society of Chemistry 2015 |





