A comparison of centrifugally-spun and electrospun regenerated silk fibroin nanofiber structures and properties
Abstract
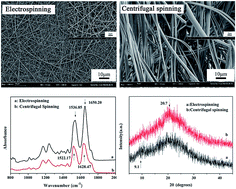
Recently, a newly-developed approach to fabricate nanofibers, centrifugal spinning, has been paid great attention for its wider material choice, higher production rate, and lower cost compared with electrospinning. However, the structure and property differences of nanofibers obtained from these two methods are unclear. In this paper, we will take regenerated silk fibroin (i.e., RSF) nanofibers as an example to reveal the differences. The centrifugally-spun and electrospun RSF nanofibers were spun under the same solution and environment. The morphology, secondary structure, orientation, and thermal properties of the two types of RSF nanofiber aggregation structures were characterized by Field-Emission Scanning Electron Microscopy (FESEM), Fourier Transform Infrared Spectroscopy (FTIR), Far Infrared (FIR), Wide Angle X-ray Diffraction (WXAD), Optical Polarizing Microscopy (OPM), Differential Scanning Calorimetry (DSC), and Thermogravimetric Analysis (TGA), respectively. The conformations of the RSF were transformed from random coil to β-sheet, and at the same time, the crystallinity, orientation, and thermostability were all enhanced through centrifugal spinning. The findings suggest that centrifugal spinning is a more promising approach to fabricate RSF fibers with stable structures and outstanding performances compared with electrospinning.

 Please wait while we load your content...
Please wait while we load your content...