Green, efficient and large-scale synthesis of benzimidazoles, benzoxazoles and benzothiazoles derivatives using ligand-free cobalt-nanoparticles: as potential anti-estrogen breast cancer agents, and study of their interactions with estrogen receptor by molecular docking†
Abstract
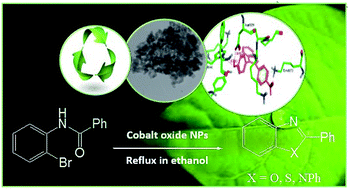
A facile and high yielding method for the synthesis of 2-aryl benzoxazoles, benzimidazole and benzothiazoles is reported employing cobalt oxide nanoparticles. The cobalt oxide nanoparticles were used as a convenient, green, easy available and highly efficient heterogeneous nano catalyst which promoted cyclization cross-coupling reactions without ligands and/or additives. This procedure has advantages such as simplicity of work-up, eco-friendly of reaction medium, low cost and mild conditions of reaction. In addition to, the binding modes of synthesized compounds to estrogen receptor were studied by molecular docking to investigate their potentially anti breast cancer activity.

 Please wait while we load your content...
Please wait while we load your content...