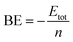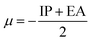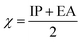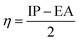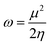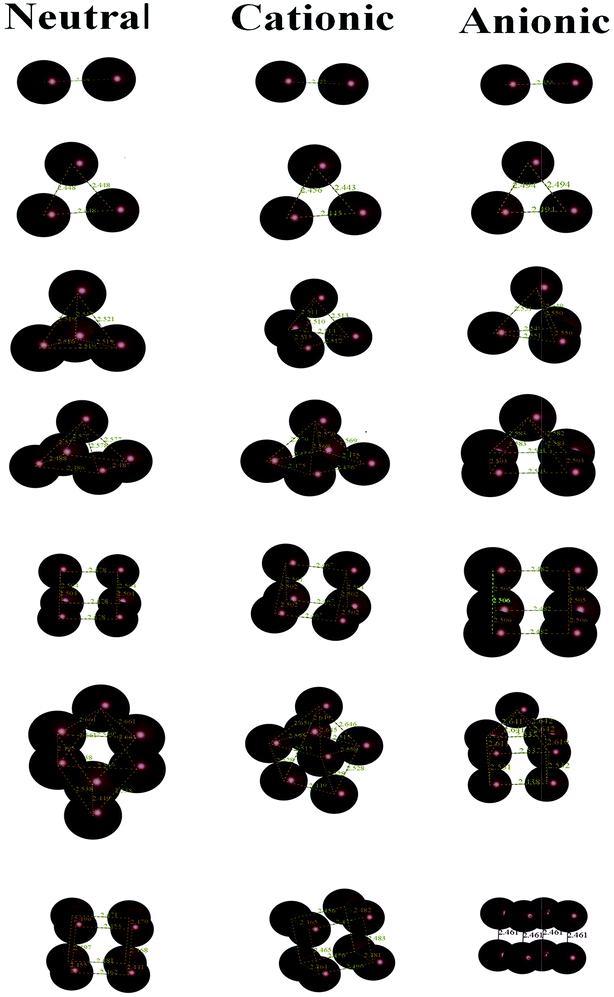Structural evolution, electronic and magnetic manners of small rhodium Rhn+/− (n = 2–8) clusters: a detailed density functional theory study†
Abhijit Dutta and
Paritosh Mondal*
Department of Chemistry, Assam University, Silchar 788011, Assam, India. E-mail: paritos_au@yahoo.co.in
First published on 14th December 2015
Abstract
A systematic investigation on the lowest energy electronic structure of neutral, cationic and anionic Rhn (n = 2–8) clusters in the gas phase is performed with an all electron relativistic method using density functional theory within the generalized gradient approximation. The lowest energy structures of neutral and ionic rhodium clusters are evaluated with different multiplicities. Neutral clusters with even atoms of rhodium and ionic clusters containing odd atoms of rhodium are optimized with multiplicities M = 1, 3 and 5, while neutral clusters containing odd rhodium atoms and ionic clusters with even atoms of rhodium are allowed to relax with multiplicities M = 2, 4 and 6. The bond length, binding energy, coordination number, bond dissociation energy, stability function, ionization potential, electron affinity, electrophilicity, LUMO–HOMO gap, chemical hardness, dipole moment and magnetic moment are evaluated from the lowest energy geometry. These studies reveal that even atom rhodium clusters are more stable than odd atom clusters. Calculated electronic properties such as the LUMO–HOMO gap, bond dissociation energy, stability function, electron affinity and electrophilicity suggest that Rh4 with a tetrahedral geometry is the most stable among all Rhn (n = 2–8) clusters. Hence, tetrahedral Rh4 may be considered as the magic number cluster.
Introduction
Atomic clusters are a very vital class of materials which are composed of a few to hundreds of atoms. Clusters show different physical1 and chemical properties2–4 from their bulk counterparts due to quantum size effects. Studies of small metal clusters provided a connecting link between molecular and bulk states. The high surface to volume ratio of smaller clusters lead to the properties differing from the bulk. The properties of nanoclusters change drastically even after the addition of a single extra atom. In recent years, transition metal clusters have been widely studied due to their unique catalytic and magnetic properties. In particular, nanoclusters of Pt, Pd and Rh are used as heterogeneous catalysts for hydrogenation as well as for the reduction of NO and oxidation of CO and unburned hydrocarbons.5 Rh on the other hand, is a vital heterogeneous catalyst used for hydrogenation as well as dehydrogenation reactions of alcohols6 and also for removing harmful gases.7 The variation of the magnetic moment with cluster size and high catalytic activity are the most significant characteristics of transition metal clusters. Many theoretical and experimental studies on the magnetic properties of 3d transition metal clusters such as Fe, Co and Ni have been extensively performed to date.8–13 These investigations suggest that 3d transition metal clusters have nonzero magnetic moments, while the bulk form shows no magnetic moment.11,14 Due to the reduced coordination and high symmetry, clusters develop a narrow d-band width that gives the possibility of larger spin multiplicities in the ground state. However, very few theoretical and experimental studies on 4d transition metal clusters are reported.15 Out of them, Rh metal clusters have been the subject of a number of experimental and theoretical studies because of their catalytic and magnetic properties. Two very important aspects are considered when investigating the magnetic properties of metal clusters: the first is to study the lowest energy geometrical structures and the second is to determine their electronic properties. Most of the electronic calculations on Rh clusters have been performed either by assuming fixed geometries16–18 or just optimizing bond lengths.15,19–21 Investigation of the electronic structure of rhodium clusters is very challenging due to a huge number of electrons and large number of achievable geometric isomers. However, some low energy rhodium clusters are derived using density functional theory calculations. Reddy et al.22 combined the techniques of MD (molecular dynamics) with DFT and anticipated that a 13 atom Rh cluster would be magnetic with a magnetic moment of 1.62 μB, while the observed value of magnetic moment per atom of Rh13 was found to be 0.48 μB.23,24 Jinglong et al.15,25 examined different small rhodium clusters and Li et al.19 evaluated the magnetic properties of Rhn (n = 6, 9, 13, 19 and 43). Cox et al. reported the permanent magnetic moment of rhodium clusters with n ≤ 60–90, while ruthenium along with palladium clusters showed nonmagnetic behavior at least for a cluster size greater than 13.24,26 Rh is the first case where magnetism is observed in the nanoclusters of a nonmagnetic solid. A remarkable size dependence of the magnetic moment per atom of rhodium is observed from experimental studies. The magnetic moment of nanoclusters oscillates as a function of cluster size along with the number of atoms. The magnetic moment of a rhodium cluster seems to be different from other ferromagnetic transition metal clusters such as Fe, Co and Ni, where the magnetic moments are extended over wide ranges.27–31 A finite size effect in the electronic structure and reduction of the local coordination number qualitatively reflect the enhancement of magnetism in small clusters.24 A spin-polarized DFT study32,33 of Ru, Rh, and Pd clusters shows a large magnetic moment for small clusters, while the magnetic moment of Ru and Rh clusters decreases gradually with increasing cluster size.Some theoretical works22,23 revealed that Rh4 with a tetrahedral geometry is stabilized in the singlet state; while Bae et al.34 showed that high spin rhombus Rh4 is the ground state. The magnetic moment of metal clusters changes with changes in their geometry. The magnetic moment of a Rh13 cluster is found to be 1.3 μB per atom with a cage like structure,34 while Stern–Gerlach evaluated a magnetic moment of 0.48 μB per atom.24,26 Different geometrical assumptions can clarify the obvious inconsistency between different theoretical and experimental works. Thus it is obvious that obtaining the ground state geometry is very much crucial for explaining the magnetic properties of metal clusters.
Most of the recent investigations related to Rh clusters are motivated by the theoretical studies of Galicia35 and Reddy et al.23 and the experimental works of Cox et al.24,26 A wide range of magnetic moments of rhodium nanoclusters are observed from theoretical studies.15,19,36,37 This indicates a quite strong understanding of the calculated magnetic properties of Rh with a theoretical method approximation. Due to indirect experimental information and insufficient precise structural determination, the correct properties of free-standing clusters are hard to pin down. Metals do not always follow the good proportionality of magnetism with coordination number and interatomic distances which is observed from the ab initio calculation of Rh2 supported on Ag (001).38 Therefore, a methodical exploration of both the geometric and magnetic properties of isolated rhodium clusters with respect to size is vital.
The magnetism as well as the bonding nature in correlation with the atomic structures of Rh nanoclusters is found to be significant for catalysis.39 Hence, it is challenging to fully recognize the magnetic properties to understand their role in catalytic applications. The correct representation of the electronic correlation for the large number of open shell electronic states which arise due to incomplete 4d states of Rh is a challenging aspect in theoretical study.40
To the best of our knowledge, probably for the first time a detailed systematic investigation on the electronic, structural and magnetic properties of neutral and ionic Rhn (n = 2–8) clusters has been done. The main focus of this study is to correlate different properties such as bond length, coordination number, binding energy, bond dissociation energy, ionization potential, electron affinity, LUMO–HOMO gap, global hardness, etc., with the size and stability of the clusters. The DOS and spin density values are evaluated in order to study the electronic and magnetic contribution of each atom to a cluster. A systematic survey on small rhodium clusters is done to determine their most stable or reactive cluster on the basis of calculated reactivity parameters. Geometry optimization of different possible structural isomers of neutral Rhn (n = 2–8) clusters is carried out with different multiplicities i.e., M = 2, 4 and 6 for odd atom clusters and M = 1, 3 and 5 for even atom clusters to evaluate the stable geometry. The geometries of Rhn+ and Rhn− clusters are also fully optimized with multiplicities of 2, 4, and 6 for even atom ionic clusters, and 1, 3 and 5 for odd atom ionic clusters, in order to determine the most stable geometry.
Computational details
All the isomers of rhodium clusters are fully optimized using a double numerical plus polarization (DNP) basis set implemented in a DMol3 program package.41,42 DFT calculations are performed under a generalized gradient approximation (GGA) with a BLYP exchange correlation functional43,44 which incorporates the exchange functional of Becke with the gradient corrected functional of Lee–Yang–Parr. A DNP45 basis set is chosen for geometry optimization. The DNP basis set is comparable to a Gaussian split-valence 6-31G** basis set. Relativistic calculations are very important for heavy metal atoms. Hence, all electron relativistic corrections to valence orbitals via a local pseudo potential are carried out for direct inversion in a subspace method (DIIS) without symmetry constraints. In this study self consistent field (SCF) procedures are adopted with a convergence criteria of energy 1 × 10−5 Ha, maximum force gradient 2 × 10−3 Ha Å−1 and displacement convergence 5 × 10−3 Å on the total energy and 10−6 a.u. on electron density are the boundary conditions applied. All neutral and charged isomers of rhodium clusters are optimized corresponding to low spin configurations. In order to compare the energy values of ground state clusters with their higher multiplicities, symmetry restricted calculations are done at higher spin states. Vibrational frequency calculations are done to ensure an energy minima. A zero point vibrational energy correction is incorporated in all the calculated energies. From further study of higher multiplicities most of the clusters are found to retain their ground state. An absence of imaginary frequencies for all geometries suggests their energy minima. To evaluate the stability of the clusters with respect to size, the binding energy per atom, second difference of binding energy, first vertical ionization potential, electron affinity and chemical hardness of the stable structures are determined.The average binding energy per atom is computed from the following equation
For ionic clusters, the binding energy is evaluated as
| BE = −Etot±/n |
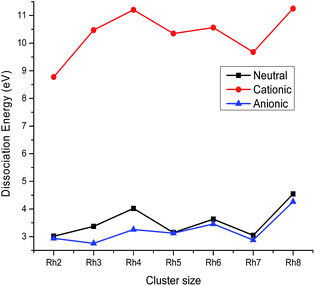
The dissociation energy of a cluster of size n has been calculated from
| D(n, n − 1) = E(Rhn − 1) + E(Rh1) − E(Rhn) |
Chemical hardness (η) can be defined as the second derivative of the energy (E) with respect to the number of electrons (N) at a constant external potential ν(![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) )
)
Using the finite difference approach46 the chemical potential, electronegativity and chemical hardness can be computed from the vertical ionization potential (IP) and electron affinity (EA).
According to Parr’s prescription, the electrophilicity index47 is defined as
Results and discussion
Geometries and energetics
In order to find out the best exchange-correlation functional to be used for the calculation, we carried out a comparative study of the bond length, vibrational frequency and binding energy of a rhodium dimer using various functionals with the available experimental results.48 The comparative results are presented in Table 1. It is noticed from Table 1 that among all the GGA functionals used, the BLYP functional provides the best assessment of all three experimental parameters considered. Hence, we chose the BLYP functional for this study.| Method | Binding energy (eV) | Frequency (cm−1) | Bond length (Å) |
|---|---|---|---|
| GGA-BLYP | 3.013 | 268 | 2.308 |
| GGA-PW91 | 0.524 | 287 | 2.269 |
| GGA-BOP | 2.203 | 277 | 2.312 |
| GGA-BP | 2.462 | 286 | 2.288 |
| GGAVWN_BP | 2.484 | 286 | 2.286 |
| GGA-HCTH | 2.021 | 284 | 2.309 |
| GGA-RP | 2.336 | 279 | 2.308 |
| GGA-PBE | 3.321 | 287 | 2.286 |
| Experiment | 2.92 ± 0.22 | 267 | 2.280 |
Neutral Rhn clusters
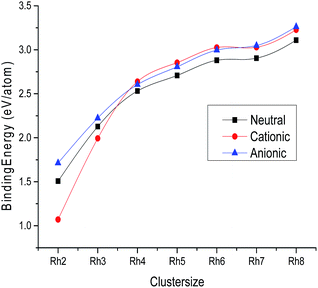
The ground state for Rh atoms is found to be a quartet, 4F (4d85s1), with an energy of 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524.7615 eV. The ground state geometries of neutral Rhn (n = 2–8) clusters evaluated at the BLYP/DNP level are shown in Fig. 1 and all other isomers with their point groups are given in Fig. S1 of the ESI.† Singlet multiplicity is the lowest energy state for neutral Rh2 with a symmetric point group D∞h and a bond length of 2.308 Å. The binding energy per atom of all the neutral clusters with different multiplicities is given in Table 2 and the bond lengths are mentioned in Table S1 of the ESI.† It is observed from Table 2 that the binding energy per atom of Rh2 is calculated to be 1.5067 eV. The energy values of the singlet and triplet states of the Rh2 cluster are determined to be comparable i.e. the triplet state possesses an energy 0.3660 eV higher than the singlet state. The variation of the binding energy per atom of neutral clusters with cluster size is shown in Fig. 2. For a neutral Rh3 cluster, a triangular geometry with a quartet state is the lowest energy isomer with a bond length of 2.448 Å and a symmetric point group, D3h. The energy difference between the doublet and the quartet state is found to be only 0.1659 eV. The binding energy per atom for the most stable Rh3 cluster with a triangular shape is 2.1276 eV. A singlet state with a tetrahedral geometry is the ground state for the neutral Rh4 cluster with an average bond length and binding energy per atom of 2.518 Å and 2.5315 eV, respectively. The energy difference between the triplet and singlet state is evaluated to be 0.3798 eV. The calculated binding energy per atom of Rh4 is found to be lower than the corresponding values reported by Jinlong et al.15 and Reddy et al.22 However, the DFT evaluated average bond length is found to be closer to the corresponding value (2.48 Å) reported by them. For a cluster containing five rhodium atoms, a doublet state with a square pyramidal geometry and symmetric point group C4v is the lowest energy state. The average bond length and binding energy per atom of the stable Rh5 cluster are 2.532 Å and 2.7091 eV, respectively. The energy difference between the singlet and triplet state of the Rh5 cluster is 0.0778 eV only. The binding energy value is very close to the reported value of 2.70 eV per atom predicted by Chien37 (GGA-DFT) but lower than the values (3.06 and 3.13 eV per atom) showed by Jinlong et al.15 and Reddy et al.22 with the symmetric point groups D3h and C4v, respectively. The bond length of 2.532 Å obtained in this study is closer to the reported values (2.52 and 2.54 Å) by Jinlong et al.15 and Chien.34 A trigonal prismatic geometry for a Rh6 neutral cluster in the singlet state with a symmetric point group D3h is observed to be the ground state with an average bond length of 2.491 Å and binding energy of 2.8809 eV per atom. The energy difference between the singlet state and triplet state is found to be 0.0797 eV. The binding energy and bond length values are evaluated to be lower than the corresponding values reported by Jinlong et al.15 (DV-LSDA) and Li19 (DV-LSDA). However, the calculated binding energy value is exactly the same as the binding energy obtained by Chien et al.37 For a Rh7 cluster, a quartet state with a capped prism structure is found to be the most stable isomer with a symmetric point group (C2v). The binding energy per atom and the average bond length of this stable cluster are evaluated to be 2.9045 eV and 2.539 Å, respectively. The binding energy value of Rh7 obtained is lower than the binding energy values of Jinlong et al.15 (3.43 eV per atom) and Reddy et al.22 (3.33 eV per atom). The average bond distance of 2.77 Å evaluated in this study is higher than the corresponding values reported by Jinlong et al.15 and Reddy et al.22 The energy difference between the doublet and quartet state of the Rh7 cluster is found to be 0.0218 eV. A cubic geometry of Rh8 with a singlet state is evaluated to be most stable isomer. This isomer has a symmetric point group (Oh), with an average bond length and binding energy per atom of 2.459 Å and 3.1092 eV, respectively. The triplet state is recorded to be slightly higher in energy (0.00370 eV) than the singlet state. The binding energy as well as the average bond distance is calculated to be lower than the values suggested by Jinlong et al.15 and Reddy et al.22
524.7615 eV. The ground state geometries of neutral Rhn (n = 2–8) clusters evaluated at the BLYP/DNP level are shown in Fig. 1 and all other isomers with their point groups are given in Fig. S1 of the ESI.† Singlet multiplicity is the lowest energy state for neutral Rh2 with a symmetric point group D∞h and a bond length of 2.308 Å. The binding energy per atom of all the neutral clusters with different multiplicities is given in Table 2 and the bond lengths are mentioned in Table S1 of the ESI.† It is observed from Table 2 that the binding energy per atom of Rh2 is calculated to be 1.5067 eV. The energy values of the singlet and triplet states of the Rh2 cluster are determined to be comparable i.e. the triplet state possesses an energy 0.3660 eV higher than the singlet state. The variation of the binding energy per atom of neutral clusters with cluster size is shown in Fig. 2. For a neutral Rh3 cluster, a triangular geometry with a quartet state is the lowest energy isomer with a bond length of 2.448 Å and a symmetric point group, D3h. The energy difference between the doublet and the quartet state is found to be only 0.1659 eV. The binding energy per atom for the most stable Rh3 cluster with a triangular shape is 2.1276 eV. A singlet state with a tetrahedral geometry is the ground state for the neutral Rh4 cluster with an average bond length and binding energy per atom of 2.518 Å and 2.5315 eV, respectively. The energy difference between the triplet and singlet state is evaluated to be 0.3798 eV. The calculated binding energy per atom of Rh4 is found to be lower than the corresponding values reported by Jinlong et al.15 and Reddy et al.22 However, the DFT evaluated average bond length is found to be closer to the corresponding value (2.48 Å) reported by them. For a cluster containing five rhodium atoms, a doublet state with a square pyramidal geometry and symmetric point group C4v is the lowest energy state. The average bond length and binding energy per atom of the stable Rh5 cluster are 2.532 Å and 2.7091 eV, respectively. The energy difference between the singlet and triplet state of the Rh5 cluster is 0.0778 eV only. The binding energy value is very close to the reported value of 2.70 eV per atom predicted by Chien37 (GGA-DFT) but lower than the values (3.06 and 3.13 eV per atom) showed by Jinlong et al.15 and Reddy et al.22 with the symmetric point groups D3h and C4v, respectively. The bond length of 2.532 Å obtained in this study is closer to the reported values (2.52 and 2.54 Å) by Jinlong et al.15 and Chien.34 A trigonal prismatic geometry for a Rh6 neutral cluster in the singlet state with a symmetric point group D3h is observed to be the ground state with an average bond length of 2.491 Å and binding energy of 2.8809 eV per atom. The energy difference between the singlet state and triplet state is found to be 0.0797 eV. The binding energy and bond length values are evaluated to be lower than the corresponding values reported by Jinlong et al.15 (DV-LSDA) and Li19 (DV-LSDA). However, the calculated binding energy value is exactly the same as the binding energy obtained by Chien et al.37 For a Rh7 cluster, a quartet state with a capped prism structure is found to be the most stable isomer with a symmetric point group (C2v). The binding energy per atom and the average bond length of this stable cluster are evaluated to be 2.9045 eV and 2.539 Å, respectively. The binding energy value of Rh7 obtained is lower than the binding energy values of Jinlong et al.15 (3.43 eV per atom) and Reddy et al.22 (3.33 eV per atom). The average bond distance of 2.77 Å evaluated in this study is higher than the corresponding values reported by Jinlong et al.15 and Reddy et al.22 The energy difference between the doublet and quartet state of the Rh7 cluster is found to be 0.0218 eV. A cubic geometry of Rh8 with a singlet state is evaluated to be most stable isomer. This isomer has a symmetric point group (Oh), with an average bond length and binding energy per atom of 2.459 Å and 3.1092 eV, respectively. The triplet state is recorded to be slightly higher in energy (0.00370 eV) than the singlet state. The binding energy as well as the average bond distance is calculated to be lower than the values suggested by Jinlong et al.15 and Reddy et al.22
| Multiplicity | ||||||
|---|---|---|---|---|---|---|
| M = 1 | M = 2 | M = 3 | M = 4 | M = 5 | M = 6 | |
| Neutral cluster size | ||||||
| Rh2 | 1.5067 | 1.3235 | 1.5060 | |||
| Rh3_1 | 1.6584 | 1.666 | 1.6644 | |||
| Rh3_2 | 2.0722 | 2.1276 | 2.0649 | |||
| Rh4_1 | 2.5315 | 2.5055 | 2.4251 | |||
| Rh4_2 | 2.3471 | 2.3565 | 2.3713 | |||
| Rh5_1 | 2.6337 | 2.6282 | 2.6082 | |||
| Rh5_2 | 2.7091 | 2.6935 | 2.6583 | |||
| Rh5_3 | 2.7086 | 2.6935 | 2.6640 | |||
| Rh5_4 | 2.7084 | 2.6935 | 2.6817 | |||
| Rh6_1 | 2.7393 | 2.7392 | 2.7333 | |||
| Rh6_2 | 2.8307 | 2.8306 | 2.8365 | |||
| Rh6_3 | 2.8307 | 2.8319 | 2.8365 | |||
| Rh6_4 | 2.7135 | 2.7121 | 2.7001 | |||
| Rh6_5 | 2.8809 | 2.8676 | 2.8381 | |||
| Rh7_1 | 2.5676 | 2.5645 | 2.5660 | |||
| Rh7_2 | 2.8867 | 2.8917 | 2.8903 | |||
| Rh7_3 | 2.9014 | 2.9045 | 2.9026 | |||
| Rh7_4 | 2.8133 | 2.8119 | 2.7964 | |||
| Rh7_5 | 2.7869 | 2.7866 | 2.8217 | |||
| Rh7_6 | 2.8868 | 2.8917 | 2.8903 | |||
| Rh8_1 | 2.9728 | 2.9541 | 2.9612 | |||
| Rh8_2 | 2.6068 | 2.6092 | 2.6103 | |||
| Rh8_3 | 2.9884 | 2.9868 | 2.9825 | |||
| Rh8_4 | 3.1092 | 3.1087 | 3.0796 | |||
| Rh8_5 | 2.9543 | 2.9492 | 2.9403 | |||
| Rh8_6 | 2.9632 | 2.8432 | 2.7890 | |||
| Rh8_7 | 2.6789 | 2.6794 | 2.5978 | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Ionic clusters | ||||||
| Rh2+ | 0.7862 | 0.9506 | 1.0707 | |||
| Rh2− | 1.5784 | 1.7128 | 1.4433 | |||
| Rh3+ | 1.9935 | 1.9207 | 1.9935 | |||
| Rh3− | 2.2245 | 2.2143 | 2.2176 | |||
| Rh4+ | 2.6381 | 2.5449 | 2.4901 | |||
| Rh4− | 2.6048 | 2.5297 | 2.4852 | |||
| Rh5+ | 2.8531 | 2.8412 | 2.8082 | |||
| Rh5− | 2.8054 | 2.7948 | 2.8069 | |||
| Rh6+ | 3.0262 | 2.9965 | 2.9557 | |||
| Rh6− | 2.9966 | 2.9850 | 2.9627 | |||
| Rh7+ | 3.0288 | 3.0278 | 3.0284 | |||
| Rh7− | 3.0491 | 3.0447 | 3.0473 | |||
| Rh8+ | 3.2271 | 3.2171 | 3.1987 | |||
| Rh8− | 3.2619 | 3.2404 | 3.2285 | |||
Cationic (Rhn+) and anionic (Rhn−) clusters
In the literature, very few reports on cationic and anionic clusters of rhodium are accessible. The ground state geometries and average bond lengths evaluated at the BLYP/DNP level are shown in Fig. 1 and Table S1 of the ESI,† respectively. Cationic and anionic rhodium clusters are generated by removing and adding one electron to the lowest energy neutral Rhn clusters. Spin unrestricted geometry optimization of these ionic clusters with different multiplicities is performed. The variation of the binding energies per atom of these ionic clusters with cluster size is shown in Fig. 2 and the binding energy values (eV per atom) are listed in Table 2. A cationic (Rh2+) cluster in a sextet state is the lowest energy state with the symmetric point group D∞h, while, the ground state for an anionic (Rh2−) cluster is a quartet with the point group D∞h. It is seen that the average bond distance and binding energy per atom for the Rh2+ and Rh2−clusters are 2.355 and 2.353 Å and 1.0707 and 1.7128 eV, respectively. The sextet Rh2+ is stabilized more by 0.56912 eV than the doublet state, while, the quartet Rh2− is stabilized more by 0.26884 eV than the geometry with doublet multiplicity. The lowest energy triangular geometry with the symmetry point group D3h is found for Rh3+ and Rh3− in the singlet state. It is seen from Table S1† that the average bond distances (Rh–Rh) in Rh3+ and Rh3− are found to be 2.448 Å and 2.494 Å, respectively. The binding energy per atom for the cationic cluster is 1.9935 eV, while for the anionic cluster it is calculated to be 2.2245 eV. The energies of Rh3−, in the singlet as well as in the triplet state, are found to be closer. Again, the doublet states of Rh4+ and Rh4− with tetrahedral geometry are found to be the ground state with a Td symmetry point group. The average bond distances of the tetrahedral cationic and anionic rhodium clusters are 2.512 Å and 2.550 Å, respectively. While for cationic and anionic Rh4, a quartet multiplicity is found to be the least stable. The binding energies per atom for the ground state of Rh4+ and Rh4− are evaluated to be 2.6381 and 2.6048 eV, respectively. Cationic and anionic Rh5 clusters with singlet and pentet multiplicities are evaluated to be the ground state with average bond lengths of 2.538 and 2.543 Å, respectively. The structure of anionic and cationic Rh5 is evaluated to be square pyramidal with the symmetric point group C4v. The binding energy values per atom for Rh5+ and Rh5− are 2.8531 and 2.8069 eV, respectively. The energy difference between the triplet and singlet states of Rh5+ is 0.05932 eV, while, between the pentet and singlet states of Rh5− it is 0.0080 eV. A doublet state with a trigonal prismatic geometry for both Rh6+ and Rh6− clusters with a point group D3h is stabilized. The average bond lengths for Rh6+ and Rh6− are calculated to be 2.484 Å and 2.497 Å, respectively. While, the quartet states of Rh6+ and Rh6− are less stabilized by 0.17796 and 0.06911 eV, respectively, with respect to the doublet state of respective ionic Rh6. The binding energy per atom values of the lowest energy Rh6+ (3.0262 eV) and Rh6− (2.9966 eV) are noticed to be close. Rh7+ and Rh7− with a singlet multiplicity and a capped prism geometry with C2v symmetry are found to be the lowest energy structures. The triplet states of Rh7+ and Rh7− are evaluated to be less stable than the corresponding singlet states. The binding energies per atom for Rh7+ and Rh7− are 3.0288 and 3.0491 eV, respectively. A cubic geometry in the doublet state with the symmetric point group D3h is the ground state for cationic and anionic Rh8 clusters. Table S1† reports that the average Rh–Rh bond lengths for Rh8+ and Rh8− are 2.474 and 2.461 Å respectively. The binding energy per atom for the Rh8+ cluster (3.2271 eV) is found to be lower than for Rh8− (3.2619 eV). The quartet states of the Rh8+ and Rh8− clusters are the least stable. However, the quartet and doublet states of ionic Rh8 clusters are found to possess very close energy values.Trends
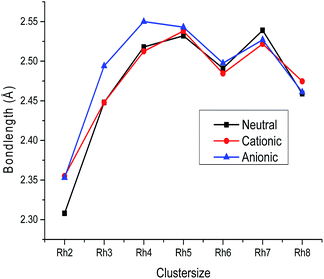
The variation of the average bond length with cluster size for the most stable cluster is shown in Fig. 3. It is observed from the figure that the average bond lengths increase with size in the case of smaller clusters, while no regular variation of geometrical parameters is noticed in the case of larger clusters. The variation of binding energy with cluster size is shown in Fig. 2. It is quite visible from Fig. 2 that the binding energy per atom of neutral and ionic (cationic and anionic) clusters increases with the increasing number of atoms and number of bonds. Hence, the energy stability of a rhodium cluster increases with the cluster size, while this variation is less prominent in the case of larger clusters. The coordination number (α) of all the clusters is evaluated at the DNP/BLYP level. The coordination number of the neutral as well as the ionic clusters is evaluated to be the same. The coordination numbers (α) of the Rh3, Rh4, Rh5, Rh6, Rh7 and Rh8 clusters are found to be 2.00, 3.00, 3.20, 3.00, 3.71, and 3.00, respectively. That is the Rh4, Rh6, and Rh8 clusters in the neutral as well as in the ionic form possess the same coordination number.Relative stability
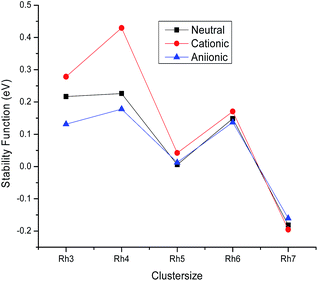
To understand the relative stability of the rhodium clusters, we have evaluated the second finite difference of total energies Δ2Eb(n), which is the measure of the stability function. The stability function is calculated from the following mathematical formulation:| Δ2Eb(n) = 2Eb(n) − Eb(n + 1) − Eb(n − 1) |
Electronic properties and reactivity parameters
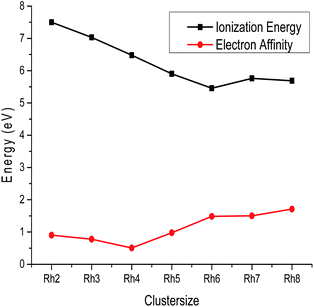
| IP = En+ − En |
| EA = En − En− |
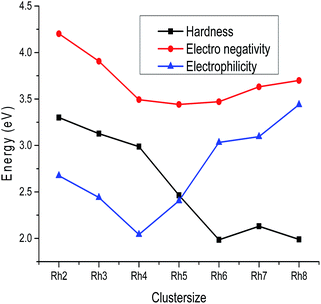
The variation of electronegativity with cluster size is plotted in Fig. 8. It is seen from Fig. 8 that the electronegativity values decrease from Rh2 to Rh5 and then increase up to Rh8 (Table S3 of the ESI†). In the case of larger clusters the electronegativity values increase with the increase of the number of metal atoms because the attraction between the nucleus and inner core electrons increases, as with the increase of atoms in a cluster the nuclear charge also increases. Electrophilicity is the measure of the stabilization energy required by clusters when they acquire an extra electronic charge. It is observed from Table S3 of the ESI† and Fig. 8 that Rh4 has lower electrophilicity values than the others i.e. it is less prone to get electrons from its surroundings, which supports the higher stability of the Rh4 cluster compared to its neighbours.
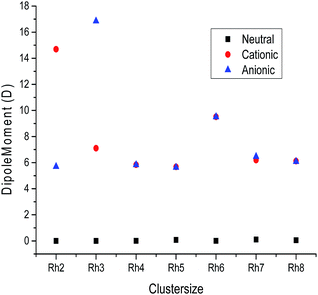
Magnetic properties
Molecular orbital study
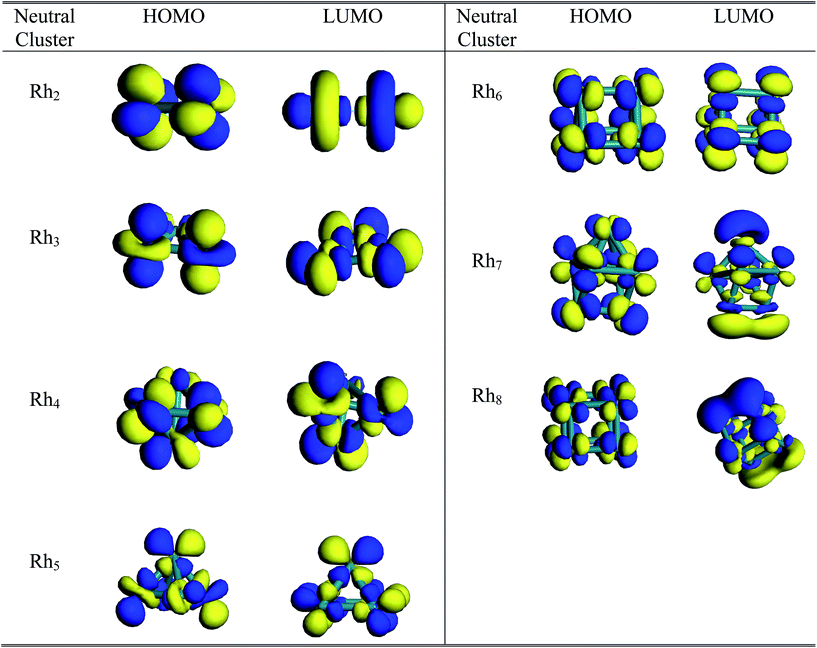
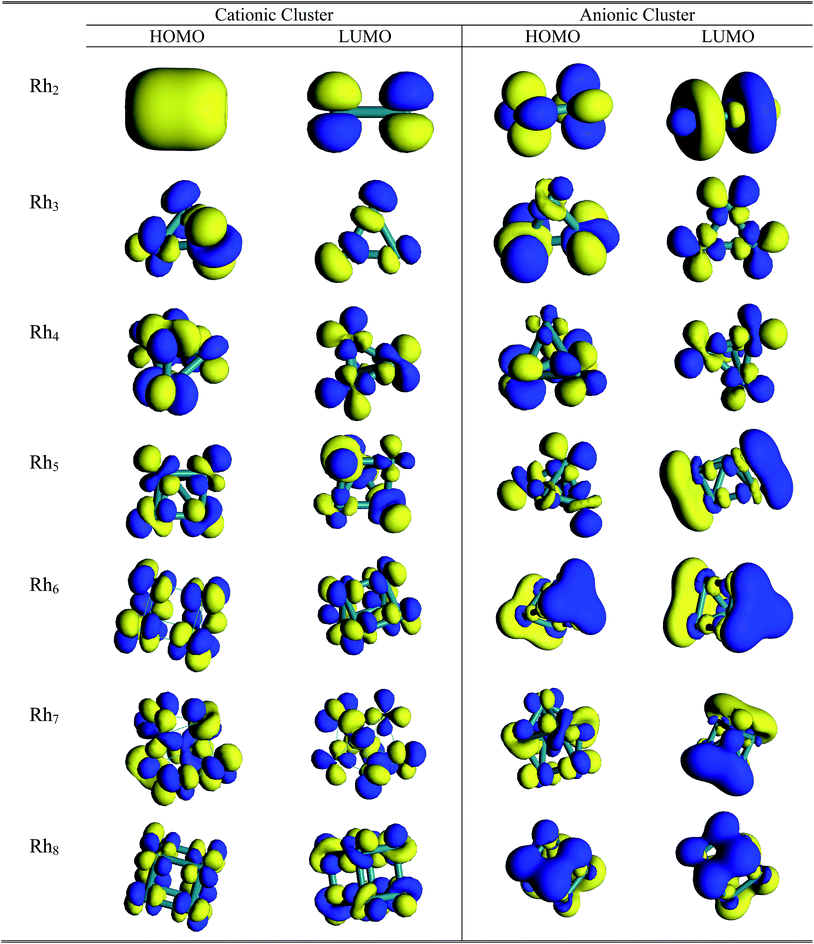
A pictorial representation of molecular orbitals can be used to understand the structural properties along with the stability and bonding properties of the nanoclusters. The 3D isosurface diagrams (LUMO and HOMO) of the most stable Rhn clusters (neutral and ionic) evaluated at the BLYP/DNP level are presented in Fig. 10 and 11. The isosurface diagrams of the LUMO and HOMO indicate the types of orbitals involved in the formation of the nanoclusters, that is which type of orbitals are responsible for bond formation. Analysis of the isosurface diagrams reveals that in most of the cases there is always sideways or π overlap between the d orbitals of the rhodium atoms. In some cases constructive or sigma overlapping is also observed among the bonding orbitals. The HOMO of neutral Rh2 (b2u) consists of a non-bonding dxy orbital but in the LUMO (a2u) hybridization is noticed with an anti-bonding dz2 orbital along the bond axis. The HOMO of the cationic Rh2 cluster suggests sigma bond overlapping while in the anionic cluster nonbonding overlap is observed similar to that of the neutral cluster. It is observed from the HOMO diagram of neutral Rh3 (e′) that ddπ bonding occurs from the hybridization of the dz2 orbital oriented perpendicular to the triangular framework where as in the LUMO (a′′2) some constructive overlapping is observed between the four lobes of the d orbitals. In the cationic cluster of Rh3, there is anti-symmetric overlapping between the dz2 orbital of one atom with the four lobes of the d orbitals of the other atoms on the HOMO isosurface. The HOMO of anionic Rh3 reveals pi bonding with the dz2 orbitals but in the LUMO limited region overlapping is noticed among the orbitals. The HOMO (t1) of Rh4 consists of four lobed either dyz or dzx orbitals where as in the LUMO (t2) ddπ occurs in between the dz2 orbitals. The isosurface diagrams of the HOMO of cationic and anionic Rh4 show bonding as well as perpendicular nonbonding overlap. In the neutral Rh5 cluster the HOMO (a2) is formed by the overlapping of either a dyz or dzx orbital with a dz2 orbital. In cationic Rh5 limited region overlapping with a dz2 orbital is observed in the HOMO, whereas a mismatch of overlapping among dx2−y2 and dz2 is seen in the LUMO. The LUMO of neutral Rh5 possesses b1 symmetry. In neutral clusters of Rh7 (a1) and Rh8 (eu), the LUMO is composed of sigma bonding overlap whereas in Rh6, both the HOMO (e′′) and LUMO (a′′1) are composed of nonbonding overlap between four lobe d orbitals. In anionic Rh6, positive bonding overlap is observed in the HOMO as well as in the LUMO. For cationic Rh7 the HOMO and LUMO and for Rh8 the HOMO consists of sideways limited region overlap among perpendicular d orbitals whereas in the LUMO of anionic Rh7 and Rh8, ddσ bonding occurs. The HOMO of Rh7 and Rh8 clusters has (t1u) and (a2) symmetry.Density of states study
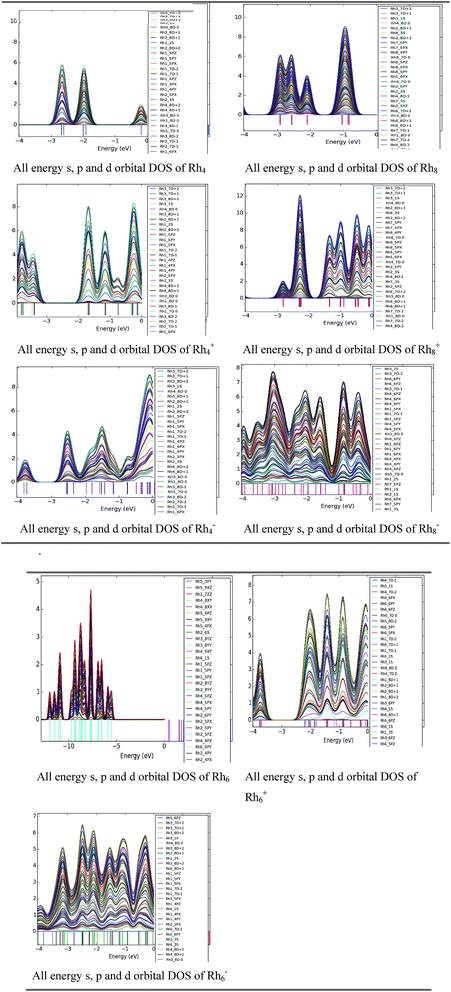
To investigate the orbital occupation per energy interval a DOS study was performed for neutral and ionic rhodium clusters. A few cases are picked up for our analysis purpose viz. neutral and ionic Rhn clusters (n = 4, 6 and 8). The DOS of the d orbitals of a rhodium atom are partially noticeable near the zero energy level which is hybridized with the s and p orbitals of rhodium. The zero energy is related to the Fermi energy level. For cationic Rh4 and Rh8 clusters, the density of states is at a maximum nearer the Fermi level than it is for the corresponding neutral clusters. For cationic clusters the intensity of the peak density is high at certain energy levels which means that there are many states available for occupation at that energy level. Hybridization along with the band width is higher for all neutral and ionic clusters of Rh8 than for Rh4. Rh6− clusters have higher mixing of orbitals whereas no DOS is observed for neutral Rh6 near the Fermi level. With the increase in the number of atoms in the cluster more overlapping occurs between the orbitals of rhodium. The width of the orbital band is noticed to be higher in anionic clusters than in cationic and neutral clusters (Fig. 12).Spin density and deformation density analysis
The spin density and deformed electron density are shown in Fig. 13 and 14, respectively. It is seen from Fig. 13 that the magnetic moment of Rh4 is found to be zero because the spin up density is neutralized by the spin down density. Again, the spin down density is high on each vertex which is cancelled out by the high spin up density in the middle region of the cluster which is the reason for the zero magnetic moment of Rh6. The odd atom clusters of Rh5 and Rh7 show nonzero magnetic moments because the spin up density is found to be higher than the spin down density. The deformation density reveals that the electron density is more deformed in cationic Rh4 than in its neutral and anionic clusters. The electron density of neutral and anionic Rh5 clusters is evaluated to be more deformed than in the cationic cluster. Again, the electron density of cationic Rh6 is more deformed than in the neutral and anionic cluster of Rh6. That is, the electron density is more deformed in the cationic clusters of Rh4 and Rh6 and in the anionic Rh5 cluster. The electron density in the cationic cluster is more deformed because of the greater nuclear charge which pulls the electrons more tightly than the anionic and neutral rhodium nanoclusters.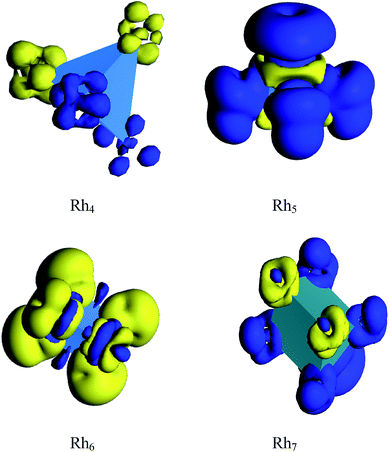 | ||
| Fig. 13 Calculated spin density of Rhn (n = 4, 5, 6 and 7) clusters. (Blue and yellow colour represent spin up and spin down density.) | ||
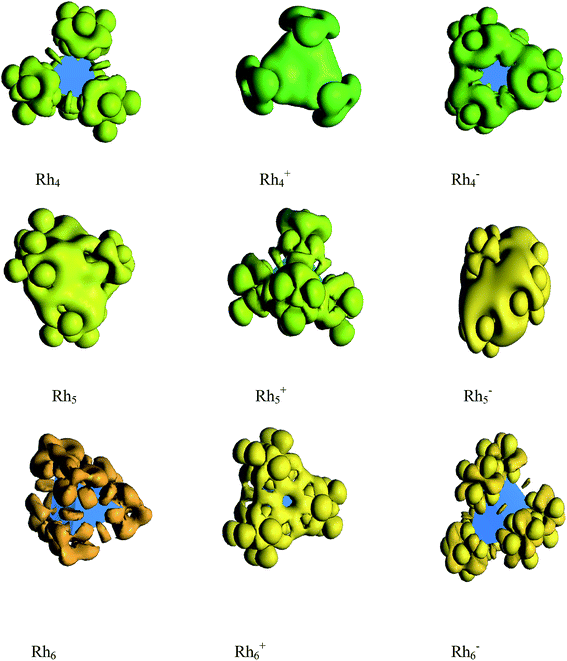 | ||
| Fig. 14 DFT evaluated deformation electron density of neutral and ionic Rhn (n = 4, 5 and 6) clusters. | ||
Conclusion
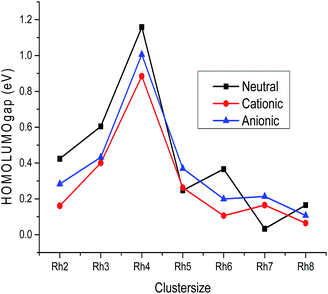
We have investigated systematically the electronic, structural and magnetic properties of neutral and ionic Rhn (n = 2–8) clusters with different multiplicities. In this study, we have evaluated different parameters such as bond length, binding energy, coordination number, stability function, bond dissociation energy, ionization potential, electron affinity, LUMO–HOMO gap, electrophilicity, electronegativity, global hardness and magnetic properties of different possible isomers of rhodium clusters. Based on the calculated reactivity parameters such as the binding energy, LUMO–HOMO gap, bond dissociation energy and electron affinity, an important inference can be drawn on the stability of the clusters. These results reveal that even atom rhodium clusters are more stable than odd atom clusters and hence, suggest the highest stability for Rh4. It is found that larger clusters have a higher binding energy than smaller clusters in all of their neutral and ionic Rhn clusters. Cationic clusters possess a higher stability function than neutral and anionic rhodium clusters. The highest LUMO–HOMO gap, the lowest electron affinity and electrophilicity, the highest stability function, the high ionization potential and high bond dissociation energy suggest that Rh4 is the magic cluster as these parameters are hallmarks to predict the magic cluster.51 Molecular orbital analysis suggests that the bonding in Rhn clusters in most of the cases is π overlapping between the d orbitals.Acknowledgements
Authors thank Department of Science and Technology (DST), New Delhi, India for financial support (SB/EMEQ-214/2013).References
- U. Sarkar and S. A. Blundell, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 79, 125441 CrossRef.
- M. Che and C. O. Bennett, Adv. Catal., 1989, 36, 55 CAS.
- K. Judai, S. Abbet, A. S. Worz, U. Heiz and C. R. Henry, J. Am. Chem. Soc., 2004, 126, 2732 CrossRef CAS PubMed.
- G. Qiu, M. Wang, G. Wang, X. Diao, D. Zhao, Z. Du and Y. Li, J. Mol. Struct.: THEOCHEM, 2008, 861, 131 CrossRef CAS.
- B. Kalita and R. C. Deka, J. Chem. Phys., 2007, 127, 244306 CrossRef PubMed.
- D. A. Hickman and L. D. Schmidt, Science, 1993, 259, 343 CAS.
- V. P. Zhdanov and B. Kasemo, Surf. Sci. Rep., 1997, 29, 31 CrossRef.
- D. R. Salahub and R. P. Messmer, Surf. Sci., 1981, 106, 415 CrossRef CAS.
- K. Lee, J. Callaway and S. Dhar, Phys. Rev. B: Condens. Matter Mater. Phys., 1985, 30, 1724 CrossRef.
- A. Rosen and T. T. Rantala, Z. Phys. D: At., Mol. Clusters, 1986, 3, 205 CrossRef CAS.
- G. M. Pastor, J. Dorantes-Davila and K. H. Benneman, Phys. Rev. B: Condens. Matter Mater. Phys., 1989, 40, 7642 CrossRef CAS.
- N. Fujima and T. Yamaguchi, J. Phys. Soc. Jpn., 1989, 58, 3290 CrossRef CAS.
- B. I. Dunlap, Phys. Rev. A, 1990, 41, 5691 CrossRef CAS.
- F. Liu, S. N. Khanna and P. Jena, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 43, 8179 CrossRef CAS.
- Y. Jinlong, F. Toigo and W. Kelin, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 7915 CrossRef CAS.
- K. Lee, Z. Phys. D: At., Mol. Clusters, 1997, 40, 164 CrossRef CAS.
- R. Guirado-López, D. Spanjaard, M. C. Desjonqueres and F. Aguilera-Granja, J. Magn. Magn. Mater., 1998, 186, 214 CrossRef.
- J. Guevara, A. M. Llois, F. Aguilera-Granja and J. M. Montejano- Carrizales, Solid State Commun., 1999, 111, 335 CrossRef CAS.
- Z. Q. Li, J. Z. Yu, K. Ohno and Y. Kawazoe, J. Phys.: Condens. Matter, 1995, 7, 47 CrossRef CAS.
- B. Piveteau, M. C. Desjonqueres, A. M. Olés and D. Span-jaard, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 53, 9251 CrossRef CAS.
- G. W. Zhang, Y. P. Feng and C. K. Ong, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 17208 CrossRef CAS.
- B. V. Reddy, S. K. Nayak, S. N. Khanna, B. K. Rao and P. Jena, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 5214 CrossRef CAS.
- B. V. Reddy, S. N. Khanna and B. I. Dunlap, Phys. Rev. Lett., 1993, 70, 3323 CrossRef CAS PubMed.
- A. J. Cox, J. G. Louderback and L. A. Bloomfield, Phys. Rev. Lett., 1993, 71, 923 CrossRef CAS PubMed.
- Y. Jinlong, F. Toigo, W. Kelin and Z. Manhong, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 7173 CrossRef CAS.
- A. J. Cox, J. G. Louderback, S. E. Apsel and L. A. Bloomfield, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 12295 CrossRef CAS.
- I. M. L. Billas, J. A. Becker, A. Châtelain and W. A. de Heer, Phys. Rev. Lett., 1993, 71, 4067 CrossRef CAS PubMed.
- I. M. L. Billas, A. Châtelain and W. A. de Heer, Science, 1994, 265, 1662 Search PubMed.
- J. P. Bucher, D. C. Douglass and L. A. Bloomfield, Phys. Rev. Lett., 1991, 66, 3052 CrossRef CAS PubMed.
- J. P. Bucher, D. C. Douglass and L. A. Bloomfield, Phys. Rev. B: Condens. Matter Mater. Phys., 1992, 45, 6341 CrossRef.
- D. C. Douglass, A. J. Cox, J. P. Bucher and L. A. Bloomfield, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 12874 CrossRef CAS.
- V. Kumar and Y. Kawazoe, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 144413 CrossRef.
- V. Kumar and Y. Kawazoe, Eur. Phys. J. D, 2003, 24, 81 CrossRef CAS.
- Y.-C. Bae, H. Osanai, V. Kumar and Y. Kawazoe, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 195413 CrossRef.
- R. Galicia, Rev. Mex. Fis. S, 1985, 32, 51 CAS.
- G. W. Zhang, Y. P. Feng and C. K. Ong, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 17208 CrossRef CAS.
- C.-H. Chien, E. Blaisten-Barojas and M. R. Pederson, Phys. Rev. A, 1998, 58, 2196 CrossRef CAS.
- V. S. Stepanyuk, W. Hergert, P. Rennert, J. Izquierdo, A. Vega and L. C. Balbás, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 57, 14020 CrossRef.
- J. Wei and E. Iglesia, J. Catal., 2004, 225, 116 CrossRef CAS.
- C. H. Chien, E. B. Barojas and M. R. Pederson, Phys. Rev. A, 1998, 58, 3 CrossRef.
- B. Delley, J. Chem. Phys., 2000, 113, 7756 CrossRef CAS.
- B. Delley, J. Chem. Phys., 1990, 92, 508 CrossRef CAS.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785 CrossRef CAS.
- B. Delly and D. E. Ellis, J. Chem. Phys., 1982, 76, 1949 CrossRef.
- R. P. Iczkowski and J. L. Margrave, J. Am. Chem., Soc., 1961, 83, 3547–3551 CrossRef CAS.
- P. K. Chattaraj, U. Sarkar and D. R. Roy, Chem. Rev., 2006, 106, 2065–2091 CrossRef CAS PubMed.
- K. A. Gingerich and D. L. Cocke, J. Chem. Soc., Chem. Commun., 1972, 1, 536 RSC.
- A. H. Larsen, J. Kleis, K. S. Thygesen, J. K. Norskov and K. W. Jacobsen, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 245429 CrossRef.
- F. Aguilera-Granja, J. L. Rodríguez-López, K. Michaelian, E. O. Berlanga-Ramírez and A. Vega, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 224410 CrossRef.
- B. K. Rao, P. Jena, S. Burkart, G. Gantefor and G. Seifert, Phys. Rev. Lett., 2001, 86, 692 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21600a |
| This journal is © The Royal Society of Chemistry 2016 |