Synthesis of substituted pyrroles using a silver-catalysed reaction between isocyanoacetates/benzyl isocyanides and chromones†
Xueyu Qi,
Haoyue Xiang,
Yuhong Yang and
Chunhao Yang*
State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zu Chong Zhi Road, Shanghai 201203, P. R. China. E-mail: chyang@simm.ac.cn; Fax: +86-21-50806770; Tel: +86-21-50806770
First published on 13th November 2015
Abstract
A novel synthetic strategy to construct substituted-pyrroles has been developed using silver-catalysed reactions between isocyanides/benzyl isocyanides and chromones. These reactions proceed under mild conditions and yield polysubstituted pyrroles with efficient yields. The silver catalyst plays a key role in sequestering and activating the isocyano group and in sequential Michael addition and cyclization reactions.
Substituted pyrroles represent an important class of nitrogen-containing heterocycles that are often observed in natural products,1 pharmaceutical substances,2 and bioactive materials.3 A large numbers of pyrrole derivatives have been found to act as biological agents, including, a tubulin polymerization inhibitor,4 a Cdc7 kinase inhibitor,5 a potassium-competitive acid blocker (P-CAB),6 and a pesticide, pyoluteorin7 (Fig. 1). As a result of these findings, considerable attention has been paid to developing efficient methods for the synthesis of pyrrole derivatives. The most frequently used methods to synthesize pyrroles employ the classical Hantzsch and Paal–Knorr procedures, but these synthetic approaches require multistep operations, harsh conditions, and do not confer regioselectivity.8 More efficient and environmentally friendly reactions, such as the Paal–Knorr reaction, have been reported.9 Although a variety of methods for the synthesis of functionalized pyrrole derivatives have recently been developed,10 direct access to polyfunctionalized pyrroles from common, commercially available starting materials, is still a challenge.
 | ||
| Fig. 1 Representative examples of pyrrole derivatives. Isocyanoacetates have been widely used in organic synthesis and are useful building blocks for the generation of N-heterocycles11 because of their multi-reaction centres, such as an isocyanide group, which includes an acidic CH fragment and a carboxylic acid with a protecting group. The cycloaddition reaction of isocyanoacetates provides an ideal route to substituted pyrroles due to the efficiency of the reaction. Historically, the Barton–Zard synthesis, which represents the reaction between an activated alkene and an activated methylene isocyanide under basic conditions, has been the most efficient method for the synthesis of substituted pyrroles (Scheme 1 eqn (1)).12 However, poor yields and unwanted by-products limit this synthesis approach. Recently, the cycloaddition of isocyanide has been extended to incorporate alkynes with the help of a transition metal-catalyst (Scheme 1 eqn (2)).13 The copper or silver salts employed increase the selective chemical transformations involving terminal alkynes. Based on these results, isocyanoacetates represent a very attractive starting material for investigations of synthetic applications. | ||
Chromones are an additional class of starting materials that are used for constructing various biological heterocycles.14 The use of 3-formylchromone in the synthesis of pyrroles has previously been reported (Scheme 1 eqn (3)).15 Based on these previous studies, we believe that chromones may be coupled with the reaction of isocyanoacetates for the synthesis of pyrrole derivatives (Scheme 1 eqn (4)).
 | ||
| Scheme 1 Methods for the synthesis of pyrroles from isocyanoacetates, including the method described in this manuscript. | ||
Our initial efforts focused on the reaction between 4H-chromen-4-one 1a and ethyl 2-isocyanoacetate 2a in the presence of silver salts and bases. Following the reaction, the corresponding pyrrole derivative 3a was produced with moderate yields (Table 1, entry 1). Notably, no by-product from the dimerization of isocyanides was observed in this reaction.
| Entry | Catalyst | Temp (°C) | Solvent | Base | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction condition: 1a (0.5 mmol, 1.0 equiv.), 2a (0.75 mmol, 1.5 equiv.), base (2.0 equiv.), and silver salts (20 mol%) in solvent (3.0 mL) under air.b Isolated yields.c The reaction was carried out without silver salt.d NR = no reaction.e Silver salts (10 mol%) was used.f The reaction was carried out without base. | |||||
| 1 | Ag2O | rt | NMP | K2CO3 | 64 |
| 2 | Ag2O | 50 | NMP | K2CO3 | 66 |
| 3 | Ag2O | 100 | NMP | K2CO3 | 76 |
| 4 | Ag2O | 130 | NMP | K2CO3 | 75 |
| 5 | Ag2O | 100 | DMSO | K2CO3 | 70 |
| 6 | Ag2O | 100 | DMF | K2CO3 | 80 |
| 7 | Ag2O | 100 | Dioxane | K2CO3 | 63 |
| 8 | Ag2O | 100 | DMF | KOAc | 73 |
| 9 | Ag2O | 100 | DMF | Cs2CO3 | 70 |
| 10 | Ag2O | 100 | DMF | DBU | 50 |
| 11 | Ag2CO3 | 100 | DMF | K2CO3 | 98 |
| 12 | AgNO3 | 100 | DMF | K2CO3 | 93 |
| 13 | AgOTf | 100 | DMF | K2CO3 | 79 |
| 14 | AgNTf2 | 100 | DMF | K2CO3 | 80 |
| 15c | — | 100 | DMF | K2CO3 | NRd |
| 16e | Ag2CO3 | 100 | DMF | K2CO3 | 77 |
| 17f | Ag2CO3 | 100 | DMF | — | 20 |
Based on these results, we attempted optimize the reaction conditions (Table 1). The investigation of different reaction temperatures indicated that the optimal temperature was 100 °C (Table 1, entries 1–4). Once the optimum temperature was defined, several solvents were screened (Table 1, entry 3, entries 5–7). DMF, instead of NMP, produced 3a in an 80% yield, which was the best result (Table 1, entry 6). Moreover the effect of bases was also studied. The results found that K2CO3 was the optimal base (Table 1, entry 6, entries 8–10); the presence of base was critical for improving the yield of the reaction (Table 1, entry 17). The effects of silver salts on the reaction were studied, and Ag2CO3 was found to be the optimal silver salt (Table 1, entry 6, entries 11–14); it is worth noting that a decrease in the concentration of Ag2CO3 resulted in a reduced yield (Table 1, entry 16). It is also worth noting that the reaction would not occur in the absence of silver salts (Table 1, entry 15). As a result of these studies, the optimal reaction conditions were found to include Ag2CO3 (20 mol%) and K2CO3 (2.0 equiv.) in DMF (3.0 mL) at 100 °C for 1 h.
The structure of 3a was established by X-ray crystal structure analysis (Fig. 2).
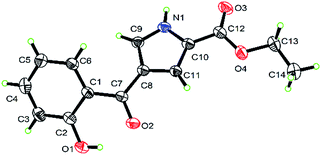 | ||
| Fig. 2 X-ray crystal structure of ethyl 4-(2-hydroxybenzoyl)-1H-pyrrole-2-carboxylate 3a (probability 30%). | ||
To study the capacity of this reaction method, a variety of chromones 1 were reacted with isocyanides 2 under the optimal reaction conditions to produce substituted-pyrroles 3. The results are shown in Table 2. When both electron-donating and -withdrawing groups were located on the benzene rings, the chromones yielded substituted-pyrroles with efficient yields (Table 2, 3a–3t). Electronic effects had little influence on the sequential reactions with isocyanoacetates. Different alkyl, halo, and methoxy groups located in the aryl ring of chromones 1 produced pyrrole derivatives 3a–3o with excellent yields. Moreover, multigroup chromones 1 were also compatible with the reaction process and produced substituted-pyrroles with excellent yields (Table 2, 3p–3r). In addition, both an aryl-substituted compound (1s) and methyl 2-isocyanoacetate (2t) were found to be suitable substrates (Table 2, 3s and 3t). Other activated methylene isocyanides, such as tosylmethyl isocyanide and benzyl isocyanide, may be used to incorporate structural diversity into the pyrrole ring. However, when a benzyl isocyanide (2u) was used, the pyrrole derivative was produced with a moderate yield and the reaction required more time (Table 2, 3u). When a tosylmethyl isocyanide was used under the optimal conditions, it failed to yield the expected pyrrole. However, on the whole, the silver-catalysed reaction of activated methylene isocyanides with a broad range of chromones provides a powerful method for the synthesis of 2,4-disubstituted pyrroles.
The proposed reaction mechanism (Scheme 2) based on previously published works. The initial step involves the formation of a silver-isocyanide complex A generated from 2a in the presence of silver catalyst. As described in previous studies, the silver salts coordinate the isocyano group and activate the isocyanides (1) for the cycloaddition reaction.13c An intermolecular Michael addition of compound 1a and complex A produces intermediate C. An intramolecular cyclization reaction occurs under alkaline conditions to produce complex D. Complex D undergoes a 1,3-hydrogen shift followed by protonation mediated by KHCO3 to yield 3a, thus completing the catalytic cycle for Ag2CO3.
Conclusions
This manuscript described the silver-catalysed synthesis of 2,4-disubstituted pyrroles from isocyanoacetates/benzyl isocyanides and a variety of chromones. This synthetic approach represents an extremely simple, efficient, and economical method of producing biologically active pyrrole derivatives with excellent yields. Further studies of the reaction mechanism and the application of these products are under investigation in our laboratory.Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant number 81321092) and SKLDR/SIMM (SIMM1403ZZ-01).Notes and references
- (a) H. Fan, J. Peng, M. T. Hamann and J.-F. Hu, Chem. Rev., 2008, 108, 264 CrossRef CAS PubMed; (b) R. Jansen, S. Sood, V. Huch, B. Kunze, M. Stadler and R. Müller, J. Nat. Prod., 2014, 77, 320 CrossRef CAS.
- (a) A. Schäfer, A. Wellner, M. Strauss, A. Schäfer, G. Wolber and R. Gust, J. Med. Chem., 2012, 55, 9607 CrossRef; (b) G. La Regina, R. Silvestri, M. Artico, A. Lavecchia, E. Novellino, O. Befani, P. Turini and E. Agostinelli, J. Med. Chem., 2007, 50, 922 CrossRef CAS PubMed.
- (a) M. Lazerges, K. Chane-Ching, S. Aeiyach, S. Chelli, B. Peppin-Donnat, M. Billon, C. Lombard, F. Maurel and M. Jouini, J. Solid State Electrochem., 2009, 13, 231 CrossRef CAS; (b) S. Hamilton, M. J. Hepher and J. Sommerville, Sens. Actuators, B, 2005, 107, 424 CrossRef CAS.
- G. La Regina, R. Bai, A. Coluccia, V. Famiglini, S. Pelliccia, S. Passacantilli, C. Mazzoccoli, V. Ruggieri, L. Sisinni, A. Bolognesi, W. M. Rensen, A. Miele, M. Nalli, R. Alfonsi, L. Di Marcotullio, A. Gulino, A. Brancale, E. Novellino, G. Dondio, S. Vultaggio, M. Varasi, C. Mercurio, E. Hamel, P. Lavia and R. Silvestri, J. Med. Chem., 2014, 57, 6531 CrossRef CAS PubMed.
- M. Menichincheri, C. Albanese, C. Alli, D. Ballinari, A. Bargiotti, M. Caldarelli, A. Ciavolella, A. Cirla, M. Colombo, F. Colotta, V. Croci, R. D'Alessio, M. D'Anello, A. Ermoli, F. Fiorentini, B. Forte, A. Galvani, P. Giordano, A. Isacchi, K. Martina, A. Molinari, J. K. Moll, A. Montagnoli, P. Orsini, F. Orzi, E. Pesenti, A. Pillan, F. Roletto, A. Scolaro, M. Tatò, M. Tibolla, B. Valsasina, M. Varasi, P. Vianello, D. Volpi, C. Santocanale and E. Vanotti, J. Med. Chem., 2010, 53, 7296 CrossRef CAS PubMed.
- Y. Arikawa, H. Nishida, O. Kurasawa, A. Hasuoka, K. Hirase, N. Inatomi, Y. Hori, J. Matsukawa, A. Imanishi, M. Kondo, N. Tarui, T. Hamada, T. Takagi, T. Takeuchi and M. Kajino, J. Med. Chem., 2012, 55, 4446 CrossRef CAS.
- D. M. Bailey, R. E. Johnson and U. J. Salvador, J. Med. Chem., 1973, 16, 1298 CrossRef CAS PubMed.
- (a) A. Hantzsch, Ber. Dtsch. Chem. Ges., 1890, 23, 1474 CrossRef; (b) L. Knorr, Ber. Dtsch. Chem. Ges., 1884, 17, 1635 CrossRef; (c) C. Paal, Ber. Dtsch. Chem. Ges., 1884, 17, 2756 CrossRef; (d) V. Amarnath, D. C. Anthony, K. Amarnath, W. M. Valentine, L. A. Wetterau and D. G. Graham, J. Org. Chem., 1991, 56, 6924 CrossRef CAS.
- (a) V. F. Ferreira, M. C. B. V. de Souza, A. C. Cunha, L. O. R. Pereira and M. L. G. Ferreira, Org. Prep. Proced. Int., 2001, 33, 411 CrossRef CAS; (b) H. Cho, R. Madden, B. Nisanci and B. Torok, Green Chem., 2015, 17, 1088 RSC; (c) A. R. Bharadwaj and K. A. Scheidt, Org. Lett., 2004, 6, 2465 CrossRef CAS PubMed; (d) G. Minetto, L. F. Raveglia and M. Taddei, Org. Lett., 2004, 6, 389 CrossRef CAS PubMed; (e) P. B. Cranwell, M. O'Brien, D. L. Browne, P. Koos, A. Polyzos, M. Pena-Lopez and S. V. Ley, Org. Biomol. Chem., 2012, 10, 5774 RSC; (f) R. U. Braun, K. Zeitler and T. J. J. Müller, Org. Lett., 2001, 3, 3297 CrossRef CAS PubMed.
- V. Estevez, M. Villacampa and J. C. Menendez, Chem. Soc. Rev., 2010, 39, 4402 RSC.
- (a) R. de la Campa, I. Ortín and D. J. Dixon, Angew. Chem., Int. Ed., 2015, 54, 4895 CrossRef CAS PubMed; (b) Z.-L. He and C.-J. Wang, Chem. Commun., 2015, 51, 534 RSC; (c) M.-X. Zhao, H.-L. Bi, R.-H. Jiang, X.-W. Xu and M. Shi, Org. Lett., 2014, 16, 4566 CrossRef CAS PubMed; (d) A. V. Lygin and A. de Meijere, Angew. Chem., Int. Ed., 2010, 49, 9094 CrossRef CAS PubMed; (e) A. V. Gulevich, A. G. Zhdanko, R. V. A. Orru and V. G. Nenajdenko, Chem. Rev., 2010, 110, 5235 CrossRef CAS PubMed.
- (a) N. C. Misra, K. Panda, H. Ila and H. Junjappa, J. Org. Chem., 2007, 72, 1246 CrossRef CAS; (b) J. L. Bullington, R. R. Wolff and P. F. Jackson, J. Org. Chem., 2002, 67, 9439 CrossRef CAS PubMed; (c) D. H. R. Barton, J. Kervagoret and S. Z. Zard, Tetrahedron, 1990, 46, 7587 CrossRef CAS.
- (a) S. Kamijo, C. Kanazawa and Y. Yamamoto, J. Am. Chem. Soc., 2005, 127, 9260 CrossRef CAS PubMed; (b) J. Liu, Z. Fang, Q. Zhang, Q. Liu and X. Bi, Angew. Chem., Int. Ed., 2013, 52, 6953 CrossRef CAS; (c) M. Gao, C. He, H. Chen, R. Bai, B. Cheng and A. Lei, Angew. Chem., Int. Ed., 2013, 52, 6958 CrossRef CAS PubMed; (d) O. V. Larionov and A. de Meijere, Angew. Chem., Int. Ed., 2005, 44, 5664 CrossRef CAS PubMed; (e) A. V. Lygin, O. V. Larionov, V. S. Korotkov and A. de Meijere, Chem.–Eur. J., 2009, 15, 227 CrossRef CAS PubMed; (f) C. Kanazawa, S. Kamijo and Y. Yamamoto, J. Am. Chem. Soc., 2006, 128, 10662 CrossRef CAS PubMed.
- (a) X. Qi, H. Xiang, Q. He and C. Yang, Org. Lett., 2014, 16, 4186 CrossRef CAS PubMed; (b) I. Savych, T. Glasel, A. Villinger, V. Y. Sosnovskikh, V. O. Iaroshenko and P. Langer, Org. Biomol. Chem., 2015, 13, 729 RSC; (c) J. Yan, M. Cheng, F. Hu and Y. Hu, Org. Lett., 2012, 14, 3206 CrossRef CAS; (d) X. Zhang, Q. He, H. Xiang, S. Song, Z. Miao and C. Yang, Org. Biomol. Chem., 2014, 12, 355 RSC.
- (a) A. O. Fitton, J. R. Frost, H. Suschitzky and P. G. Houghton, Synthesis, 1977, 133 CrossRef CAS; (b) A. O. Fitton, M. Kosmirak, H. Suschitzky and J. L. Suschitzky, Tetrahedron Lett., 1982, 23, 3953 CrossRef CAS; (c) A. S. Plaskon, S. V. Ryabukhin, D. M. Volochnyuk, A. N. Shivanyuk and A. A. Tolmachev, Tetrahedron, 2008, 64, 5933 CrossRef CAS; (d) P. D. Clarke, A. O. Fitton, M. Kosmirak, H. Suschitzky and J. L. Suschitzky, J. Chem. Soc., Perkin Trans. 1, 1985, 1747 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1412634. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra21915a |
| This journal is © The Royal Society of Chemistry 2015 |




