Study on the accelerated Gutknecht self-cyclocondensation of amino-sugars under atmospheric pressure chemical ionization conditions†
Hao-Yang Wang*a,
Jun-Ting Zhanga,
Shi-Hao Sunb,
Shu-Sheng Zhanga,
Fang Zhanga,
Hui Zhua and
Yin-Long Guo*a
aState Key Laboratory of Organmetallic Chemistry and National Center for Organic Mass Spectrometry in Shanghai, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai, 200032, China. E-mail: haoyangwang@sioc.ac.cn; ylguo@sioc.ac.cn
bZhengzhou Tobacco Research Institute, China National Tobacco Corporation, 2 Fengyang Road, Zhengzhou 450001, China
First published on 1st December 2015
Abstract
An unexpected gas phase Gutknecht self-condensation of D-glucosamine hydrochloride to 2,5-deoxyfructosazine (2,5-DOF) in atmospheric pressure chemical ionization mass spectrometry (APCI-MS) was described. Mechanistic studies indicated that the thermospray conditions in APCI largely accelerate the irreversible Gutknecht self-cyclocondensation reaction of amino-sugars. Our observations provide a promising clue for a new borate-free synthetic method of 2,5-DOF by mimicking the APCI conditions.
In 1879, H. Gutknecht first reported the method for synthesis of pyrazine by reduction of the α-oximino ketone.1 Since then many variations of Gutknecht pyrazine synthesis were developed, but the basic principle of these methods was the self-cyclocondensation of α-amino carbonyl compound to a dihydropyrazine and ultimately formed the aromatic molecule pyrazine by further oxidation or dehydration.2,3 Just recently, we found an unexpected Gutknecht self-cyclocondensation reaction of D-glucosamine to 2,5-deoxyfructosazine (2,5-DOF) showing the protonated signal at m/z 305 by consequent loss of 3H2O in atmospheric pressure chemical ionization mass spectrometry (APCI-MS) condition, whereas the electrospray mass spectrometry (ESI-MS) analysis only showed the signals of M·H+ at m/z 180 and 2 M·H+ at m/z 359. Then, in order to fully understand the scientific questions behind such interesting APCI-MS experiments of amino-sugars, we need to answer the following three important questions: (1) the specific reaction mechanism of such Gutknecht self-cyclocondensation reaction in APCI conditions and its difference with the typical borate-catalyzed Gutknecht pyrazine synthesis in solution phase; (2) why APCI conditions are suitable for such reaction, but ESI not; (3) the APCI-based Gutknecht self-condensation is only an interesting ambient gas phase reaction or it also provides clues for new synthetic methods of 2,5-DOF.
As a major ionization method bridged the atmosphere condition to vacuum environment of mass spectrometry, APCI shares some common aspects with ESI. However, compared with ESI, APCI has its unique ionization mechanism initiated by ion–molecule reactions at atmospheric pressure. The first step of APCI requires an effective thermal vaporization or desorption to generate the free neutral gas phase molecules, which are further ionized by a corona discharge to corresponding ionic species.4 The most significant character of APCI is generating ions from the neutrals, but ESI prefers to directly release ionic species from reaction solution by desolvation.4,5 The unique characters of APCI make it an ideal method for studying certain reaction mechanism,6,7 as well as monitoring reaction process.8 At the same time some useful APCI-based ambient gas-phase reactions have been studied and reported.9–11 Therefore, in some special cases, the ionic species from APCI have same m/z values with their counterparts from ESI but their ionization positions12 and even detailed structures, as well as their configurations, would be different.
D-glucosamine is a prominent amino-sugar in the biochemical synthesis of glycosylated proteins and lipids. And it is also an important dietary supplement in treatment for osteoarthritis.13 Its three possible configurations might exist at certain equilibrium in solution (Scheme 1). Nowadays, the advantages of IM-MS (ion-mobility mass spectrometry) provide us a valuable chance to reveal the structural information of the shape and size for ionic species14 and attract lots of attentions from chemists.15 Besides elemental compositions obtained with high resolution measurement and fragmentation patterns acquired by CID (collision induced dissociation), the IM-Q-TOF MS (ion-mobility quadrupole time-of-flight mass spectrometry) could provide another dimensional information-drift time for the characterization of the ionic species generated in ambient reactions, even in the trace amount level. In another words, IM-MS could work as a specific “chromatographic method” for fast analysis ionic species based on their size and shape in extremely short time scale (normally less than 60 ms). Therefore, IM-Q-TOF MS would become a valuable tool for exploration of the mechanisms for ion–molecule reactions at ambient condition.14 Just as the concept of “reactions on the fly in ambient mass spectrometry” presented by Cooks group recently,16 we want to achieve “characterization of the reaction products and even intermediates on the fly in ambient mass spectrometry” with the help of IM-MS.
 | ||
| Scheme 1 Three amino-sugars: D-glucosamine (1), D-mannosamine (2), D-galactosamine (3) and the three possible configurations of D-glucosamine: α-, β- and open chain forms. | ||
First we studied the mass spectrometry behaviors of three amino-sugar hydrochloride salts: D-glucosamine (1), D-mannosamine (2), D-galactosamine (3) by ESI-IM-Q-TOF MS. The results of D-glucosamine hydrochloride showed major ions 1·H+ at m/z 180 and its dehydration signal at m/z 162, 1·Na+ at m/z 202, 2(1)·H+ at m/z 359 and 2(1)·Na+ at m/z 381 (Fig. 1a). The similar results were obtained for compounds 2·HCl and 3·HCl (Fig. S1†). However, to our big surprise, APCI-IM-Q-TOF-MS analysis of the D-glucosamine hydrochloride salt (1·HCl) showed no signals of 1·H+ at m/z 180 and 2(1)·H+ at m/z 359 at all, but giving the significantly large amount of ion at m/z 305 (Fig. 1b). The analogous results could also be obtained from the APCI analysis of compounds 2·HCl and 3·HCl (Fig. S1 and Table S1†). The ion at m/z 305 in APCI of 1·HCl was primarily assigned as the protonated 2,5-deoxyfructosazine (2,5-DOF, 4) formed by the Gutknecht self-cyclocondensation reaction (Scheme 2). Compared with D-glucosamine 1, D-mannosamine 2 has the different 2-position-amino group configuration, but their rest sugar chain structures remain the same. They should give the same Gutknecht reaction product in APCI-condition. The authentic 2,5-DOF (4) was synthesized and its APCI-IM-Q-TOF-MS spectrum showed 4·H+ at m/z 305, which was quite similar to APCI mass spectra of 1·HCl and 2·HCl (Fig. 1c and S1†). The detailed MS/MS dissociation patterns of ions at m/z 305 from APCI analysis of 1·HCl and 2·HCl and the authentic 4·H+ at m/z 305 are nearly identical and they also have quite similar drift time at about 21.50 ms (Fig. 2).
 | ||
| Fig. 1 (a) ESI-MS spectrum of D-glucosamine hydrochloride (1·HCl), (b) APCI-MS spectrum of 1·HCl and (c) APCI-MS spectrum of 2,5-DOF (4). | ||
 | ||
| Scheme 2 The Gutknecht self-condensation of amino-sugars (1–3) to 2,5-deoxyfructosazine (4) or its isomer 5. | ||
 | ||
| Fig. 2 APCI-MS/MS spectra and the drift time spectra of ion at m/z 305 from the APCI-analysis of: (a) 1·HCl; (b) 2·HCl; (c) the 2,5-DOF (4). | ||
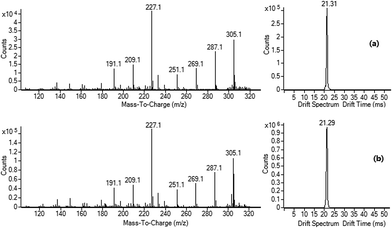
Compared with D-glucosamine 1, D-galactosamine 3 has the different configuration at 4-position-hydroxyl group, therefore it would give a different Gutknecht self-condensation reaction product compound 5 (isomer of 2,5-DOF). Then the experimental results from the same IM-Q-TOF MS conditions show that the ion at m/z 305 from APCI analysis of 3·HCl has slightly difference in the fragment ion distributions of MS/MS spectrum to that of 4·H+ at m/z 305 and also has shorter drift time at 21.31 ms. The authentic compound 5 was synthesized and the IM-Q-TOF MS experiments supported that the Gutknecht self-condensation reaction product of 3·HCl was the compound 5 (Fig. 3, S2 and S3†). Therefore, with the help of IM-Q-TOF MS experiments, the ionic specie at m/z 305 in the APCI analysis of amino-sugars were confirmed to be the protonated Gutknecht self-condensation products, but not the products or complexes formed by random dehydrations. Meanwhile the obvious difference in the detailed MS/MS dissociation patterns of 4·H+ and 5·H+ at m/z 305 also provides extra proof to differentiate them. The ESI-IM-Q-TOF-MS experiments of compounds 4 and 5 showed that the drift time and the MS/MS dissociation patterns of 4·Na+ (20.51 ms) and 5·Na+ (20.31 ms) at m/z 327 were also different (Fig. S3†). The possible fragmentation patterns of 4·H+/5·H+ at m/z 305 and 4·Na+/5·Na+ at m/z 327 were proposed and depicted in Scheme 3 and Scheme S1 and S2,† respectively.
It has been reported that D-glucosamine can be converted to complicated products, involving the deoxyfructosazines as major components, in aqueous condition.17–19 Addition of borate or phenylboronate could effectively prevent the random dehydrations as side reactions, because borate could chelate with the diol moieties of D-glucosamine, giving 2,5-DOF as the major product in base condition.17 From previous studies, the open form of D-glucosamine has the highest affinity for borate and in turn the borate or phenylboronate help the configuration change of amino-sugar to the active open chain structure.20 But the presence of borate or phenylboronate in the reaction solution makes the purification taking time and energy, as well as decreasing the total yields. It is proposed that the similar point of the Gutknecht self-cyclocondensation reaction in APCI and the solution phase reaction in presence of base and borate relied on the liberation and activation of the hydrochloride salts of amino-sugars to the highly reactive free neutral open chain state. According to such hypothesis, the Gutknecht self-cyclocondensation reaction tendency would be retarded or even blocked when the ring-chain tautomerization to the active open chain α-amino aldehyde structure was restrained by acylation of the 2-position-amino group and protection of the 1-position hemiacetal-hydroxyl group. The following experiments confirmed our hypothesis.
The N-acetyl-D-glucosamine (6) also gave rise to the ion at m/z 305 via Gutknecht self-condensation at same APCI-conditions. Such results showed that the acylation of the amino group could not completely stop the ring-chain tautomerization in gas phase of the APCI condition (Fig. 4). The possible formation process of ion at m/z 305 from N-acetyl-D-glucosamine at APCI was depicted in Scheme 4. The drift time comparison and MS/MS experiments of such ion by IM-Q-TOF confirmed it to be the protonated 2,5-DOF (4). The 4-methylumbelliferyl N-acetyl-β-D-glucosaminide (7) is the derivate of D-glucosaminide with acylation of the 2-position-amino group and the protection of 1-position hemiacetal-hydroxyl group (Fig. 4). Thus, its ring-chain tautomerization process was completely blocked by such chemical modifications. Just as our expectation, the APCI-IM-Q-TOF-MS spectrum of 7 showed no signal at m/z 305, but giving the 7·H+ at m/z 380, the dehydration signal at m/z 362 and its fragment ion at m/z 177 (Scheme 4). All the experimental results showed that the most important two driving forces for the interesting Gutknecht self-cyclocondensation reaction of unprotected amino-sugar hydrochloride salts in APCI condition were: (1) expulsion of HCl in thermospray process of APCI conditions; (2) the ring-chain tautomerization to the highly active neutral open chain α-amino aldehyde structure.
 | ||
| Scheme 4 The chemical structures of the N-acylated amino-sugars 6 and 7, and their possible gas-phase transformation reactions at APCI condition. | ||
The amino-sugars in this research were obtained as their hydrochloride salts. The protonation of amino-group in 2-position makes them quite stable in both solid form and solution. At the same time, aldehyde group prefers to react with the 5-position intramolecular OH-group to form hemiacetal. Although the equilibrium of the ring-chain tautomerization of these salts might give rise to their open chain form, the protonation of the α-amino group entirely restrained the first bimolecular imine formation step of Gutknecht pyrazine synthesis. Thus, the release of the neutral α-amino aldehyde open chain structure by expulsion of HCl is the most critical requirement for such Gutknecht self-cyclocondensation reaction of amino-sugars hydrochloride. The unique thermal vaporization step (desolvation temperature at 350 °C) in APCI not only helped the amino-sugar hydrochloride salts to get rid of HCl giving their neutral state but also could activate the neutral state to the highly active α-amino aldehyde open chain structure. On the other hand, the thermalization condition of APCI facilities the dehydration steps in forming the imine and dihydropyrazine intermediates. All these factors in APCI largely accelerate the irreversible Gutknecht self-cyclocondensation reaction of amino-sugars and make the reaction finished in the amazing millisecond timescale in APCI ionization process.
Thus, APCI-IM-MS provides all the suitable conditions for Gutknecht pyrazine synthesis and detection of 2,5-DOF directly and immediately from amino-sugar hydrochloride salts without adding extra catalysts and reagents. ESI ionization of amino-sugar hydrochloride salts more prefer to transfer the protonated amino-sugars directly from solution to gas phase. For this reason, ESI analysis of amino-sugar hydrochloride salts only gave rise to M·H+ and 2 M·H+. Increasing the desolvation temperature of ESI ion source to 350 °C only led to the generation of dehydration fragment ions of [M·H − H2O]+ at m/z 162 and [2 M·H − H2O]+ at m/z 341 of glucosamine but not helped to generate the ionic species of 2,5-DOF [M + H]+ at m/z 305 (Fig. S4†). This study enriched our knowledge of the difference in the ionization mechanism between ESI and APCI. In traditional opinions, APCI was more suitable for less polar organic compounds and its applications for polar carbohydrate compounds were limited by the possible decomposition or fragmentation reactions that happened in APCI. However, the unique capacity of APCI for activation of amino-sugars might have its own alternative potentials for developing new on-line APCI-based derivatization method for analysis of small molecular sugars and amino-sugars. Such idea provides chemists more choices in ionization methods when they study small molecular carbohydrate compounds by mass spectrometry. Meanwhile, the IM-MS method was applied to provide the drift-time as an elegant proof for the occurrence of ambient Gutknecht self-condensation reaction of amino-sugars at APCI and such capacity of IM-MS is also useful to probe the reaction mechanism for some other ambient organic reactions.
In 2012, Cooks group developed the ESI-based synthetic methods and reported that the ambient reaction condition could significantly increase the reaction rate of some condensation reactions.21 Herein, our experimental results showed that APCI conditions could also significantly increase the Gutknecht condensation reaction of amino-sugars even in absence of borate or phenylboronate. We even could collect small amount solid residuals in the APCI ion source (Fig. S5†). The MS (Fig. S6†) and NMR analysis (Fig. 5 and S7†) showed that they contained 2,5-DOF. The typical signals of two H atoms in pyrazine ring at 8.64 and 8.47 ppm and signals of CH2 near pyrazine ring at 2.94–2.88 and 3.13–3.17 ppm (in Fig. 5b) also clearly confirmed the occurrence of the Gutknecht self-cyclocondensation of amino-sugars at APCI-ion source. Therefore, the APCI-based synthesis of 2,5-DOFs directly from amino-sugar hydrochloride salts provides a promising clue for a more clean and green boron-free synthesis strategy. Nowadays, more and more attentions were paid for developing new synthesis methods of the highly-value increasing deoxyfructosazine derivates, because these compounds were widely used as the flavour agent in food and tobacco industry and had the pharmacological and physiological applications for diabetes, cancers and immunological and inflammatory diseases.22 The next step of our researches is to design and build a more practical and efficient thermospray device by mimicking our APCI experiment conditions to realize such borate-free Gutknecht synthesis of 2,5-DOF from D-glucosamine hydrochloride directly.
Acknowledgements
We are grateful for financial supports from the National Natural Science Foundation of China (21532005, 21475145 and 21472228) and Youth Innovation Promotion Association CAS (2013171) and Opening Projects of Key Laboratory of Tobacco Flavor Basic Research of CNTC (DZ2013047). Thanks for the helps of Man-Yu Zhang, Qiang Han and Xiang-Dong Jiang of Agilent Technologies (China) Co., Ltd.Notes and references
- (a) H. Gutknecht, Chem. Ber., 1879, 12, 2290–2292 CrossRef; (b) H. Gutknecht, Chem. Ber., 1880, 13, 1116–1119 CrossRef.
- (a) K. L. Hoy and W. H. Hartung, J. Org. Chem., 1958, 23, 967–971 CrossRef CAS; (b) A. G. Myers, D. W. Kung and B. Zhong, J. Am. Chem. Soc., 2000, 122, 3236–3237 CrossRef CAS.
- J. J. Li, Name reactions in heterocyclic chemistry II, Wiley, Hoboken, 2011, pp. 430–439 Search PubMed.
- J. H. Gross, Mass spectrometry: A textbook, Springer, Heidelberg, 2nd edn, 2011, pp. 604–606 Search PubMed.
- F. J. Andrade, J. T. Shelley, W. C. Wetzel, M. R. Webb, G. Gamez, S. J. Ray and G. M. Hieftje, Anal. Chem., 2008, 80, 2646–2653 CrossRef CAS PubMed.
- E. C. Meurer, L. S. Santos, R. A. Pilli and M. N. Eberlin, Org. Lett., 2003, 5, 1391–1394 CrossRef CAS PubMed.
- S. Fürmeier and J. O. Metzger, J. Am. Chem. Soc., 2004, 126, 14485–14492 CrossRef PubMed.
- (a) J. Auld and D. R. Hastie, Int. J. Mass Spectrom., 2009, 282, 91–98 CrossRef CAS; (b) C. H. Hsieh, C. S. Chao, K. K. T. Mong and Y. C. Chen, J. Mass Spectrom., 2012, 47, 586–590 CrossRef CAS PubMed.
- E. C. Meurer, A. A. Sabino and M. N. Eberlin, Anal. Chem., 2003, 75, 4701–4709 CrossRef CAS PubMed.
- Z. X. Zhao, H. Y. Wang and Y. L. Guo, Curr. Org. Chem., 2011, 15, 3734–3749 CrossRef CAS.
- (a) Y. H. Song, H. Chen and R. G. Cooks, Rapid Commun. Mass Spectrom., 2005, 19, 3493–3499 CrossRef CAS; (b) H. Chen, O. Y. Zheng and R. G. Cooks, Angew. Chem., Int. Ed., 2006, 45, 3656–3660 CrossRef CAS PubMed; (c) H. Chen, L. S. Eberlin, M. Nefliu, R. Augusti and R. G. Cooks, Angew. Chem., Int. Ed., 2008, 47, 3422–3425 CrossRef CAS PubMed.
- Y. F. Chai, N. Hu and Y. J. Pan, J. Am. Soc. Mass Spectrom., 2013, 24, 1097–1101 CrossRef CAS PubMed.
- W. W. Pigman, D. Horton and J. D. Wander, The Carbohydrates, Academic Press, New York, 2nd Edn, 1980, vol 1B, pp. 727–728 Search PubMed.
- C. Lapthorn, F. Pullen and B. Z. Chowdhry, Mass Spectrom. Rev., 2013, 32, 43–71 CrossRef CAS PubMed.
- (a) Á. Révész, D. Schröder, T. A. Rokob, M. Havlík and B. Dolenský, Angew. Chem., Int. Ed., 2011, 50, 2401–2404 CrossRef; (b) D. Schröder, M. Budešínský and J. Roithová, J. Am. Chem. Soc., 2012, 134, 15897–15905 CrossRef PubMed; (c) C. J. Shaffer, D. Schröder, C. Gütz and A. Lützen, Angew. Chem., Int. Ed., 2012, 51, 8097–8100 CrossRef CAS.
- R. D. Espy, M. Wleklinski, X. Yan and R. G. Cooks, TrAC, Trends Anal. Chem., 2014, 57, 135–146 CrossRef CAS.
- J. Rohovec, J. Kotek, J. A. Peters and T. Maschmeyer, Eur. J. Org. Chem., 2001, 3899–3901 CrossRef CAS.
- F. W. Lichtenthaler, Acc. Chem. Res., 2002, 35, 728–737 CrossRef CAS.
- L. Y. Jia, Y. X. Wang, Y. Qiao, Y. Q. Qi and X. L. Hou, RSC Adv., 2014, 4, 44253–44260 RSC.
- (a) K. Sumoto, M. Irie, N. Mibu, S. Miyano, Y. Nakashima, K. Watanabe and T. Yamaguchi, Chem. Pharm. Bull., 1991, 39, 792–794 CrossRef CAS PubMed; (b) A. Zhu, J. B. Huang, A. Clark, R. Romero and H. R. Petty, Carbohydr. Res., 2007, 342, 2745–2749 CrossRef CAS PubMed.
- T. Müller, A. Badu-Tawiah and R. G. Cooks, Angew. Chem., Int. Ed., 2012, 51, 11832–11835 CrossRef PubMed.
- (a) M. Vanduin, J. A. Peters, A. P. G. Kieboom and H. Vanbekkum, Tetrahedron, 1985, 41, 3411–3421 CrossRef CAS; (b) R. Vandenberg, J. A. Peters and H. Vanbekkum, Carbohydr. Res., 1994, 253, 1–12 CrossRef CAS; (c) Q. Li, Y. H. Ye, A. X. Yan, Y. W. Zhou, H. Shen and Q. Y. Xing, Chem. Res. Chin. Univ., 2001, 22, 1824–1828 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental conditions and related data and spectra. See DOI: 10.1039/c5ra22331h |
| This journal is © The Royal Society of Chemistry 2015 |