Analysis of model Pd- and Pt-containing contaminants in aqueous media using ESI-MS and the fragment partitioning approach†
Leonid V. Romashovab,
Gleb D. Rukhovichb and
Valentine P. Ananikov*a
aZelinsky Institute of Organic Chemistry, Russian Academy of Sciences, Leninsky prospect 47, 119991 Moscow, Russia. E-mail: val@ioc.ac.ru
bMoscow State High School No 192, Leninsky prospect 34A, 119334 Moscow, Russia
First published on 7th December 2015
Abstract
Ubiquitous usage of Pd- and Pt-containing nanoparticles in automotive catalytic converters is an important potential threat to the environment. The unavoidable release of transition metal species to the environment and their contact with water give rise to the poisoning of ecosystems by heavy metal compounds. Electrospray ionization mass spectrometry and the newly-developed fragment partitioning approach show that a variety of metal species may be formed upon contact of metal salts with water. A series of monometallic complexes, homonuclear clusters and heteronuclear clusters of palladium and platinum were detected and characterized. The study has revealed a critical danger of metal contamination due to easy formation of transition metal clusters, which may be much more toxic than corresponding monometallic complexes.
1. Introduction
Due to their unique chemical and physical properties, platinum group metals (PGM) have broad applications in various areas of industry, advanced technologies, and research.1,2 The three platinum group metals – Pd, Pt and Rh – are of paramount importance in the automobile industry as components of catalytic converters. It is important to realize the scale of precious metal consumption in the manufacturing of autocatalysts. As a representative example, >70% of the approximately 200 tons of palladium produced annually world-wide are used in the manufacturing of catalytic converters (Fig. 1a). | ||
| Fig. 1 Palladium demand by application (a)3 and a general scheme of automotive catalyst operation (b).4 | ||
The goal of the catalytic converter is to transform toxic exhaust gases CO, CxHy and NOx into harmless CO2, H2O, and N2 (Fig. 1b). Conversion occurs on the surface of nanosized precious metal catalyst (Fig. 1b) and is indeed very efficient for environmental protection.5 However, this fascinating technology has a dangerous drawback. Small amounts of the metals may leave the surface of the catalyst during catalytic converter operation. This process is known as leaching and is readily facilitated by the presence of coordinating molecules that are available in the surrounding gas phase (H2O, CO, NO, CO2 etc.).6 The outstanding growth of the automotive industry has resulted in increased environmental pollution by palladium (together with platinum and rhodium), which is emitted with exhaust gases from catalytic converters. The emitted palladium is deposited along roadways, on soil and plants, and in the water.7 Ecotoxicological measurements have shown that the palladium content in road dust reaches 250–300 μg kg−1.8 The negative effects of platinum group metals on the biosphere cannot be excluded due to their increasing accumulation in the environment, high biological availability of transition metals, and unknown toxicological and ecological influence.9 It has been shown that solid waste released from autocatalysts to the environment retains high chemical reactivity.5,10
Therefore, the investigation of PGM behavior under environmental conditions is an important problem. Electrospray ionization mass spectrometry (ESI-MS) is the method of choice for studying environmental behavior of transition metal complexes, enabling the direct analysis of water contamination. In the present work, we studied model palladium and platinum compounds in aqueous media using the highly sensitive and soft ESI-MS as an analytical technique11–13 to examine the forms in which these metals can be present in surface water. In addition to monometallic species, we also addressed the possibility of the formation of polynuclear transition metal clusters in solutions, which are known to exhibit high chemical reactivity14 and have significant biological influence.15
2. Results and discussions
2.1. ESI-MS data analysis based on fragment partitioning
ESI-MS is a highly sensitive and informative analytical tool with numerous advantages for practical application. However, for transition metal compounds, even a simple analyzed sample can produce rather complicated spectra with hundreds of signals.11 A routine ESI-MS analysis of the model solution of Na2PdCl4 in water carried out in the present work showed that 1536 spectral lines are present. The presence of numerous signals in the spectra is caused by the easy coordination of various ligands and the association of solvent molecules with the metal center, as well as the facile degradation and transformation of the transition metal species. In addition, each individual compound gives rise to multiple spectral lines in the high-resolution ESI-MS spectrum due to isotopic distribution.A conventional mass spectrum analysis for the studied sample is based on the calculation of the brutto formula Cn1Hn2On3Cln4Nn5Pdn6 and variations of coefficients n1–n6. In spite of significant time and effort, it was not possible to carry out a complete analysis of the registered mass spectrum using this standard approach. Several brutto-formula alternatives for each signal complicate the spectra assignment, and it is often difficult to unambiguously connect the brutto-formula with a reliable structure of the complex.
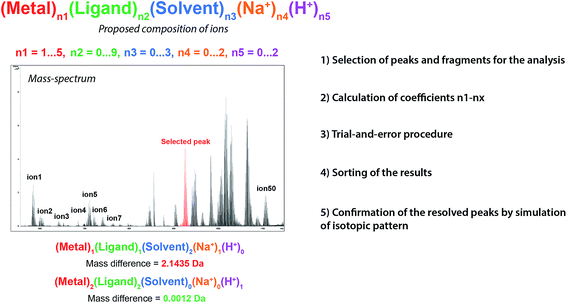
To solve this problem, we utilized another approach based on the partitioning of the molecular structures into a possible set of possible fragments and making variations based on fragment analysis (Fig. 2) rather than per-element analysis. To illustrate the idea, the composition of the detected species was analyzed in the form of (Metal)n1(Solvent)n2(Na+)n3(H+)n4(Ligand_1)n5(Ligand_2)n6…(Ligand_X)nx, which significantly simplifies and speeds up the analysis compared with the fitting of the general brutto-formula Pdn1Cn2Hn3On4Cln5Nn6. Resolving the data in terms of coordinated ligands and solvents directly connects the detected MS signal with the chemical nature of the species. The fragment partitioning approach is not limited to particular structures, and the data analysis can be carried out in a general form of (Metal_1)n1(Metal_2)n2…(Fragment_1)n3(Fragment_2)n4.
The developed algorithm involves the following steps (Fig. 2): (1) the selection of peaks and fragments for the analysis, (2) the calculation of coefficients n1 – nx, (3) the evaluation of possible structural variations in the fragments, (4) the sorting and scoring of the results, and (5) the confirmation of the resolved peaks by simulations of isotopic patterns. A dedicated software program was created to implement the developed algorithm (see ESI†).
2.2. ESI-MS study of an aqueous solution of Na2PdCl4
Sodium tetrachloropalladate(II) was chosen as the model palladium compound for the study. The ESI-MS analysis of its aqueous solution showed the existence of a variety of species in solution, both in positive (Fig. 3) and negative (Fig. 4) ion modes. In spite of the simple structure of the starting metal salt, Na2PdCl4, rather complicated spectra containing 1291 and 245 signals were recorded for the positive and negative ion modes, respectively. A detailed analysis of the ESI-MS spectra using the developed fragment partitioning approach enabled the identification of a variety of metal species in solution (Table 1).| Positive ion mode | Negative ion mode | ||||
|---|---|---|---|---|---|
| Composition | m/z | Rel. int., % | Composition | m/z | Rel. int., % |
| NaPdCl2(CH3CN) | 241.86 | 1.08 | PdCl3 | 214.81 | 0.76 |
| Na2PdCl3 | 258.79 | 1.64 | NaPdCl4 | 270.77 | 0.40 |
| NaPdCl2(CH3CN)2 | 282.88 | 2.96 | Na2PdCl5 | 328.73 | 3.66 |
| Na2PdCl3(CH3CN) | 301.81 | 1.53 | Pd2Cl5 | 390.65 | 100.00 |
| NaPdCl3(CH3CN)2 | 316.75 | 2.26 | NaPd2Cl6 | 448.61 | 70.11 |
| Pd2Cl3(CH3CN)2 | 402.78 | 3.13 | Na2Pd2Cl7 | 508.57 | 10.83 |
| NaPd2Cl4(CH3CN) | 419.70 | 12.08 | Pd3Cl7 | 568.50 | 21.05 |
| NaPd2Cl4(CH3CN)2 | 460.72 | 49.74 | NaPd3Cl8 | 624.45 | 61.78 |
| Na2Pd2Cl5(CH3CN) | 477.66 | 14.62 | Na2Pd3Cl9 | 684.41 | 34.08 |
| Na3Pd2Cl6 | 502.19 | 70.36 | Pd4Cl9 | 742.38 | 7.00 |
| NaPd3Cl6(CH3CN)2 | 633.47 | 3.40 | NaPd4Cl10 | 800.31 | 6.44 |
| Na2Pd3Cl7(CH3CN)2 | 653.50 | 3.22 | Na2Pd4Cl11 | 861.26 | 5.16 |
| Na3Pd3Cl8 | 672.43 | 4.82 | Pd5Cl11 | 918.22 | 8.08 |
| Na4Pd3Cl9 | 732.39 | 3.47 | NaPd5Cl12 | 977.17 | 8.23 |
| Na5Pd3Cl10 | 790.35 | 2.21 | |||
| Na6Pd3Cl11 | 855.74 | 3.43 | |||
In positive ion mode, the ESI-MS spectrum (Fig. 3, Table 1) is dominated by binuclear palladium species of various compositions. Because positive ions of transition metal complexes formed during electrospray ionization tend to be electrophilic and coordinatively unsaturated, several signals corresponding to solvent adducts can be detected in the spectrum. The ESI-MS spectrum in negative ion mode (Fig. 4, Table 1) was more informative because the Na2PdCl4 starting material can easily generate anionic species following the dissociation of the sodium cations. Additionally, compared with the spectrum in positive ion mode, a significantly lower signal overlap was observed. The following trends can be distinguished: signals of sodium-free anions (red colored) show the highest intensity in the case of binuclear species; for monosodium adducts (green colored), signals of dinuclear and trinuclear species are nearly the same intensity; and for disodium adducts, the most intensive signal corresponds to trinuclear clusters.
Thus, it may be proposed that sodium ions stabilize trinuclear chloropalladate anions during electrospray ionization because in the case of larger cluster anions (Pd4, Pd5), there is no significant difference in signal intensity between the sodium-free chloropalladates and the sodium containing adducts; therefore, the stability of polynuclear clusters does not significantly depend on the presence of sodium. Comparisons between the spectra in both ESI-MS ion modes show that binuclear chloropalladates are prevalent in solution because the corresponding signals show the highest intensity in the spectra regardless of ion mode and particle composition.
2.3. ESI-MS study of an aqueous solution of Na2PtCl6
Sodium tetrachloroplatinate(II) was chosen as the model compound for the spectral study of platinum complexes. As in the previous sample, in spite of the simple structure of the metal salt Na2PtCl6, complicated spectra containing numerous signals were detected (Fig. 5 and 6). The nature of the signals in the spectra was elucidated using the approach described above (Table 2).| Positive ion mode | Negative ion mode | ||||
|---|---|---|---|---|---|
| Composition | m/z | Rel. int., % | Composition | m/z | Rel. int., % |
| Na3(OH)PtCl3 | 386.84 | 16.64 | PtCl3 | 300.87 | 1.87 |
| Na3PtCl4 | 404.81 | 14.38 | PtCl4 | 335.84 | 34.11 |
| Na3(OH)PtCl5 | 456.78 | 80.20 | Pt(OH)Cl4 | 352.84 | 1.89 |
| Na3PtCl6 | 476.74 | 37.40 | NaPtCl4 | 358.83 | 21.04 |
| Na4(OH)PtCl6 | 516.74 | 9.43 | PtCl5 | 372.81 | 38.80 |
| Na4PtCl7 | 534.70 | 4.92 | NaPtCl5 | 398.82 | 2.33 |
| Na5Pt2(OH)2Cl10 | 892.57 | 6.76 | NaPt(OH)Cl5 | 410.80 | 27.52 |
| Na5Pt2(OH)Cl11 | 912.54 | 7.65 | NaPtCl6 | 430.76 | 100.00 |
| Na5Pt2Cl12 | 930.50 | 4.91 | Na2Pt(OH)Cl6 | 470.75 | 27.12 |
| Na6Pt2(OH)2Cl11 | 952.53 | 4.20 | Na2PtCl7 | 488.72 | 6.61 |
| Na6Pt2(OH)Cl12 | 970.49 | 4.73 | Na3Pt(OH)Cl7 | 528.71 | 3.60 |
| Na6Pt2Cl13 | 988.46 | 2.57 | Na3PtCl8 | 546.68 | 2.05 |
| Na7Pt2(OH)2Cl12 | 1008.48 | 1.33 | Na4Pt(OH)Cl8 | 586.67 | 0.44 |
| Na7Pt2(OH)Cl13 | 1026.45 | 1.47 | Na5Pt2Cl8 | 788.62 | 1.64 |
| Na7Pt3(OH)3Cl15 | 1328.37 | 1.21 | Na2Pt2Cl11 | 824.55 | 1.99 |
| Na7Pt3(OH)2Cl16 | 1347.33 | 2.24 | Na6Pt2Cl9 | 848.58 | 3.49 |
| Na7Pt3(OH)Cl17 | 1366.29 | 2.80 | Na3Pt2Cl12 | 884.51 | 9.31 |
| Na7Pt3Cl18 | 1382.26 | 1.85 | |||
| Na8Pt3(OH)3Cl16 | 1405.28 | 1.78 | |||
| Na8Pt3(OH)2Cl17 | 1423.25 | 2.05 | |||
| Na8Pt3(OH)Cl18 | 1442.20 | 0.99 | |||
| Na8Pt3Cl19 | 1463.25 | 0.83 | |||
In positive ion mode, mononuclear platinum species dominate in the ESI-MS spectrum (Fig. 5, Table 2). Another significant feature of the spectrum is the presence of a large number of signals corresponding to OH-containing ions; this leads us to suggest that chloroplatinates are more susceptible to hydrolysis and reactions with water than chloropalladates. This fact is in accordance with the reported potentiometric, spectrophotometric and NMR data on the hydrolysis of [PtCl6]2−.16 The intensity of the signals corresponding to species containing 4–6 Pt atoms is very low; thus, their analysis results are unreliable, and the composition data for these ions are not shown in Table 2.
The ESI-MS spectrum of Na2PtCl6 in negative ion mode (Fig. 6, Table 2) also differs significantly from the analogous Na2PdCl4 spectrum. Again, the signals of the mononuclear species dominate the spectra, and the presence of OH-containing ions is clearly observed. In the case of the Na2PtCl6 solution, the signals correspond to Na+-free species (red color; Fig. 6) are lower in intensity than those for the sodium-adducts (green and blue color; Fig. 6). Thus, by comparing the spectra of Na2PtCl6 in both ESI-MS ion modes, we can propose that a solution of chloroplatinate in water gives rise to predominately mononuclear Pt species with appreciable amounts of partially hydrolyzed ions.
2.4. ESI-MS study of the mixture of Na2PdCl4 and Na2PtCl6 in aqueous solution
The release of both metals to environment may initiate the formation of heterometallic species in water solutions. To examine the possibility of the formation of heteronuclear Pd–Pt clusters in the solution containing both transition metals, an aqueous mixture of Na2PdCl4 and Na2PtCl6 was studied using ESI-MS.In positive ion mode (Table 3), the signals of the heteronuclear species showed very low intensity, whereas in negative ion mode, the intensity of the heteronuclear species signals was rather high (Table 3). It is important to note that all detected heteronuclear clusters contained a single Pt atom, whereas the quantity of Pd atoms in the clusters varied from 1 to 3. This finding is in accordance with the observed difference between Pd and Pt in the tendency to form polynuclear species (cf. Fig. 3 and 5). Thus, these experiments have shown that polynuclear heterometallic clusters can be easily formed if both metal ions are presented in solution.
| Positive ion mode | Negative ion mode | ||||
|---|---|---|---|---|---|
| Composition | m/z | Rel. int., % | Composition | m/z | Rel. int., % |
| Na3PdPtCl8 | 654.59 | 1.34 | NaPdPtCl8 | 606.59 | 16.16 |
| Na4PdPtCl9 | 712.54 | 1.86 | Na2PdPtCl9 | 666.55 | 26.25 |
| Na5PdPtCl10 | 768.50 | 0.86 | NaPd2PtCl10 | 784.43 | 1.28 |
| Na6PdPtCl11 | 828.45 | 0.34 | Na2Pd2PtCl11 | 844.38 | 3.28 |
| Na7PdPtCl12 | 886.40 | 0.30 | Na3Pd2PtCl12 | 902.34 | 6.07 |
| Na5Pd2PtCl12 | 946.33 | 0.66 | NaPd3PtCl12 | 962.29 | 0.39 |
| Na6Pd2PtCl13 | 1006.30 | 0.24 | |||
As a representative example, comparisons of the simulated isotope pattern distribution and the experimentally measured spectrum for the heterometallic cluster Na2PdPtCl9− are shown in Fig. 7. Excellent agreement between the measured and calculated spectra confirms the identified composition of the ions. Verification of the peak assignment was carried out for all resolved signals in the present study (see ESI†). It should be noted that the assessment of the signal fine structure is a well-established and reliable tool in mass-spectrometric analysis.
Overall, ESI-MS is a very efficient analytic tool for studying solutions of transition metal complexes. Nevertheless, it is important to mention some known drawbacks. The outcome of the analysis may be concentration dependent12 and may be very sensitive to the presence of organic contaminants.17 The impact of the initial presence of the ions in solution and the possibility of ion generation during the electrospray process are also important considerations.12–14,18
The mechanism of formation of polynuclear clusters in solution is of much importance to understand reactivity of metal particles and to reveal possible pathways under catalytic conditions. It was proposed that leaching of metal species and oriented attachment of metal species are the key processes for tuning the performance of catalytic reactions and they have a large impact on metal contamination.6,19 More detailed studies on this fascinating topic will be a subject of out future research work.
3. Conclusions
Although studied palladium and platinum salts have simple composition, the corresponding solutions possess rather complicated ESI-MS spectra. Detailed spectral analysis is an important task for studying potential risk of environmental pollution, as well as for the fields of catalysis and organometallic chemistry. These Pd and Pt salts are ubiquitously used as catalyst precursors and staring materials for preparation of more complex compounds. Signal assignment in the spectra of starting materials is required for mechanistic studies and reaction monitoring.The ESI-MS study of aqueous solutions of Na2PdCl4 and Na2PtCl6 has revealed that the platinum salt predominantly consists of mononuclear species in solution, whereas binuclear species predominate in palladium salt solutions. An analysis of the aqueous mixture of two salts has shown the possibility of the formation of heteronuclear chlorinated clusters with the general formula of the metal core PtPdn (n = 1…3), which can be detected in positive and negative ESI-MS ion modes. These findings are highly important from an ecotoxicological point of view because the chemical properties and biological activity of monometallic complexes, homonuclear clusters and heteronuclear clusters may differ substantially. The implementation of the fragment partitioning approach and development of corresponding software enabled rapid analysis of complicated ESI-MS spectra of metal-containing compounds. The present study has revealed a potential danger of metal contamination due to easy formation of transition metal clusters, which can be much more toxic, as compared to the corresponding monometallic complexes.
The overall amount of metals can be determined by known methods (ICP-MS, spectroscopy, etc.), while revealing the structure and understanding reactivity of metal contaminants is a challenge. As far as environmental issues are concerned, it should be noted that the power of ESI-MS is to reveal the nature of metal complexes and to study transformations of transition metal complexes in solution. We anticipate the rapid development of the ecotoxicological analysis of Pd- and Pt-contamination of the environment, and high performance analytic methods will provide valuable insights into the molecular mechanisms of these processes.
4. Experimental part
4.1. Preparation of the solutions
A total of 0.1 mmol of a corresponding salt (Na2PdCl4 or Na2PtCl6·6H2O) was dissolved in 10 mL of HPLC-grade water (Sigma) and stirred overnight at room temperature. In the mixed metal cluster formation experiment, equal volumes of two solutions were stirred overnight at room temperature. Prior to the ESI-MS measurements, each sample was diluted 100-fold by HPLC-grade acetonitrile (Merck).4.2. ESI-MS measurements
High resolution mass spectra were recorded using a Bruker maXis instrument (Bruker Daltonik GmbH, Bremen, Germany) equipped with an electrospray ionization (ESI) ion source. The measurements were performed in positive (+MS) and negative (−MS) ion modes (HV capillary: 4500 V (for positive) and 3000 V (for negative); HV end plate offset: −500 V) with the following scan range in m/z: 50–3000. External calibration of the mass spectrometer was performed with Electrospray Calibrant Solution (Fluka). Direct syringe injection was used for the analyzed solutions in MeCN (flow rate: 3 μL min−1). Nitrogen was used as the nebulizer gas (0.4 bar) and dry gas (4.0 L min−1); the drying temperature was set at 180 °C. The recorded spectra were processed using the Bruker DataAnalysis 4.0 software package.4.3. Software development
The program used to perform the fragment partitioning approach was developed in Qt Creator 5.5 using C++ language. The target mass was decomposed as a linear combination of fragment exact masses with natural coefficients. The maximum coefficient of the fragment was limited by the integer part of the quotient obtained by dividing the target mass by the mass of the fragment. Because the coefficients are natural and bounded from above, it was possible to check all variants using restriction criteria (target mass and tolerance).Acknowledgements
ESI-MS and chemical studies were supported by Russian Science Foundation (RSF Grant 14-13-01030). Development of mathematical procedures was partially supported RFBR (Grant 14-03-31465). The authors gratefully acknowledge Dr Julia V. Burykina for the assistance and helpful discussions.References
- (a) H. Renner, G. Schlamp, I. Kleinwächter, E. Drost, H. M. Lüschow, P. Tews, P. Panster, M. Diehl, J. Lang, T. Kreuzer, A. Knödler, K. A. Starz, K. Dermann, J. Rothaut, R. Drieselmann, C. Peter and R. Schiele, in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2000 Search PubMed; (b) L. B. Hunt and F. M. Lever, Platinum Met. Rev., 1969, 13, 126 CAS; (c) Chemistry of the Platinum Group Metals. Recent Developments, ed. F. R. Hartley, Elsevier Science, 1991 Search PubMed; (d) Precious Metals for Biomedical Applications, ed. N. Baltzer and T. Copponnex, Woodhead Publishing, 2014 Search PubMed.
- Catalytic applications: (a) A. R. Muci and S. L. Buchwald, in Practical palladium catalysts for C–N and C–O bond formation, ed. N. Miyaura, Springer, Berlin Heidelberg, 2002, pp. 131–209 Search PubMed; (b) J. Hartwig, Organotransition Metal Chemistry: from Bonding to Catalysis, University Science Books, Sausalito, 2009 Search PubMed; (c) Metal-Catalyzed Cross-Coupling Reactions and More, ed. A. de Meijere, S. Bräse and M. Oestreich, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2013 Search PubMed; (d) Olefin Metathesis: Theory and Practice, ed. K. Grela, John Wiley & Sons Inc., Hoboken, New Jersey, 2014 Search PubMed; (e) Homogeneous Catalysis for Unreactive Bond Activation, ed. Z.-J. Shi, John Wiley & Sons Inc., Hoboken, New Jersey, 2015 Search PubMed; (f) J. M. Thomas and W. J. Thomas, Principles and Practice of Heterogeneous Catalysis, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2015 Search PubMed; (g) Hydrofunctionalization, ed. V. P. Ananikov and M. Tanaka, Springer, Berlin, 2013 Search PubMed.
- A. Cowley, PLATINUM 2013 Interim Review, Johnson Matthey PLC, Royston, 2013 Search PubMed.
- Adapted from http://www.preciousmetals.umicore.com/recyclables/SAC/catalyticconverter/.
- (a) J. Kašpar, P. Fornasiero and N. Hickey, Catal. Today, 2003, 77, 419 CrossRef; (b) P. J. Millington and A. P. E. York, Platinum Met. Rev., 2012, 56, 58 CrossRef; (c) C. Morgan, Johnson Matthey Technol. Rev., 2014, 58, 217 CrossRef.
- A. S. Kashin and V. P. Ananikov, J. Org. Chem., 2013, 78, 11117 CrossRef CAS PubMed.
- (a) G. C. Lough, J. J. Schauer, J.-S. Park, M. M. Shafer, J. T. DeMinter and J. P. Weinstein, Environ. Sci. Technol., 2005, 39, 826 CrossRef CAS PubMed; (b) Palladium Emissions in the Environment: Analytical Methods, Environmental Assessment and Health Effects, ed. F. Zereini and F. Alt, Springer, 2006 Search PubMed.
- K. Boch, M. Schuster, G. Risse and M. Schwarzer, Anal. Chim. Acta, 2002, 459, 257 CrossRef CAS.
- (a) K. Ravindra, L. Bencs and R. Van Grieken, Sci. Total Environ., 2004, 318, 1 CrossRef CAS; (b) Immunotoxicology of Environmental and Occupational Metals, ed. J. T. Zelicoff and P. Thomas, CRC Press, 1998 Search PubMed.
- E. Mieczynska, A. Gniewek and A. M. Trzeciak, Appl. Catal., A, 2012, 421–422, 148 CrossRef CAS.
- (a) R. B. Cole, Electrospray Ionization Mass Spectrometry; Fundamentals, Instrumentation, and Applications, John Wiley, New York, 1997 Search PubMed; (b) J. H. Gross, Mass Spectrometry, Springer, Berlin, 2004 Search PubMed.
- (a) L. P. E. Yunker, R. L. Stoddard and J. S. McIndoe, J. Mass Spectrom., 2014, 49, 1 CrossRef CAS PubMed; (b) K. L. Vikse, Z. Ahmadi and J. S. McIndoe, Coord. Chem. Rev., 2014, 279, 96 CrossRef CAS.
- D. Schröder, Acc. Chem. Res., 2012, 45(9), 1521 CrossRef PubMed.
- L. V. Romashov, L. L. Khemchyan, E. G. Gordeev, I. O. Koshevoy, S. P. Tunik and V. P. Ananikov, Organometallics, 2014, 33, 6003 CrossRef CAS.
- M. Tsoli, H. Kuhn, W. Brandau, H. Esche and G. Schmid, Small, 2005, 8–9, 841 CrossRef PubMed.
- (a) C. M. Davidson and R. F. Jameson, Trans. Faraday Soc., 1965, 61, 2462 RSC; (b) B. Shelimov, J. F. Lambert, M. Che and B. Didillon, J. Am. Chem. Soc., 1999, 121, 545 CrossRef CAS.
- D. B. Eremin and V. P. Ananikov, Organometallics, 2014, 33, 6352 CrossRef CAS.
- V. B. Di Marco and G. G. Bombi, Mass Spectrom. Rev., 2006, 25, 347 CrossRef CAS PubMed.
- (a) R. H. Crabtree, Chem. Rev., 2012, 112, 1536 CrossRef CAS PubMed; (b) N. Y. S. Phan, M. van Der Sluys and C. W. Jones, Adv. Synth. Catal., 2006, 348, 609 CrossRef CAS; (c) C. Deraedt and D. Astruc, Acc. Chem. Res., 2014, 47, 494 CrossRef CAS PubMed; (d) A. Trzeciak and J. J. Ziolkowski, Coord. Chem. Rev., 2007, 251, 1281–1293 CrossRef CAS; (e) E. O. Pentsak, A. S. Kashin, M. V. Polynski, K. O. Kvashnina, P. Glatzel and V. P. Ananikov, Chem. Sci., 2015, 6, 3302 RSC; (f) V. P. Ananikov, ACS Catal., 2015, 5, 1964 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Description of the developed program and ESI-MS spectra are available in the electronic supplementary information. See DOI: 10.1039/c5ra22257e |
| This journal is © The Royal Society of Chemistry 2015 |