Modulation of stability and functionality of a phyto-antioxidant by weakly interacting metal ions: curcumin in aqueous solution
Damayanti Bagchia,
Siddhi Chaudhuria,
Samim Sardara,
Susobhan Choudhurya,
Nabarun Polleya,
Peter Lemmensbc and
Samir Kumar Pal*a
aDepartment of Chemical, Biological and Macromolecular Sciences, S. N. Bose National Centre for Basic Sciences, Block JD, Sector III, Salt Lake, Kolkata 700 098, India. E-mail: skpal@bose.res.in; Fax: +91 033 2335 3477; Tel: +91 033 2335 5706-08
bInstitute for Condensed Matter Physics, TU Braunschweig, Mendelssohnstraße 3, 38106 Braunschweig, Germany
cLaboratory for Emerging Nanometrology, TU Braunschweig, 38106 Braunschweig, Germany
First published on 24th November 2015
Abstract
The natural polyphenol curcumin and its metal coordinated complexes show obvious benefits in the medical therapies of cancer and several neurodegenerative diseases. On the other side their stability and bioavailability are critical issues. The present study is an attempt to address the stability and functionality of curcumin upon complexation with transition metal ions. We have synthesized and optically characterized metallo–curcumin complexes with Cu(II) and Zn(II). From femtosecond resolved upconversion studies an interaction at the molecular level is revealed based on an observed photoinduced electron transfer from curcumin to the metal ions. In order to investigate the antioxidant activity of the complexes, we have performed a 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay in dark. The Cu(II)–curcumin complex exhibits an enhanced and recyclable activity, more pronounced compared to that of the Zn(II)–curcumin complex, which can be attributed to the weaker O–H bond present in the former case. In contrast, the Zn(II) complex has a higher solubility and stability in aqueous media than the Cu(II) complex. To address stability vs. functionality issues, we have suggested a facile method that enhances the solubility and stability of curcumin in aqueous media by metalation with Zn(II) and a successional replacement of Zn(II) in the complex by Cu(II) through a simple route to enhance the activity prior to its use. We have also used the complex in a model anti-bacteriological assay experiment where it shows significantly higher activity compared to pure curcumin. The dichlorofluorescin (DCFH) oxidation indicates an enhancement in ROS generation, which in turn is responsible for the enhanced antioxidative property of the Cu(II)–curcumin complex. Our results provide a promising method to use metallo–curcumin complexes in diverse biological applications.
1. Introduction
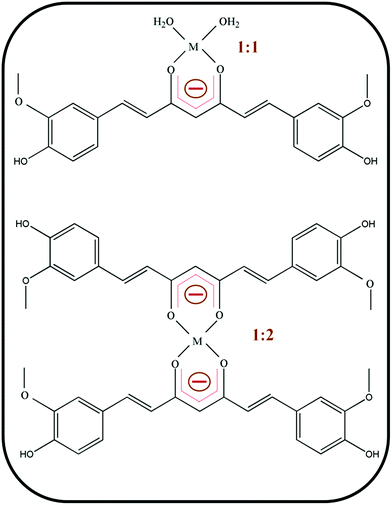
Curcumin is a natural yellowish orange diarylheptanoid derived from the rhizomes of Curcuma longa L. popularly known as turmeric, a member of the ginger family.1 The diverse pharmacological applications of curcumin towards various diseases includes Alzheimer's disease,2 breast cancer,3 pancreatic cancer,4 colon cancer,5 arthritis6 and oxidative stress induced pathogenesis.7 Furthermore its promising antioxidant activity8 anticipates its possible use as a novel drug for other lethal diseases.9 Curcumin is a linear polyphenol consisting of two o-methoxy phenolic groups which are connected by a seven carbon linker consisting of an α,β-unsaturated β-diketo moiety10 (Scheme 1a). The diketo group exhibits keto–enol tautomerism (Scheme 1b) and can exist in different types of conformers depending on the nature of the solvent.11 In most of non-polar and moderately polar solvents, the enol form is generally more stable than the keto form by 5 to 8 kcal mol−1 due to strong intramolecular H-bonding.12 Curcumin is hydrophobic in nature and almost insoluble in water at physiological pH values.13 This may be due to the presence of β-diketone linkers in the seven carbon chain. This leads to poor bio-availability, limited absorption in body, rapid metabolism and excretion.14 Therefore it is an important task to improve the bio-availability and solubility of curcumin in water in order to further exploit its medicinal benefits.15To overcome the problem of low bioavailability of curcumin, several methods have been proposed including conjugation to water-soluble polymers16 or encapsulation in colloidal carriers such as gold nanoparticles,17 silver nanoparticles18 and polymer nanoparticles.19 Recent report suggests that the introduction of dimethylaminomethyl group as substituents on aromatic rings in curcumin improves their aqueous solubility as the basic nitrogen atom will be responsible for converting the target compounds into the salt forms.20 Another efficient strategy to improve the bioavailability of curcumin is the formation of complexes with transition metal ions, such as Zn2+, Cu2+, Mn2+ and Fe2+ which attract interest in the contemporary literature.21 Some of these complexes are of much higher stability compare to free curcumin.22 Curcumin acts as a monobasic bidentate ligand in which the α,β-unsaturated β-diketo moiety acts as a chelating agent for complexation with metal ions.23 Moreover, the stability of curcumin is increased by a factor of 20 after its complexation with Zn(II) ions at a buffer pH of 7.0.24 Stable metallo–curcumin complexes are useful in a dual manner: firstly, the complexations increase the drug solubility and can also act as new metal based antioxidants which reduce the cytotoxicity of metal ions.25 Moreover, complexation of curcumin to palladium(II) metal centre and its conjugation to another functionalized bioactive ligand, significantly enhance cell-death in prostate cancer cell line through apoptosis signal transduction route due to increased aqueous solubility and hence indicate the possibility of use of metallo–curcumin complexes as potential metal based anti-cancer drugs.26 Recent studies reveal that deprotonation of the hydroxyl group in the keto–enol moiety leads to the formation of a bidentate β-diketonate which strongly chelates with transitional metal ions namely Cu(II) and Zn(II) in two different square planer geometries forming 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes respectively (Scheme 2).27 Among the different metal bound complexes of curcumin, Zn(II) and Cu(II) complexes received immense importance for their diverse biological applications. The latest studies have shown that the Cu(II) and curcumin interaction may be important for its anti-cancer28 and anti-amyloid properties.29 It has also been reported that due to the reversible electron transfer reactions with superoxide ions, Cu(II) complexes of curcumin can act as superoxide dismutase enzyme mimics.30 Zn(II)–curcumin complexes show anti-cancer, gastro-protective and antidepressant effects and are also very much effective for oxidative stress reduction.31 These metallo–curcumin complexes are receiving increasing attentions due to their enhanced antioxidant activity. However, the complexes can induce DNA damage due to binding and consequently showing pro-oxidant activity.32 Therefore, detailed mechanistic investigation of activity of metal complexes is needed. Moreover, both the aqueous solubility and stability are not profoundly altered for a particular metallo–curcumin complex and an alternative approach is required.
2 complexes respectively (Scheme 2).27 Among the different metal bound complexes of curcumin, Zn(II) and Cu(II) complexes received immense importance for their diverse biological applications. The latest studies have shown that the Cu(II) and curcumin interaction may be important for its anti-cancer28 and anti-amyloid properties.29 It has also been reported that due to the reversible electron transfer reactions with superoxide ions, Cu(II) complexes of curcumin can act as superoxide dismutase enzyme mimics.30 Zn(II)–curcumin complexes show anti-cancer, gastro-protective and antidepressant effects and are also very much effective for oxidative stress reduction.31 These metallo–curcumin complexes are receiving increasing attentions due to their enhanced antioxidant activity. However, the complexes can induce DNA damage due to binding and consequently showing pro-oxidant activity.32 Therefore, detailed mechanistic investigation of activity of metal complexes is needed. Moreover, both the aqueous solubility and stability are not profoundly altered for a particular metallo–curcumin complex and an alternative approach is required.
In the present study, we have synthesized and optically characterised two metallo–curcumin complexes of Zn(II) and Cu(II). Femtosecond resolved fluorescence transient studies of the complexes have clearly unravelled the key time components associated with the excited state electron transfer dynamics. The role of metal ions in antioxidant activity of the complex is evaluated in detail using well-known radical scavenger 2,2-diphenyl-1-picrylhydrazyl (DPPH) for aqueous media in dark condition. Herein, we suggest a new method for both increasing aqueous solubility by incorporation of Zn(II) and then to enhance the activity, where the metal ion can effectively be altered by Cu(II). Thus, we propose a novel approach in which both stability for storage and activity prior to use can be achieved in aqueous solvent. We have also used the active Cu–curcumin complex in a model bacteriological culture experiment to evaluate the effect of the synthesized complex as an antimicrobial agent. Our studies on ROS marker including dichlorofluorescin (DCFH) in aqueous solution is in consonance with the antibacterial activity of the complex. This study clearly indicates the mechanism of higher free-radical scavenging activity by incorporation of Cu(II) ions in curcumin followed by its action as an antimicrobial agent. The newly suggested method to first increase the water solubility by incorporation of Zn(II) and then replacing it by Cu(II) for higher activity of the metallo–curcumin complex might be useful in future for further in vivo experiments.
2. Experimental section
2.1. Materials
In this study analytical grade chemicals without further purification were used for synthesis. Curcumin, Zn(OAc)2·2H2O, Cu(OAc)2·H2O, CuCl2·2H2O were purchased from Sigma-Aldrich. As a suitable solvent for synthesis of metallo–curcumin complexes methanol was used (Merck). Millipore water was used as aqueous solvent. For femtosecond upconversion studies and bacteriological assay dimethyl sulfoxide (DMSO, from Spectrochem) was used as a solvent.2.2. Synthesis of metallo–curcumin complexes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.33 50 mL of 2 mM methanolic solution of curcumin was prepared and heated at 60 °C for dissolution. Zinc acetate dihydrate (2 mmol) was dissolved in 100 mL methanol by heating. The solution was added into the curcumin solution and a red powder precipitate was produced immediately. The mixture was refluxed for 2 h.33 The red solid product was filtered and washed firstly by cold methanol and then by water to remove the residue reactants. The purified product was dried in vacuum overnight and the final appearance of the product was a red, crystalline, and solid powder. The synthesized product exhibits 1
1.33 50 mL of 2 mM methanolic solution of curcumin was prepared and heated at 60 °C for dissolution. Zinc acetate dihydrate (2 mmol) was dissolved in 100 mL methanol by heating. The solution was added into the curcumin solution and a red powder precipitate was produced immediately. The mixture was refluxed for 2 h.33 The red solid product was filtered and washed firstly by cold methanol and then by water to remove the residue reactants. The purified product was dried in vacuum overnight and the final appearance of the product was a red, crystalline, and solid powder. The synthesized product exhibits 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio of Zn(II) and curcumin as reported in earlier literature.21b
1 stoichiometric ratio of Zn(II) and curcumin as reported in earlier literature.21b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry as reported in earlier literature.21b
1 stoichiometry as reported in earlier literature.21b2.3. Synthesis of Cu(II)–curcumin complex from Zn(II)–curcumin complex
The synthesized Zn(II)–curcumin complex was dissolved in water by using successive cyclomixing and bath-sonication for 3 h. The solution was centrifuged at 5000 rpm for 5 min and the supernatant (orangish-red) solution was separated. An aqueous solution of CuCl2·2H2O was added to the collected supernatant solution and vortexed for 2 h. A brown solution was generated.2.4. Characterization methods
3. Results & discussion
Metalation of organic ligands causes significant changes in their electronic structures. UV-visible spectroscopy is a useful technique to understand the complexation due to metalation. Fig. 1b shows the absorption maxima of curcumin at 422 nm with a shoulder at 348 nm in water. The absorption peak can be attributed to electronic transition typically a π–π* in nature from (HOMO) to (LUMO) and (HOMO−1) to (LUMO) respectively.36 With extensively delocalized π-electrons curcumin exhibits a bright yellow colour (Fig. 1a). After successful attachment of a metal ion to curcumin, a distinct red shift is observed in the absorption spectra. For the Zn(II)–curcumin, a sharp peak was observed at 440 nm, with a small peak at 485 nm. The dark brown Cu(II)–curcumin complex shows more prominent red shift with a broad peak at 512 nm. The red shift may be correlated with the formation of a new, lower energy charge-transfer state by electronic transition from the ligand curcumin to the metal ions. The delocalized π-electron density over the ligand curcumin moiety behaves as a donor to positively charged metal centres with empty d-orbitals behaving as an acceptor, exhibiting a ligand to metal charge transfer band (LMCT).37 The extent of interaction can be explained on the basis of number of d-electrons present in the system. Zn(II) having a filled d-orbital shows lower interactions compared to Cu(II), a d9 system which is prone to be involved in LMCT due to the presence of low-lying empty d orbitals.27a Moreover, Fig. 1b demonstrates curcumin and its metal complexes show extended absorbance in near infra-red region. The room temperature PL spectra (Fig. 1c) of curcumin depict a peak at 530 nm upon excitation at 400 nm. However, after metalation with Zn(II) and Cu(II) steady-state emission was significantly decreased although the corresponding absorbance spectra of these solutions are similar. This indicates involvement of non-radiative processes. The inset of Fig. 1c shows excitation spectra of all the samples and a clear quenching is observed. The significant quenching in both emission and excitation spectra suggest internal quenching due to complexation.Femtosecond resolved fluorescence decay transients of metallo–curcumin samples have been collected to understand the excited state interaction between metal and curcumin. The decay of curcumin was monitored at 530 nm upon excitation at 400 nm, in absence and presence of Cu(II) and Zn(II) in DMSO. The decay profiles are shown in Fig. 2. The fluorescence transient of curcumin is fitted with biexponential decay with lifetime of 3.2 ps (shorter component: signature of solvation dynamics) and 73.5 ps (longer component: indication for excited state intramolecular H atom transfer ESIHT),38 with an average lifetime of 50.4 ps. The decay profile of curcumin in presence of Cu(II) and Zn(II) show shorter time component of 0.7 ps with an average lifetime of 30.8 ps and 23.0 ps, respectively. The shorter excited state lifetime for Cu(II)–curcumin and Zn(II)–curcumin suggest the photoinduced electron-transfer process from curcumin to metal ions.27b In order to confirm the electron migration process, well-known electron acceptor benzoquinone (BQ) has been attached to curcumin. The possible excited state interaction in curcumin–BQ was monitored and the electron transfer timescale (∼0.7 ps) was found to be similar to that of metallo–curcumin samples. It has to be noted that BQ attached curcumin structure might not be similar to that of metallo curcumin complexes but excited state electron transfer timescale is similar due to proximity between two entities in both the cases. Thus, the shorter timescale in presence of metal ions can be rationalized as an electron transfer from curcumin to the attached metal ion. The lifetime components of transients with their relative percentages are presented in a tabular form (Table 1).
| Samples | τ1 (ps) | τ2 (ps) | τ3 (ps) | τavg (ps) |
|---|---|---|---|---|
| a The emission (monitored at 530 nm) was detected with 400 nm laser excitation. Numbers in parentheses indicate relative contributions. | ||||
| Curcumin | — | 3.2 (32.8%) | 73.5 (67.2%) | 50.4 |
| Cu–curcumin | 0.7 (35.5%) | 5.0 (25.5%) | 75.0 (39.0%) | 30.8 |
| Zn–curcumin | 0.7 (52.6%) | 7.5 (20.5%) | 78.9 (26.9%) | 23.0 |
| Curcumin–BQ | 0.7 (45.5%) | 5.0 (38.9%) | 75.0 (15.6%) | 14.0 |
The analysis regarding the stability and antioxidant activity of metallo–curcumin complexes in bio-compatible solvent water is essential for application purposes. In this regard, the UV-Vis peak maxima at 430 nm for curcumin in the presence and absence of metal ions were monitored in kinetic mode for 10 min (Fig. 3a) to establish aqueous stability of the complexes. All the solutions under consideration are clear and there is no scattering effect. Zn(II)–curcumin complex shows maximum stability followed by curcumin whereas Cu(II)–curcumin solution shows lowest stability. Fig. 3b demonstrates antioxidant activity of curcumin and metallo–curcumin complexes in dark under stirring condition. Antioxidant activities of the samples are monitored by the decolourization kinetics of stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) in ethanol–water mixture.39 DPPH, a violet coloured radical is reduced to DPPH2 which is yellow in colour due to donation of an H-atom from the polyphenolic antioxidant to the radical.40 The absorbance of all the samples is maintained to 0.1 at 430 nm and DPPH is added in such a manner so that the absorbance at 520 nm becomes 0.5 just after addition of DPPH which is much higher compared to self-absorption of the complexes at that particular wavelength. Moreover, the assays were performed under stirring conditions which suggest that there is no decrease in absorbance due to precipitation of the complexes. The free radical quenching kinetics data have been fitted with biexponential decay functions. The time constants are calculated to be 46.5 min, 38.5 min and 6.5 min for curcumin, Zn(II)–curcumin and Cu(II)–curcumin, respectively (Table 2). The increase in the radical scavenging activity for metallo–curcumin samples is clearly evident from Fig. 3b. The enhanced anti-oxidant property for Cu(II)–curcumin can be attributed to weaker ArO–H bond present in o-methoxy phenolic group (red circled in Scheme 1) and a consequent easier H-atom loss process. Due to the presence of stronger O–H bond in case of Zn(II) complex, the radical scavenging activity is many folds lesser compared to Cu(II)-complex.21b Furthermore, to confirm the reusability of the highly active radical scavenger Cu(II)–curcumin, recycling experiments have been performed. Three consecutive cycles were conducted (Fig. 3c) which show that the rate of radical scavenging remains almost constant indicating the high activity of Cu(II)–curcumin. Moreover, curcumin and Zn(II)–curcumin complex did not produce any such recyclability. This observation suggests that only for Cu(II)–curcumin complex, radical scavenging activity become rejuvenated after each cycle. Fig. 3c depicts that in the first cycle, the scavenging activity (57% DPPH degradation) is obtained in the presence of a very small amount of Cu(II)–curcumin (OD at 430 nm = 0.1), and the degradation become 41% after third cycle. The time constants are shown in Table 3. The results demonstrate that Cu(II)–curcumin serve as a highly effective recyclable free-radical scavenger than Zn(II)–curcumin and curcumin in water.
| Samples | t1 (min) | t2 (min) | tavg (min) |
|---|---|---|---|
| a Numbers in parentheses indicate relative contributions. | |||
| DPPH | 30.0 (25%) | 75.0 (75%) | 63.8 |
| Curcumin | 4.7 (36%) | 70.0 (64%) | 46.5 |
| Zn–curcumin | 4.1 (25%) | 50.0 (75%) | 38.5 |
| Cu–curcumin | 1.5 (46%) | 10.8 (54%) | 6.5 |
| Zn–curcumin + CuCl2 | 6.3 (27%) | 9.1 (73%) | 8.3 |
| Sample | Cycle | Efficiency | |||
|---|---|---|---|---|---|
| t1 (min) | t2 (min) | tavg (min) | Degradation percentage (%) | ||
| a Numbers in parentheses indicate relative contributions. | |||||
| Curcumin | 1st | 6.8 (28.6%) | 70.0 (71.4%) | 52.0 | 11.0 |
| 2nd | 7.8 (29.4%) | 70.5 (70.6%) | 52.1 | 7.0 | |
| 3rd | 6.5 (28.6%) | 71.0 (71.4%) | 52.6 | 6.0 | |
| Zn–curcumin | 1st | 4.6 (26.0%) | 50.3 (74.0%) | 38.5 | 28.0 |
| 2nd | 33.7 (80.4%) | 45.1 (19.6%) | 36.0 | 15.0 | |
| 3rd | 4.1 (18.0%) | 51.0 (82.0%) | 42.6 | 10.0 | |
| Cu–curcumin | 1st | 6.6 (50.0%) | 6.6 (50.0%) | 6.6 | 57.0 |
| 2nd | 6.9 (5.0%) | 9.9 (95.0%) | 9.8 | 41.0 | |
| 3rd | 9.0 (50.0%) | 9.0 (50.0%) | 9.1 | 41.0 | |
From Fig. 3, it is clear that Zn(II)–curcumin complex is more stable in water whereas the less stable Cu(II)–curcumin is more active. In order to clarify this issue, an alternative approach is followed. Before using as an antioxidant more stable Zn–curcumin should be kept for greater aqueous stability and then addition of CuCl2·2H2O followed by vortex for 2 h can replace Zn(II) by Cu(II) which eventually enhances the activity. Fig. 4a illustrate the visible colour change by addition of CuCl2·2H2O in Zn(II)-complex from orange to brown, indicating formation of Cu(II)-complex. Fig. 4b shows the UV-Vis spectra of Zn–curcumin after addition of CuCl2 solution, which is distinctly different from Zn(II)–curcumin and CuCl2·2H2O (data not shown) and more likely to the absorbance spectra of Cu(II)–curcumin complex. To evaluate the antioxidant properties, DPPH degradation kinetics was monitored. Fig. 4c indicates that there is a clear increase in antioxidant property compared to Zn–curcumin and CuCl2·2H2O (data not shown). The time constant is calculated to be 8.3 min (fitted with biexponential function) for the complex which is comparable to that of Cu(II)–curcumin (Table 2). This result is in agreement with the fact that Cu(II) has higher affinity for complexation than Zn(II), due to d-electron density distribution.36 Altogether, Fig. 4 suggests a simple methodology for both the enhancement of aqueous stability and antioxidant property in metallo–curcumin complexes.
In the view to investigate the anti-microbial action, highly active Cu(II)–curcumin was used as a potential antibacterial agent in dark for the inhibition of growth of Escherichia coli (E. coli). The upper panel of Fig. 5 shows picture of E. coli cultures treated with DMSO, Cu(OAc)2·H2O, curcumin and Cu(II)–curcumin in dark. The inhibition in growth of the bacterial culture for Cu(II)-complex is clearly visible. The culture treated with Cu(II)–curcumin complex contains a smaller number of colonies with respect to the control samples and cultures containing curcumin and Cu(OAc)2. In control and Cu(OAc)2 treated samples, the colony forming units (CFU) are almost similar. In case of curcumin treated samples, the bacterial growth was inhibited to 35% whereas maximum inhibition is obtained for Cu(II)–curcumin treated samples. The decrease of CFU is 60%. In order to explain the detailed mechanistic view of both antioxidant and antimicrobial action of Cu(II)–curcumin complex, the ROS generation was investigated directly by dichlorofluorescin–dichlorofluorescein (DCFH–DCF) conversion in aqueous medium. Nonfluorescent DCFH is a well-known ROS marker, which is oxidized to fluorescent DCF in presence of ROS.41 The emission intensity at 520 nm of DCF was monitored with time to evaluate the extent of ROS generation. Fig. 6a shows that there is maximum increase in fluorescence intensity in presence of Cu(II)–curcumin complex. Moreover no significant enhancement of fluorescence intensity was observed for both curcumin and its Zn(II) complex. To confirm the ROS generation, H2O2, which is a proper electron acceptor can act as a source of OH˙ radical was added to the system. This results in a huge enhancement of fluorescence intensity in case of the Cu(II)–curcumin complex (Fig. 6b). The enhancement of ROS formation can be anticipated with the chain reaction in presence of H2O2.42 The antioxidant effect of ROS generator Cu(II)–curcumin can be explained by the fact that the ROS influence the activity of electron releasing substituents present in the phenolic anti-oxidant like Cu(II)–curcumin (ArOH). This results in breaking of the O–H bond and release of hydrogen radical as reported in the earlier literature.9a,43 The hydrogen radicals can react with nucleophilic free-radicals like DPPH and quench their activity as shown in the following equation.
| ArOH + X˙ = ArO˙ + XH (X˙ = DPPH˙) |
 | ||
| Fig. 5 Bacteriological assay of cell, Cu-control, curcumin and Cu–curcumin in dark. The upper panel shows images of E. coli plates in presence of the corresponding samples. | ||
Thus Cu(II)–curcumin can act as a ROS generator as well as the by-product during ROS generation (H˙) can quench the free radicals to show antioxidant activity.
4. Conclusion
We have synthesized and optically characterized the metallo–curcumin complexes of Cu(II) and Zn(II). The femtosecond resolved upconversion technique confirms excited state electron transfer processes from the ligand curcumin to the metal ions. 2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay in dark proved the enhanced antioxidant property of curcumin by incorporation of Cu(II) ion. The rejuvenating antioxidant properties of Cu(II)–curcumin have been illustrated, which reveal recyclability and highly efficient antioxidant property of the complex. Moreover, the complex shows greater antimicrobial effect for the inhibition of E. coli growth compared to that of free curcumin. To get a more detailed mechanistic view, we performed dichlorofluorescin (DCFH) oxidation, which clearly shows ROS generation is more in case of Cu(II)–curcumin complexes. These results suggest that the presence of ROS induces the O–H bond breaking which is responsible for showing free-radical scavenging activity. Thus, the Cu(II)–curcumin complex behaves both as an anti-oxidant and anti-microbial agent. Moreover, to take care of both the factor of stability and activity of metallo–curcumin complexes, we propose a new method where aqueous stability is enhanced by Zn(II) complex and the activity can be increased by replacing Zn(II) with Cu(II) through a simple route prior to its use. The shift in absorbance spectra clearly demonstrates the replacement of metal ion at the reaction site. The overall observation is schematically represented in Scheme 3. We believe that our study will effectively lead to the development of multifunctional, stable metallo–curcumin complexes having excellent potential to be used for in vivo studies and as future medicines. | ||
| Scheme 3 Schematic representation of metal exchange process leading to duality in action: enhancement of both aqueous stability and anti-oxidant property. | ||
Acknowledgements
S. C. and S. C. thank CSIR (India) for a fellowship. N. P. thanks DST, India for Inspire Research Fellowship. We thank DST (India) for financial grants DST-TM-SERI-2k11-103 and SB-S1-PC-011-2013. We also thank DAE (India) for financial grant, 2013-37P-73-BRNS. PL thanks the NTH-School “Contacts in Nanosystems” and the Braunschweig International Graduate School of Metrology.References
- B. B. Aggarwal, A. Kumar and A. C. Bharti, Anticancer Res., 2003, 23, 363–398 CAS.
- (a) F. Yang, G. P. Lim, A. N. Begum, O. J. Ubeda, M. R. Simmons, S. S. Ambegaokar, P. P. Chen, R. Kayed, C. G. Glabe, S. A. Frautschy and G. M. Cole, J. Biol. Chem., 2005, 280, 5892–5901 CrossRef CAS PubMed; (b) T. Jiang, G.-R. Zhou, Y.-H. Zhang, P.-C. Sun, Q.-M. Du and P. Zhou, RSC Adv., 2012, 2, 9106–9113 RSC.
- C. D. Mock, B. C. Jordan and C. Selvam, RSC Adv., 2015, 5, 75575–75588 RSC.
- (a) K. I. Priyadarsini, Molecules, 2014, 19, 20091–20112 CrossRef PubMed; (b) M. Salem, S. Rohani and E. R. Gillies, RSC Adv., 2014, 4, 10815–10829 RSC.
- M. Tan, J. Luo and Y. Tian, RSC Adv., 2014, 4, 61948–61959 RSC.
- T. Esatbeyoglu, P. Huebbe, I. M. Ernst, D. Chin, A. E. Wagner and G. Rimbach, Angew. Chem., Int. Ed. Engl., 2012, 51, 5308–5332 CrossRef CAS PubMed.
- J. Wang, H. Wang, R. Zhu, Q. Liu, J. Fei and S. Wang, Biomaterials, 2015, 53, 475–483 CrossRef CAS PubMed.
- L. R. Barclay, M. R. Vinqvist, K. Mukai, H. Goto, Y. Hashimoto, A. Tokunaga and H. Uno, Org. Lett., 2000, 2, 2841–2843 CrossRef CAS PubMed.
- (a) G. Litwinienko and K. U. Ingold, J. Org. Chem., 2004, 69, 5888–5896 CrossRef CAS PubMed; (b) S. V. Jovanovic, C. W. Boone, S. Steenken, M. Trinoga and R. B. Kaskey, J. Am. Chem. Soc., 2001, 123, 3064–3068 CrossRef CAS PubMed.
- B. B. Aggarwal and B. Sung, Trends Pharmacol. Sci., 2009, 30, 85–94 CrossRef CAS PubMed.
- K. I. Priyadarsini, Curr. Pharm. Des., 2013, 19, 2093–2100 CAS.
- L. Nardo, R. Paderno, A. Andreoni, M. Másson, T. Haukvik and H. H. TØnnesen, Spectroscopy, 2008, 22, 187–198 CrossRef CAS.
- K. Bairwa, J. Grover, M. Kania and S. M. Jachak, RSC Adv., 2014, 4, 13946–13978 RSC.
- P. Basnet and N. Skalko-Basnet, Molecules, 2011, 16, 4567–4598 CrossRef CAS PubMed.
- H. Hatcher, R. Planalp, J. Cho, F. M. Torti and S. V. Torti, Cell. Mol. Life Sci., 2008, 65, 1631–1652 CrossRef CAS PubMed.
- C. Banerjee, S. Maiti, M. Mustafi, J. Kuchlyan, D. Banik, N. Kundu, D. Dhara and N. Sarkar, Langmuir, 2014, 30, 10834–10844 CrossRef CAS PubMed.
- D. K. Singh, R. Jagannathan, P. Khandelwal, P. M. Abraham and P. Poddar, Nanoscale, 2013, 5, 1882–1893 RSC.
- S. Kundu and U. Nithiyanantham, RSC Adv., 2013, 3, 25278–25290 RSC.
- S. Bisht, G. Feldmann, S. Soni, R. Ravi, C. Karikar, A. Maitra and A. Maitra, J. Nanobiotechnol., 2007, 5, 1–18 CrossRef PubMed.
- X. Fang, L. Fang, S. Gou and L. Cheng, Bioorg. Med. Chem. Lett., 2013, 23, 1297–1301 CrossRef CAS PubMed.
- (a) S. Wanninger, V. Lorenz, A. Subhan and F. T. Edelmann, Chem. Soc. Rev., 2015, 44, 4986–5002 RSC; (b) X.-Z. Zhao, T. Jiang, L. Wang, H. Yang, S. Zhang and P. Zhou, J. Mol. Struct., 2010, 984, 316–325 CrossRef CAS; (c) T. M. Kolev, E. A. Velcheva, B. A. Stamboliyska and M. Spiteller, Int. J. Quantum Chem., 2005, 102, 1069–1079 CrossRef CAS; (d) S. Sardar, S. Sarkar, M. T. Myint, S. Al-Harthi, J. Dutta and S. K. Pal, Phys. Chem. Chem. Phys., 2013, 15, 18562–18570 RSC; (e) P. Kar, S. Sardar, E. Alarousu, J. Sun, Z. S. Seddigi, S. A. Ahmed, E. Y. Danish, O. F. Mohammed and S. K. Pal, Chem.–Eur. J., 2014, 20, 10475–10483 CrossRef CAS PubMed.
- D. Pucci, A. Crispini, B. S. Mendiguchia, S. Pirillo, M. Ghedini, S. Morelli and L. De Bartolo, Dalton Trans., 2013, 42, 9679–9687 RSC.
- E. Ferrari, R. Benassi, S. Sacchi, F. Pignedoli, M. Asti and M. Saladini, J. Inorg. Biochem., 2014, 139, 38–48 CrossRef CAS PubMed.
- B. Zebib, Z. Mouloungui and V. Noirot, Bioinorg. Chem. Appl., 2010, 2010, 292760 Search PubMed.
- S. Daniel, J. L. Limson, A. Dairam, G. M. Watkins and S. Daya, J. Inorg. Biochem., 2004, 98, 266–275 CrossRef CAS PubMed.
- A. Valentini, F. Conforti, A. Crispini, A. De Martino, R. Condello, C. Stellitano, G. Rotilio, M. Ghedini, G. Federici, S. Bernardini and D. Pucci, J. Med. Chem., 2009, 52, 484–491 CrossRef CAS PubMed.
- (a) M. A. Addicoat, G. F. Metha and T. W. Kee, J. Comput. Chem., 2011, 32, 429–438 CrossRef CAS PubMed; (b) M. H. Leung, D. T. Pham, S. F. Lincoln and T. W. Kee, Phys. Chem. Chem. Phys., 2012, 14, 13580–13587 RSC.
- V. D. John, G. Kuttan and K. Krishnankutty, J. Exp. Clin. Cancer Res., 2002, 21, 219–224 CAS.
- L. Rossi, S. Mazzitelli, M. Arciello, C. R. Capo and G. Rotilio, Neurochem. Res., 2008, 33, 2390–2400 CrossRef CAS PubMed.
- A. Barik, B. Mishra, A. Kunwar, R. M. Kadam, L. Shen, S. Dutta, S. Padhye, A. K. Satpati, H. Y. Zhang and K. Indira Priyadarsini, Eur. J. Med. Chem., 2007, 42, 431–439 CrossRef CAS PubMed.
- X. Mei, D. Xu, S. Xu, Y. Zheng and S. Xu, Chem.–Biol. Interact., 2012, 197, 31–39 CrossRef CAS PubMed.
- H. Ahsan, N. Parveen, N. U. Khan and S. M. Hadi, Chem.–Biol. Interact., 1999, 121, 161–175 CrossRef CAS PubMed.
- R. Hariharan, S. Senthilkumar, A. Suganthi and M. Rajarajan, Mater. Res. Bull., 2012, 47, 3090–3099 CrossRef CAS.
- (a) R. Saha, P. K. Verma, S. Rakshit, S. Saha, S. Mayor and S. K. Pal, Sci. Rep., 2013, 3, 1580 Search PubMed; (b) S. Choudhury, P. K. Mondal, V. K. Sharma, S. Mitra, V. G. Sakai, R. Mukhopadhyay and S. K. Pal, J. Phys. Chem. B, 2015, 10849–10857 CrossRef CAS PubMed.
- S. Sardar, S. Chaudhuri, P. Kar, S. Sarkar, P. Lemmens and S. K. Pal, Phys. Chem. Chem. Phys., 2015, 17, 166–177 RSC.
- G. B. Kaufman, J. Chem. Educ., 1993, 70, A279 CrossRef.
- A. Giri, N. Goswami, C. Sasmal, N. Polley, D. Majumdar, S. Sarkar, S. N. Bandyopadhyay, A. Singha and S. K. Pal, RSC Adv., 2014, 4, 5075–5079 RSC.
- R. Adhikary, P. Mukherjee, T. W. Kee and J. W. Petrich, J. Phys. Chem. B, 2009, 113, 5255–5261 CrossRef CAS PubMed.
- S. Chaudhuri, S. Batabyal, N. Polley and S. K. Pal, J. Phys. Chem. A, 2014, 118, 3934–3943 CrossRef CAS PubMed.
- S. Chaudhuri, S. Sardar, D. Bagchi, S. S. Singha, P. Lemmens and S. K. Pal, J. Phys. Chem. A, 2015, 119, 4162–4169 CrossRef CAS PubMed.
- C. P. LeBel, H. Ischiropoulos and S. C. Bondy, Chem. Res. Toxicol., 1992, 5, 227–231 CrossRef CAS PubMed.
- J. Nakamura, E. R. Purvis and J. A. Swenberg, Nucleic Acids Res., 2003, 31, 1790–1795 CrossRef CAS PubMed.
- P. Malik and T. K. Mukherjee, Chin. J. Biol., 2014, 2014, 396708 Search PubMed.
| This journal is © The Royal Society of Chemistry 2015 |