Nitrogen and sulfur codoped graphene quantum dots as a new fluorescent probe for Au3+ ions in aqueous media†
Tingting Yang,
Fei Cai,
Xiaodan Zhang and
Yuming Huang*
The Key Laboratory of Eco-environments in Three Gorges Reservoir Region, Ministry of Education, College of Chemistry and Chemical Engineering, Southwest University, Chongqing 400715, China. E-mail: ymhuang@swu.edu.cn; Fax: +86-23-68254843; Tel: +86-23-68254843
First published on 7th December 2015
Abstract
Nitrogen and sulfur codoped graphene quantum dots (N,S-GQDs) were facilely fabricated by a one-step hydrothermal treatment of citric acid as the carbon source in the presence of cysteine as the doping agent. The resulting N,S-GQDs were characterized by TEM, AFM, Raman, FT-IR, UV-vis, XPS and fluorescence spectroscopy. The N,S-GQDs display a luminescence quantum yield of 35.4%, which was about 14 times greater than that of the undoped graphene quantum dots (GQDs). The AFM image showed a typical topographic height of 0.5 to 1.5 nm with 1–4 graphene layers. The fluorescent emission spectra of N,S-GQDs display an excitation-dependent behavior, and the emission peaks shift from 413 to 440 nm on increasing the excitation wavelength from 310 to 380 nm. The N,S-GQDs possess a storage stability of at least 2 months and are stable in the presence of high concentrations of salt. Due to the strong specific reactivity between Au3+ and amine groups on the N,S-GQDs, the addition of Au3+ ions to the N,S-GQDs suspension results in the formation of gold nanoparticles, which strongly quench the fluorescence of N,S-GQDs. While, other common cations and anions result in negligible changes in the fluorescence of N,S-GQDs. On this basis, a sensitive fluorometric method for the detection of Au3+ was developed that has a 50 nM detection limit and a linear range that extends from 0.1 μM to 50 μM. The method has been successfully used for the determination of Au3+ in real aqueous samples and for gold content in auranofin.
Introduction
As one of the most important noble metals, gold is widely used in different fields due to its excellent physical and chemical properties. For example, gold-based catalysts have received great attention due to their superior catalytic performance.1–3 Though Au(0) is stable and biocompatible, its salt displays high reactivity and shows some biological effects.4,5 For instance, gold salt-based compounds have been used as drugs to treat various diseases, including tuberculosis, asthma, malaria, cancer, HIV, and arthritis.6–8 Au3+ was reported to cause damage to the liver, kidneys, and the peripheral nervous system due to its strong affinity to DNA and several enzymes.9–11 Owing to the therapeutic significance and biological effect of Au3+, it is desirable to develop a sensitive and selective method for its detection. Typically, atomic absorption spectrophotometry12 and inductively coupled plasma-mass spectroscopy (ICP-MS)13 were used for the sensitive and selective analysis of Au3+. However, these technologies are usually in need of complex sample preparation steps and expensive instrument. The fluorescence (FL) methods as technically simple yet effective techniques have received considerable attention due to their high sensitivity, selectivity, fast response and simplicity. A number of fluorescent organic probes have been developed for the detection of Au3+.5,14–22 However, the synthesis of these organic probes are usually complicated.5 In addition, organic solvents or aqueous-organic media are needed for sensing of gold ions (Table 1).15–22| Fluorescent probes | Reaction media | Detection limit (μM) | Linear range (μM) | Ref. |
|---|---|---|---|---|
| a NM: not mentioned. | ||||
| Rhodamine-alkyne derivative | HEPES buffer (50% ethanol) | 0.5 | NMa | 15 |
| An aryl alkyne compound | Ethanol | 0.32 | 0.1–0.5 | 16 |
| Acylsemicarbazides | PBS buffer (0.3% DMF) | 0.29 | 0.5–70 | 17 |
| A boron dipyrromethene derivative | PBS buffer (50% ethanol) | 0.32 | 0.1–0.6 | 18 |
| Coumarin-alkyne derivative | HEPES buffer–DMF (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, v/v) 6, v/v) |
0.44 | 0.5–3 | 19 |
| 4-Propargylamino-1,8-naphthalimide | PBS buffer (4% MeOH) | 8.44 | 0–60 | 20 |
| Thioamide-phenyl-substituted alkynes | PBS buffer–ethanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) 7, v/v) |
0.39 | 0.5–15 | 21 |
| Ferrocenyl derivative of 1,8-naphthalimide | PBS buffer–CH3CN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) 1, v/v) |
0.48 | 0.5–3 | 22 |
| CTPA linked Si NPs | Tris–HCl buffer (30% ethanol) | 0.023 | 0.5–100 | 44 |
| Rhodamine-polyacrylic acid-FeNPs | TBAPF6 in DMSO | 0.85 | 0.7–1.7 | 45 |
| Carbon dot cluster | An aqueous medium | 0.48 | 0–75 | 32 |
| Graphene oxide-poly(vinyl alcohol) hybrid | An aqueous medium | 0.7 | 0.7–300 | 46 |
| N,S-GQDs | An aqueous medium | 0.05 | 0.1–50 | This work |
Owing to their low toxicity, excellent water solubility and photoluminescence (PL) properties, graphene quantum dots (GQDs) have attracted extensive attention in recent years. These excellent properties make them great potential applications in bioimaging, drug delivery, DNA cleavage system, sensor development, and catalysis etc.23,24 However, GQDs also have some shorting comings, for instance, the quantum yield (QY) of the reported GQDs is still usually low and much lower than that of semiconductor QDs.24 Therefore, the facile synthesis of GQDs with high QY via simple routes is still a challenge work for their wide applications. The heteroatom-doping, in particular the nitrogen (N) or/and sulfur (S) (co)doped into GQDs, are an efficient way to elevate their QY. Different methods including hydrothermal method,25,26 microwave-assisted solvothermal method27 and microwave-assisted solid-phase synthesis method28 have been developed for the preparation of the nitrogen and sulfur co-doped graphene quantum dots (N,S-GQDs).
Herein, we employed N,S-GQDs as new fluorescent probes for the detection of Au3+. With a modification of the method by Dong et al., the N,S-GQDs were facially prepared through hydrothermal treatment of citric acid as the carbon source in the presence of cysteine as a doping agent.26 The resulting N,S-GQDs are highly blue-luminescent with a QY of 35.4%. It was found that Au3+ caused efficiently quenching of the blue emission of N,S-GQDs. Au3+ was reduced in the presence of N,S-GQDs, leading to the formation of Au nanoparticles (NPs), which quench the fluorescence of N,S-GQDs. The quenching mechanism was discussed. This new fluorescent probe can be employed for the sensitive and selective detection of Au3+. The N,S-GQDs probe was successfully applied for the detection of Au3+ level in aqueous samples. To our knowledge, although GQDs have been widely used to detect various metal ions24,29 and N,S-GQDs have been reported for cellular imaging,28 no attention has been paid to use of N,S-GQDs as a fluorescent probe for label-free detection of Au3+.
Experimental section
Chemicals and reagents
HAuCl4 and citric acid (CA) were obtained from Signopharm Chemical Reagent Co. Ltd. (Shanghai, China). NaOH, HCl, H2SO4, H3PO4 and cysteine were purchased from Chongqing Taixin Chemical Co. Ltd. (Chongqing, China). Mercaptosuccinic acid (MSA) was purchased from Aladdin Chem. Co. Ltd (Shanghai, China). Auranofin was purchased from Chemsynlab Pharmaceutical Sci. Technol. Co. Ltd (Beijing, China). All chemicals used in this work were of analytical grade and used as received without further purification. Ultra pure water with a resistivity of 18.2 MΩ cm was used in all experiments. All glasswares were soaked in the diluted HNO3 and thoroughly cleaned before use.Synthesis of N,S-GQDs
The method for N,S-GQDs preparation was from Dong et al.,26 with some modifications. In detail, for the preparation of N,S-GQDs, 2 g citric acid and 0.2 g cysteine were mixed with 5 mL pure water, then the mixture was transferred to a 25 mL Teflon-lined stainless steel autoclave and heated at 200 °C for 8 h. After cooling down to room temperature, the resulting suspension was centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min to remove the large nanoparticles, and the supernatant was dialyzed in a 3500 Da dialysis bag against water for 2 days to remove un-reacted small molecules. Finally the resulting suspension was diluted to 100 mL with pure water for further use. The N,S-GQDs yield from citric acid is approximately 75%, corresponding to 15.0 mg mL−1 N,S-GQDs.
000 rpm for 5 min to remove the large nanoparticles, and the supernatant was dialyzed in a 3500 Da dialysis bag against water for 2 days to remove un-reacted small molecules. Finally the resulting suspension was diluted to 100 mL with pure water for further use. The N,S-GQDs yield from citric acid is approximately 75%, corresponding to 15.0 mg mL−1 N,S-GQDs.
Instrumentation
The fluorescence spectrum and intensity were recorded on a Hitachi F-7000 spectrofluorometer (Kyoto, Japan) at room temperature. The UV-visible spectra were measured on a U-4100 spectrophotometer (Hitachi, Japan). Transmission electron microscopy (TEM) image was obtained on a Tecnai G2 F20 (FEI, USA) electron microscope at 200 kV. Raman spectrum was measured on a Renishaw Raman microscope (Renishaw inVia Raman microscope, UK). Atomic force microscopy (AFM) images were collected on a Bruker Multimode 8 AFM/SPM system (Bruker, Germany). X-ray photoelectron spectroscopy (XPS) characterizations were conducted by using a VG Multilab 2000X instrument (Thermal Electron, USA). Infrared spectrum was recorded with a Tenson 27 Fourier Transform Infrared spectrometer (Bruker, Germany).Fluorescent detection of Au3+
For the detection of Au3+, 400 μL of 1.5 mg mL−1 N,S-GQDs suspension and 500 μL of 0.05 M phosphate buffer (pH 5.0) were added into Au3+ with different concentrations ranging from 0 to 200 μM. After the mixture was diluted to 10 mL with pure water and incubated at 45 °C for 15 min, FL spectra measurements and photographs were carried out. The relative fluorescence intensity (F0 − F)/F0 versus Au3+ concentration was used for calibration. Here, F0 and F are the fluorescence intensities of the N,S-GQDs in the absence and presence of Au3+, respectively. Each experiment was repeated three times.Fluorescent detection of gold content in auranofin
Fluorescent detection of gold content in auranofin was performed through the following steps: (1) 10 mg auranofin was dissolved with 50 mL of ethanol; (2) 5 mL of the resulting solution was transferred to a glass beaker and heated to near dry; followed by addition of 4 mL of aqua regia (1 mL of concentrated nitric acid and 3 mL of concentrated HCl) to digest the sample by heating until a near dry digest was obtained; (3) the obtained aqua regia digest was treated again by 4 mL of aqua regia until a near dry digest was obtained; (4) the resultant aqua regia digest was diluted to 50 mL with water, then 1 mL of the obtained solution was used for the analysis of gold following the procedures in previous section.Results and discussion
Characterizations of the N,S-GQDs
Fig. 1A and S1† show the TEM image and corresponding size distribution histogram of the synthesized N,S-GQDs, respectively. As can be seen, the prepared N,S-GQDs were well dispersed with a narrow size distribution of 1.0–3.5 nm with an average diameter of 2.1 ± 0.4 nm based on the statistic result of 69 particles (Fig. S1†). There is a crystallinity region with a lattice spacing of 0.21 nm (Fig. 1A, inset), corresponding to the (1120) lattice fringes of graphene.30 The AFM image (Fig. 1B) showed a typical topographic height of 0.5 to 1.5 nm (inset of Fig. 1B), indicating that most of the N,S-GQDs consist of 1–4 graphene layers, similar to that of the previously reported GQDs with 1–5 graphene layers.24,31The Raman spectra of the N,S-GQDs display two peaks located at ca. 1340 and 1530 cm−1 (Fig. S2†), which are ascribed to the characteristic D and G bands of graphitic structure, respectively. It was found that the N,S-GQDs have an ID/IG ratio of 0.87, indicating their relatively high quality.24,25,32 Fig. S3† shows the FT-IR spectra of the N,S-GQDs. The broad peak located at 3461 cm−1 can be assigned to O–H or N–H stretching band. This suggests that there were lots of amine and hydroxyl groups on the surface of the N,S-GQDs. The absorption peak at 1640 cm−1 corresponds to stretching vibration of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O band. However, compared with GQDs, significant absorption peaks at about 1050 to 1100 cm−1 (corresponding to the characteristic C–N vibration mode),33 2100 cm−1 (corresponding to C
O band. However, compared with GQDs, significant absorption peaks at about 1050 to 1100 cm−1 (corresponding to the characteristic C–N vibration mode),33 2100 cm−1 (corresponding to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N stretching vibration mode),34 and 1399 cm−1 (corresponding to C–N stretching mode)34 appeared in the spectra of N,S-GQDs, suggesting successful synthesis of the cysteine functionalized GQDs.
N stretching vibration mode),34 and 1399 cm−1 (corresponding to C–N stretching mode)34 appeared in the spectra of N,S-GQDs, suggesting successful synthesis of the cysteine functionalized GQDs.
XPS survey spectra indicates a predominant graphitic C 1s peak at ca. 285 eV, an O 1s peak at ca. 533 eV, N 1s peak at ca. 400.1 eV, and a S 2p peak at ca. 163.1 eV. It is noted that a pronounced N 1s peak and a S 2p peak were observed for the resultant N,S-GQDs (Fig. 1C), demonstrating the successful incorporation of nitrogen and sulfur atoms into the GQDs through cysteine functionalization in the pyrolysis of citric acid. The high-resolution N 1s spectrum of the N,S-GQDs shows two peaks at 400.2 and 401.3 eV, suggesting the presence of the pyrrole-like N (C–N–C) and graphitic N or N–H bands25 in the N,S-GQDs (Fig. 1D). The high resolution S 2p spectrum of the N,S-GQDs shows two peaks at 164.2 eV and 163.1 eV (Fig. S4†), suggesting the presence of C–S–C units.26 The high-resolution C 1s spectrum of N,S-GQDs (Fig. S4†) shows four peaks at 284.7, 285.1, 285.5 and 289 eV, which were attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/C–C in graphene, C–N(O, S), C
C/C–C in graphene, C–N(O, S), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and O–C
O and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups,26,34 respectively. The O 1s spectrum of N-GQDs displays three peaks at 531.9 eV, 532.7 eV and 533.5 eV, which were attributed to C
O groups,26,34 respectively. The O 1s spectrum of N-GQDs displays three peaks at 531.9 eV, 532.7 eV and 533.5 eV, which were attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or O–C
O or O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and N–C
O and N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively (Fig. S4†). The results of FTIR and XPS demonstrate that the N,S-GQDs were rich in various functional groups, such as amino, hydroxyl, carbonyl and carboxylic acid groups on their surfaces.
O, respectively (Fig. S4†). The results of FTIR and XPS demonstrate that the N,S-GQDs were rich in various functional groups, such as amino, hydroxyl, carbonyl and carboxylic acid groups on their surfaces.
UV-vis absorption and fluorescence property of the N,S-GQDs were studied. As shown in Fig. 2A, UV-vis absorption spectrum of the N,S-GQDs shows a typical absorption peak at ca. 340 nm, which was almost the same as that of the maximum excitation peak of the N,S-GQDs. The N,S-GQDs solution emitted strong blue FL under excitation at 365 nm by a UV lamp (inset of Fig. 2A). However, it was light yellow, transparent and clear under daylight (inset of Fig. 2A). As shown in Fig. 2B, the N,S-GQDs exhibited an excitation-dependent FL behavior. As the excitation wavelength varied from 310 nm to 380 nm, the associated FL peak shifted from 413 nm to 440 nm. This excitation-dependent behavior was similar to the previously reported GQDs.35 The N,S-GQDs exhibit a maximum emission wavelength at ca. 425 nm at the excitation wavelength of 350 nm. The QY of N,S-GQDs was determined to be 35.4% when quinine sulfate was used as the reference, which increased by about 13-fold compared with the undoped GQDs (Table S1†). This suggests that N and S codoping through cysteine significantly enhance the QY of GQDs. By changing the content of cysteine during pyrolysis of CA for the synthesis of the N,S-GQDs, the effect of cysteine content on the PL emission of the N,S-GQDs was investigated. Fig. S5A† shows the effect of the cysteine content on FL response of the N,S-GQDs. The N,S-GQDs with different cysteine contents were synthesized by simply varying the amount of cysteine (0, 0.1, 0.15, 0.2, 0.3 g) while kept the amount of CA unchanged (2.0 g in the present work). As can be seen, very weak FL was found when only CA was used (Fig. S5A†). However, the FL intensity of the N,S-GQDs increases when the cysteine content increases from 0.1 to 0.2 g, above which it decreases. The result demonstrated the key role of cysteine in improving the fluorescent QY of the GQDs by successful incorporation of N and S atoms into the GQDs through cysteine functionalization in the pyrolysis of citric acid. Also, the effect of reaction time between cysteine and CA was investigated ranging from 4 h to 12 h (Fig. S5B†). When the reaction time was 8 h, the maximum FL response of the N,S-GQDs was obtained. Further increase in reaction time caused decrease in the FL response. Finally, 8 h was chosen as the reaction time for the preparation of the N,S-GQDs in the present work.
The stability of the as-synthesized N,S-GQDs plays an important role for their practical applications. Fig. S6† shows the FL response of the N,S-GQDs suspensions during two months storage at room temperature. No obvious change in fluorescence intensity of the N,S-GQDs could be found, indicating good storage stability of the as-prepared N,S-GQDs. The N,S-GQDs exhibited good stability after continuous irradiation of a Xe lamp for 60 min, demonstrating the good photostability of the as-prepared N,S-GQDs (Fig. S7†). Importantly, the FL intensity shows almost no change even though they were dispersed in an aqueous solution with an ionic strength of 1.0 M NaCl (Fig. S8†), indicating that the N,S-GQDs could resist high ionic strength and not aggregate in high salt medium.
Possible quenching mechanism
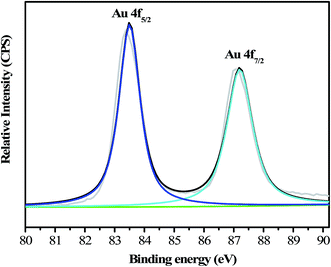
As indicated previously and shown in Fig. 2A, the N,S-GQDs dispersion was light yellow in color and exhibited a maximum emission wavelength at ca. 425 nm (blue curve) at the excitation wavelength of 350 nm. The addition of Au3+ to this dispersion leads to a significant fluorescence quenching of the blue emission from the N,S-GQDs dispersion (green curve). The possible quenching mechanism is interpreted in terms of the structural characteristics of the N,S-GQDs. The surface properties of the N,S-GQDs revealed that there were plenty of amine, hydroxyl and carboxylic groups on their surface, suggesting the possible electrostatic interaction between N,S-GQDs and Au3+, which was confirmed by the result of the measurement of zeta potential. The N,S-GQDs had a zeta potential (ζ) of −24.6 ± 0.4 mV, showing that the N,S-GQDs are negatively surface charged. Also, electron transfer occurred between N,S-GQDs and Au3+,36 which was confirmed by the result of XPS. After reaction with Au3+, the peak intensity at 401.3 eV was reduced with a small shift to 401.6 eV in the N 1s spectrum of the N,S-GQDs (Fig. 1D), suggesting that the electron cloud density of the N atom decreased and resulted in the higher binding energy peak observed. This means the electron transfer from nitrogen to Au3+ occurred. Hence, both electrostatic interaction and electron transfer could lead to the decrease of the N,S-GQDs emission, but the latter effect may play a leading role. In this work, when an aqueous solution of 50 μM HAuCl4 was blended with 150 mg L−1 N,S-GQDs suspensions at room temperature for 80 min, giving the final product as a purple red solution (inset of Fig. S9†). The absorption spectrum of resulting solution displays the characteristic surface plasmon resonance (SPR) peak of Au NPs,37 suggesting the formation of Au NPs. This was confirmed by the results of TEM and XPS. As can be seen from Fig. S1B,† after reaction of Au3+ with N,S-GQDs, the average size of the N,S-GQDs increased from 2.1 ± 0.4 nm to 4.3 ± 1.0 nm (Fig. S1C and D†). The high-resolution XPS spectrum shows two intense peaks located at 83.5 eV and 87.2 eV (Fig. 3), which are assigned to 4f7/2 and 4f5/2 features of Au(0), respectively.38 The formation of Au NPs is probably attributed to the lone electron pair of the amine group in cysteine, which can reduce Au3+ ions to Au NPs or Au cluster without the need of additional reducing agent.39,40 However, the reaction rate is relatively slow. As a control experiment, 50 μM HAuCl4 solution was reduced in the presence of 60 mg L−1 N,S-GQDs suspensions under different temperature. It is obviously that reaction time for getting a purple red solution decreased sharply with increase in reaction temperature (Fig. S10†). When reaction temperature was increased to 45 °C, only 15 min was needed for complete reduction of Au3+. Because Au NPs can quench the fluorescence of GQDs,41–43 the observed FL quenching of N,S-GQDs in the presence of Au3+ was attributed to the formation of Au NPs on the surface of N,S-GQDs.Optimization of experimental conditions
To obtain the optimal analytical conditions for the N,S-GQDs–Au3+ system, several factors affecting the fluorescence response were optimized, such as concentration of N,S-GQDs, solution pH and reaction temperature. The effect of the N,S-GQDs concentration was investigated in the range of 15–210 mg L−1 (Fig. S11†). It was found that the FL emission of the N,S-GQDs increased with increase in their concentration. However, the quenching efficiency of N,S-GQDs by Au3+ decreased with increase in the concentration of N,S-GQDs to 22.5 mg L−1. When the concentration of N,S-GQDs increased from 22.5 to 90 mg L−1, the quenching efficiency kept minor change. Further increase in the concentration of N,S-GQDs caused decrease in the quenching efficiency of N,S-GQDs by Au3+. Hence, 60 mg L−1 of the N,S-GQDs was used in the present work. The effect of solution pH was studied in the range from 2.2 to 9.0. The result indicated that FL quenching efficiency increased with increase in pH from 2.2 to 4.0, then remained almost unchanged in the range of pH from 4.0 to 5.0, above which it was decreased (Fig. S12†). Finally, pH 5.0 was chosen for further study. Fig. S10† shows the result of temperature effect on reaction time. The time for reduction of Au3+ onto the N,S-GQDs was decreased from 180 min to 15 min when the reaction temperature was increased from 10 °C to 45 °C. However, further increase in temperature did not reduce the reaction time. Thus, it can be concluded that the time for reduction of Au3+ onto the N,S-GQDs depends on reaction temperature. When the reaction temperature was set at 45 °C, quenching equilibrium reached within 15 min (Fig. S10†). Hence, 45 °C and 15 min were selected as reaction temperature and time in the subsequent experiments.Fluorescent detection of Au3+
Under optimum conditions, the sensitivity of the N,S-GQDs for the determination of Au3+ was explored. The standard solutions containing various concentration levels of Au3+ ions were added to the N,S-GQDs suspension, then the mixed solution was incubated for 15 min at 45 °C. The fluorescence intensity of the N,S-GQDs displayed a decrease at 425 nm with increasing concentration of Au3+ ions from 0 to 200 μM (Fig. 4A). The calibration curve of the values of (F0 − F)/F0 against Au3+ concentration was linear in the range from 0.1 μM to 50 μM (Fig. 4B) with a detection limit (3σ) of 50 nM. The obtained linear equation was (F0 − F)/F0 = 0.0121C + 0.028 (correlation coefficient r2 = 0.9900, n = 14), where C was the concentration of Au3+ (μM). As can be seen from Table 1, the detection limit obtained by this method is comparable with that by 2-((7-oxo-7H-furo[3,2-g]chromen-9-yl)oxy)-N-(3-(triethoxysilyl) propyl)acetamide (CTPA) linked Si NPs,44 but is much lower than many previously reported detection limits with organic fluorescence probes.15–22,45,46 This suggested that the method was more sensitive than most of the previously reported assays for Au3+ ions. The above result suggested that the method could be used for sensitive detection of Au3+ ions in a wide concentration range.To test the selectivity of the N,S-GQDs for the detection of Au3+, fluorescence response of the N,S-GQDs towards various metal ions was investigated. The tolerance limits were defined as the concentrations of coexisting ions resulting in less than 5% signal variation. As shown in Fig. 5 and S13,† except for Fe3+, no significant interferences from 1 mM K+, Na+, Ca2+, Mg2+, Al3+, Cr3+, Cd2+, Zn2+, Ba2+, F−, Cl−, Br−, NO3−, SO42−, Ac−; 0.6 mM Ni2+ and Fe2+; 0.2 mM Cu2+, Ag+, Pb2+, Co2+, and As(III); 100 μM CO32−, PO43−, S2O82−, ClO−, ClO3−, and SO32−; 8 μM Cr(VI) were observed. However, the interference from Fe3+ can be effectively eliminated by 150 μmol L−1 MSA as masking reagent (Fig. 5). It is noted that GQDs have been used as fluorescence probe for the detection of free chlorine because the free chlorine can quench the FL of GQDs by affecting their surface states.47 However, in our work, it was found 100 μM free chlorine caused <5% fluorescence quenching of the N,S-GQDs. This is probably because the preparation methods for graphene quantum dots are different; therefore, it is reasonable to believe that their surface states are different. Moreover, some neutral species, such as 500 μM hydrogen peroxide; 2 mM ascorbic acid, citric acid and oxalic acid presented only a minor effect on the fluorescence response of the N,S-GQDs (Fig. S13†). The result demonstrated that the N,S-GQDs was highly selective for Au3+ over the other substances.
Analytical application of the probe
Gold can be found in waters as various species, including gold nanoparticle, and soluble complexes of Au(I) and Au(III), and its concentration in surface waters ranged from as high as 19.3 μg L−1 in drainage from a gossan tailings pile to as low as 50 pg L−1 in open ocean water.48 To assess the applicability of the proposed method, the N,S-GQDs was used as fluorescent probe to detect Au3+ ions in real water samples. The water samples included tap water, river water and lake water. Before analysis, all water samples were filtered through a 0.22 μm membrane. The results suggested that no Au3+ in the employed water samples was detected by this method (Table 2). This is reasonable because there are no areas of gold deposit near our city. Thus, a recovery test was performed on the water samples spiked with different concentration levels of Au3+. As can be seen from Table 2, the recoveries ranged from 96.1% to 103.6%, demonstrating that the N,S-GQDs-based fluorescent method was potentially applicable for the determination of Au3+ ions in environmental water samples. The utility of the N,S-GQDs was further demonstrated by the detection of gold content in the antirheumatic drug auranofin. Auranofin is a monomeric Au(I) species in which the triethyl phosphine group stabilizes the gold–thiol complex. Thus, the aqua regia was to digest auranofin sample. The measured gold content in auranofin for three determinations was (28.92 ± 0.23)%. On a weight basis, auranofin contains 29.03% gold.49 Hence, the result given by the proposed method is in good agreement with theoretical value.| Added (μM) | Tap water | Lake water | Jialing River water | |||
|---|---|---|---|---|---|---|
| Founda (μM) | Recoverya (%) | Founda (μM) | Recoverya (%) | Founda (μM) | Recoverya (%) | |
| a Average of three measurements (mean ± SD, n = 3); ND: not detected. | ||||||
| 0 | ND | — | ND | — | ND | — |
| 0.5 | 0.51 ± 0.03 | 102.0 | 0.48 ± 0.02 | 96.1 | 0.52 ± 0.02 | 103.6 |
| 2.5 | 2.50 ± 0.01 | 100.4 | 2.46 ± 0.04 | 98.6 | 2.58 ± 0.01 | 103.0 |
Conclusion
In summary, N and S codoped GQDs were facilely prepared by an one-pot hydrothermal treatment of citric acid as the carbon source in the presence of cysteine as a doped agent. It was found that the N,S-GQDs exhibited a strong blue emission with a quantum yield of 35.4%. Interestingly, the N,S-GQDs can be used as reducing agent for the direct reduction of Au3+, leading to the formation of gold nanoparticles without additional reductant and further leading to the quenching of the fluorescent N,S-GQDs. On this basis, for the first time, we have developed a simple and yet effective fluorescent method for the sensitive and selective detection of Au3+ with a low detection limit of 50 nM and a wide detection linear range from 0.1 to 50 μM. The method promises advantages such as easy preparation of the N,S-GQDs as fluorescent probe, high sensitivity and selectivity. Hence, the fluorescence method reported here provides a new application of the functionalized GQDs and will open a novel pathway to the detection of metal ions.Acknowledgements
Financial support from the National Natural Science Foundation of China (No. 21277111) is gratefully acknowledged.References
- Y. Tao, Y. Lin, Z. Huang, J. Ren and X. Qu, Adv. Mater., 2013, 25, 2594–2599 CrossRef CAS PubMed.
- H. Yamamoto, H. Yano, H. Kouchi, Y. Obora, R. Arakawa and H. Kawasaki, Nanoscale, 2012, 4, 4148–4154 RSC.
- A. Primo and F. Quignard, Chem. Commun., 2010, 46, 5593–5595 RSC.
- E. Nyarko, T. Hara, D. J. Grab, A. Habib, Y. Kim, O. Nikolskaia, T. Fukuma and M. Tabata, Chem.-Biol. Interact., 2004, 148, 19–25 CrossRef CAS PubMed.
- Y. Yang, Q. Zhao, W. Feng and F. Li, Chem. Rev., 2013, 113, 192–270 CrossRef CAS PubMed.
- M. Navarro, Coord. Chem. Rev., 2009, 253, 1619–1626 CrossRef CAS.
- L. Messori and G. Marcon, Met. Ions Biol. Syst., 2004, 41, 279–304 CAS.
- I. Ott, Coord. Chem. Rev., 2009, 253, 1670–1681 CrossRef CAS.
- W. D. Block and E. L. Knapp, J. Pharmacol. Exp. Ther., 1945, 83, 275–278 CAS.
- A. Habib and M. Tabata, J. Inorg. Biochem., 2004, 98, 1696–1702 CrossRef CAS PubMed.
- E. E. Connor, J. Mwamuka, A. Gole, C. J. Murphy and M. D. Wyatt, Small, 2005, 1, 325–327 CrossRef CAS PubMed.
- E. Kazemi, S. Dadfarnia and A. M. H. Shabani, Talanta, 2015, 141, 273–278 CrossRef CAS PubMed.
- O. T. Butler, J. M. Cook, C. F. Harrington, S. J. Hill, J. Rieuwerts and D. L. Miles, J. Anal. At. Spectrom., 2007, 22, 187–221 RSC.
- M. Dong, Y.-W. Wang and Y. Peng, Org. Lett., 2010, 12, 5310–5313 CrossRef CAS PubMed.
- M. Jung Jou, X. Chen, K. M. K. Swamy, H. N. Kim, H.-J. Kim, S.-G. Lee and J. Yoon, Chem. Commun., 2009, 7218–7220 RSC.
- J. H. Do, H. N. Kim, J. Yoon, J. S. Kim and H.-J. Kim, Org. Lett., 2010, 12, 932–934 CrossRef CAS PubMed.
- L. Yuan, W. Lin, Y. Yang and J. Song, Chem. Commun., 2011, 47, 4703–4705 RSC.
- J.-B. Wang, Q.-Q. Wu, Y.-Z. Min, Y.-Z. Liu and Q.-H. Song, Chem. Commun., 2012, 48, 744–746 RSC.
- B. Wang, T. Fu, S. Yang, J. Li and Y. Chen, Anal. Methods, 2013, 5, 3639–3641 RSC.
- J. Y. Chio, G.-H. Kim, Z. Guo, H. Y. Lee, K. M. K. Swamy, J. Pai, S. Shin, I. Shin and J. Yoon, Biosens. Bioelectron., 2013, 49, 438–441 CrossRef PubMed.
- X. Cao, W. Lin and Y. Ding, Chem.–Eur. J., 2011, 17, 9066–9069 CrossRef CAS PubMed.
- P. Chinapang, V. Ruangpornvisuti, M. Sukwattanasinitt and P. Rashatasakhon, Dyes Pigm., 2015, 112, 236–238 CrossRef CAS.
- J. Shen, Y. Zhu, X. Yang and C. Li, Chem. Commun., 2012, 48, 3686–3699 RSC.
- L. Li, G. Wu, G. Yang, J. Peng, J. Zhao and J.-J. Zhu, Nanoscale, 2013, 5, 4015–4039 RSC.
- D. Qu, M. Zheng, P. Du, Y. Zhou, L. Zhang, D. Li, H. Tan, Z. Zhao, Z. Xie and Z. Sun, Nanoscale, 2013, 5, 12272–12277 RSC.
- Y. Dong, H. Pang, H. B. Yang, C. Guo, J. Shao, Y. Chi, C. M. Li and T. Yu, Angew. Chem., Int. Ed., 2013, 52, 7800–7804 CrossRef CAS.
- Z. Luo, D. Yang, G. Qi, J. Shang, H. Yang, Y. Wang, L. Yuwen, T. Yu, W. Huang and L. Wang, J. Mater. Chem. A, 2014, 2, 20605–20611 CAS.
- Y. Wang, Q. Zhuang and Y. Ni, Chem.–Eur. J., 2015, 21, 13004–13011 CrossRef CAS PubMed.
- X. T. Zheng, A. Ananthanarayanan, K. Q. Luo and P. Chen, Small, 2015, 11, 1620–1636 CrossRef CAS PubMed.
- J. Peng, W. Gao, B. K. Gupta, Z. Liu, R. Romero-Aburto, L. Ge, L. Song, L. B. Alemany, X. Zhan, G. Gao, S. A. Vithayathil, B. A. Kaipparettu, A. A. Marti, T. Hayashi, J.-J. Zhu and P. M. Ajayan, Nano Lett., 2012, 12, 844–849 CrossRef CAS PubMed.
- F. Cai, X. Liu, S. Liu, H. Liu and Y. Huang, RSC Adv., 2014, 4, 52016–52022 RSC.
- C. Hu, Y. Liu, Y. Yang, J. Cui, Z. Huang, Y. Wang, L. Yang, H. Wang, Y. Xiao and J. Rong, J. Mater. Chem. B, 2013, 1, 39–42 Search PubMed.
- Z. Qian, J. Ma, X. Shan, L. Shao, J. Zhou, J. Chen and H. Feng, RSC Adv., 2013, 3, 14571–14579 Search PubMed.
- Z. Wu, M. Gao, T. Wang, X. Wan, L. Zheng and C. Huang, Nanoscale, 2014, 6, 3868–3874 RSC.
- Y. Dong, J. Shao, C. Chen, H. Li, R. Wang and Y. Chi, Carbon, 2012, 50, 4738–4743 CrossRef CAS.
- J. Gu, D. Hu, W. Wang, Q. Zhang, Z. Meng, X. Jia and K. Xi, Biosens. Bioelectron., 2015, 68, 27–33 CrossRef CAS PubMed.
- J. Zhang, X. Wang and X. Yang, Analyst, 2012, 137, 2806–2812 RSC.
- D. K. Singh, R. Jagannathan, P. Khandelwal, P. M. Abraham and P. Poddar, Nanoscale, 2013, 5, 1882–1893 RSC.
- X. Yang, M. Shi, R. Zhou, X. Chen and H. Chen, Nanoscale, 2011, 3, 2596–2601 RSC.
- S. Fujii, K. Sakurai, T. Okobira, N. Ohta and A. Takahara, Langmuir, 2013, 29, 13666–13675 CrossRef CAS PubMed.
- Y. Liu, W. Q. Loh, A. Ananthanarayanan, C. Yang, P. Chen and C. Xu, RSC Adv., 2014, 4, 35673–35677 RSC.
- A. Cayuelaa, M. L. Sorianoa, M. C. Carriónb and M. Valcárcela, Anal. Chim. Acta, 2014, 820, 133–138 CrossRef PubMed.
- J. Deng, Q. Lu, Y. Hou, M. Liu, H. Li, Y. Zhang and S. Yao, Anal. Chem., 2015, 87, 2195–2203 CrossRef CAS PubMed.
- N. Niamsa, C. Kaewtong, W. Srinonmuang, B. Wanno, B. Pulpoka and T. Tuntulani, Polym. Chem., 2013, 4, 3039–3046 RSC.
- Z. Song, C. Xiao, Y. Dai, Q. Fei, Y. Huan and G. Feng, Nanotechnology, 2012, 23, 425501 CrossRef PubMed.
- A. Kundu, R. K. Layek, A. Kuila and A. K. Nandi, ACS Appl. Mater. Interfaces, 2012, 4, 5576–5582 CAS.
- Y. Dong, G. Li, N. Zhou, R. Wang, Y. Chi and G. Chen, Anal. Chem., 2012, 84, 8378–8382 CrossRef CAS PubMed.
- A. R. Lucas, S. U. Salmon, A. W. Rate, S. Larsen and K. Kilminster, Geochim. Cosmochim. Acta, 2015, 171, 156–173 CrossRef CAS.
- W. F. Kean, L. Hart and W. W. Buchanan, Br. J. Rheumatol., 1997, 36, 560–572 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Supporting Tables and figures. See DOI: 10.1039/c5ra20060a |
| This journal is © The Royal Society of Chemistry 2015 |