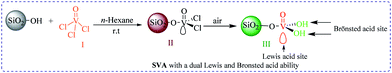
Silica vanadic acid [SiO2–VO(OH)2] as an efficient heterogeneous catalyst for the synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and 2,4,6-triarylpyridine derivatives via anomeric based oxidation†
Mohammad Ali Zolfigol*a,
Maliheh Safaieeb,
Fatemeh Afsharnaderya,
Neda Bahrami-Nejada,
Saeed Bagherya,
Sadegh Salehzadeha and
Farahnaz Malekia
aDepartment of Organic Chemistry, Faculty of Chemistry, Bu-Ali Sina University, Hamedan 6517838683, Iran. E-mail: zolfi@basu.ac.ir; mzolfigol@yahoo.com; Fax: +98 8138380709; Tel: +98 8138282807
bDepartment of Aromatic and Medicinal Plants, Nahavand University, P.O. Box 6591678546, Nahavand, Iran
First published on 18th November 2015
Abstract
Silica-bonded vanadic acid [SiO2–VO(OH)2] (SVA) efficiently catalyzed the synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-ones via reacting aromatic aldehydes and β-naphthol and urea under solvent-free condition. Additionally, 2,4,6-triarylpyridine derivatives were prepared through the condensation of ammonium acetate, aromatic aldehyde and various acetophenone in the presence of a catalytic amount of [SiO2–VO(OH)2] (SVA) under a same condition. A new anomeric based oxidation was proposed for the final step of the 2,4,6-triarylpyridine derivatives synthesis which was supported by theoretical studies. So the described new mechanistic approach can open up a new and promising insight in the course of rational design, synthesis and applications of novel hydride releasing compounds. The significant features of the present procedure are short reaction time, high yields, easy work-up, and recyclability of the SVA catalyst.
Introduction
Solid acids have been used as catalysts in various functional groups transformations because catalysis has played a major role in preventing pollution in our environment. Recently, a wide range of solid acids and their supported forms reported in the last few years for various chemical processes have been reviewed.1 Owing to their possible utilizations as an alternative for liquid inorganic acids in industry, solid acid catalysts have received main attention, they reveal the advantages of easy recycle of the catalyst from the liquid reaction media, reusability, least corrosion, green chemical procedure, and increase product selectivity.2–5Molecules with oxazine moieties have significant biological properties, counting anti-convulsant, anti-bacterial, anti-tubercular, analgesic and anti-cancer,6–11 and possess attracted far more attention because of their therapeutic potential for the treatment of Parkinson's illness.12,13 Moreover, they can be applied as intermediates in the preparation of N-substituted amino alcohols or in enantioselective syntheses of chiral amines. The tautomeric character of 1,3-O,N-heterocycles suggestions a great number of synthetic potentials.14–16 [BMIM]HSO4 as an ionic liquid, n-tetrabutylammonium bromide as a phase transfer catalyst in water and sodium hydrogen sulfate (NaHSO4) have been applied as mild catalysts for the cyclocondensation of formalin, β-naphthol and aromatic amines to give the corresponding 2,3-dihydro-2-phenyl-1H-naphth[1,2–e][1,3]oxazines.17 Furthermore, using the three-component system (β-naphthol, benzaldehyde and urea) using HClO4–SiO2.18 Additional reports exhibited the condensation of β-naphthol with hetero aryl aldehydes or substituted benzaldehydes using ammonia.19
Pyridine ring system, mainly 2,4,6-triarylpyridine is of enormous importance due to its unique position in medicinal chemistry.20,21 Moreover, the superb thermal stabilities of these pyridines have prompted a developing attention for their use as monomeric building blocks in thin films and organometallic polymers.22 These compounds have also been evoked significant consideration recently as these donated with broad range of pharmaceutical activities for example anesthetic, vasodilator, anti-convulsant, antimalarial, antiepileptic and agrochemicals for example pesticidal, fungicidal and herbicidal.21 These compounds have now been prepared through reaction of N-phenacylpyridinium salts with α,β-unsaturated ketones using ammonium acetate.23,24 Though, the pyridinium salts and the unsaturated ketones have to be prepared first, so this process is relatively expensive. Lately, numerous novel developed approaches and processes for preparation of 2,4,6-triarylpyridines have been studied, such as reaction of α-ketoketene dithioacetals with methyl ketones using ammonium acetate,25 solvent-free reaction of chalcones with NH4OAc,26 reaction of N-phosphinylethanimines with aldehydes,27 solvent-free reaction of benzaldehydes, acetophenones and NH4OAc using several catalysts, such as I2,28 HClO4–SiO2,29 Preyssler type heteropolyacid30 and the one-pot reaction of benzaldehydes, acetophenones and NH4OAc under catalyst-free microwave irradiation.31
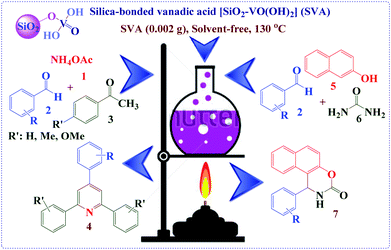
Afterward, to overcome those difficulties and in our continuous attention to the design, synthesis, applications and knowledge-based development of solid acids,32 inorganic acid salts,33 nano-sphere silica with acidic ionic liquid tag,34 novel nano structure, green and benign acidic ionic liquids, molten salts and organo catalysts for organic functional group transformations,35 and also our investigations in the synthesis of aromatic-condensed oxazinones and 2,4,6-triarylpyridine derivatives,36 we herein consider an suitable approach for silica-bonded vanadic acid [SiO2–VO(OH)2] (SVA)37 as an effective catalyst for the preparation of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and 2,4,6-triarylpyridine derivatives under solvent-free conditions (Scheme 1).
 | ||
| Scheme 1 The synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one and 2,4,6-triarylpyridine derivatives in the presence of silica vanadic acid [SiO2–VO(OH)2] (SVA) catalyst. | ||
Results and discussion
In continuation of our studies on the knowledge-based development of new silica-based resins,32 we synthesized SVA according to the our previously reported procedure as follow (Scheme 2).37Initially, to optimize the reaction conditions, the condensation reaction of 4-chlorobenzaldehyde, 4-methoxyacetophenone and ammonium acetate were selected as a model reaction and different amounts of [SiO2–VO(OH)2] as a catalyst at temperature range of 25–150 °C were verified on it under solvent-free conditions (Table 1). As revealed in Table 1, the best results were achieved when the reaction was attained using 2 mg of SVA catalyst at 130 °C (Table 1, entry 10). No improvement was known in the yield of reaction through increasing the amount of catalyst and temperature (Table 1, entries 11–14). Table 1 visibly exhibitions that in the absence of catalyst, the product was in low yield synthesized (Table 1, entries 1 and 2). An excess of the ammonium acetate was established to be satisfactory and hence the molar ratio of 4-chlorobenzaldehyde and 4-methoxyacetophenone to ammonium acetate was studied at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.
5.
| Entry | Catalyst amount (mg) | Reaction temperature (°C) | Reaction time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1 mmol), 4-methoxyacetophenone (2 mmol), ammonium acetate (5 mmol).b Isolated yields. | ||||
| 1 | — | 100 | 120 | 21 |
| 2 | — | 130 | 120 | 33 |
| 3 | 1 | 100 | 120 | 45 |
| 4 | 1 | 130 | 120 | 57 |
| 5 | 2 | r.t. | 120 | — |
| 6 | 2 | 50 | 120 | 18 |
| 7 | 2 | 75 | 120 | 43 |
| 8 | 2 | 100 | 120 | 75 |
| 9 | 2 | 125 | 60 | 88 |
| 10 | 2 | 130 | 45 | 88 |
| 11 | 2 | 150 | 45 | 88 |
| 12 | 5 | 100 | 120 | 75 |
| 13 | 5 | 130 | 45 | 88 |
| 14 | 5 | 150 | 45 | 88 |
To compare the result of the solution in comparison with solvent-free conditions, a mixture of 4-chlorobenzaldehyde, 4-methoxyacetophenone and ammonium acetate as a typical reaction, in the presence of 2 mg of [SiO2–VO(OH)2] as a catalyst in several solvents for example H2O, C2H5OH, CH3CN, CH3CO2Et, CH2Cl2, n-hexane and toluene was studied under reflux conditions. Moreover, reaction was done under solvent-free condition at 130 °C. The results are reported in Table 2. As it can be exposed in Table 2, solvent-free condition was the best condition for this purpose.
| Entry | Solvent | Reaction time (min) | Yieldb (%) |
|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1 mmol), 4-methoxyacetophenone (2 mmol), ammonium acetate (5 mmol).b Isolated yields. | |||
| 1 | Solvent-free | 45 | 88 |
| 2 | H2O | 60 | 45 |
| 3 | C2H5OH | 45 | 73 |
| 4 | CH3CN | 60 | 73 |
| 5 | CH3CO2Et | 60 | 67 |
| 6 | CH2Cl2 | 60 | 58 |
| 7 | n-Hexane | 60 | 65 |
| 8 | Toluene | 60 | 63 |
Subsequently recognizing the optimized reaction conditions, the investigation was followed by performing the reaction of aromatic aldehydes with acetophenones and ammonium acetate. To demonstrate the general applicability of this process, several aldehydes were efficiently reacted with acetophenones and ammonium acetate under similar conditions. These results encouraged us to investigate the scope and the outline of this procedure for numerous aldehydes under optimized conditions. As shown in Table 3, a wide range of aromatic aldehydes underwent electrophilic substitution reaction with acetophenones and ammonium acetate to afford various substituted 2,4,6-triarylpyridines in good yields. The nature and electronic properties of the substituents on the aromatic ring effect the alteration rate, and aromatic aldehydes having electron-withdrawing groups on the aromatic ring react faster than electron-donating groups. Also, according to the reaction time and isolated yields, the order of reactivity in the acetophenones are as follows: 4-methoxyacetophenone > 4-methylacetophenone > acetophenone. In order to evaluate the efficiency of SVA catalyst on the synthesis of 2,4,6-triarylpyridines TOF values were calculated via the equation TOF = yield (%)/[time (min) × catalyst amount (mol%)] (2 mg of catalyst is equivalent to 0.0156 mol% of catalyst37).
| Entry | Aldehyde | Ketone | Time (min) | Yieldb (%) | TOF | Mp (°C) [lit.]Ref. |
|---|---|---|---|---|---|---|
| a Reaction conditions: aldehydes (1 mmol), acetophenones (2 mmol), ammonium acetate (5 mmol), [SiO2–VO(OH)2] as a catalyst (2 mg), solvent-free, 130 °C.b Isolated yields. | ||||||
| 1 | 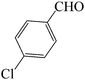 |
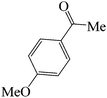 |
45 | 88 | 125.36 | 110–112 [113.8–115]38a (yellow solid) |
| 2 | 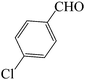 |
 |
45 | 85 | 121.08 | 198–200 [200.6–202]31 (yellow solid) |
| 3 | 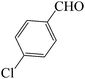 |
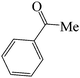 |
45 | 81 | 115.38 | 133–135 [129–130]27 (yellow solid) |
| 4 | 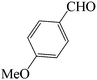 |
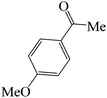 |
60 | 83 | 88.68 | 135–137 [136–137]38b (yellow solid) |
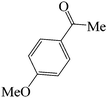
| 5 | 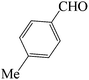 |
 |
60 | 82 | 87.61 | 175–177 [178–180]38c (yellow solid) |
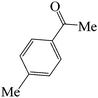
| 6 | 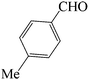 |
 |
60 | 82 | 87.61 | 153–155 (ref. 38d) (yellow solid) |
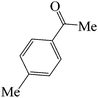
| 7 | 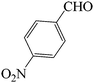 |
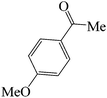 |
45 | 88 | 125.36 | 140–142 [143.1–144.7]31 (yellow solid) |
| 8 | 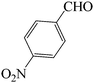 |
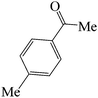 |
45 | 85 | 121.08 | 233–235 (green solid) |
| 9 | 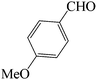 |
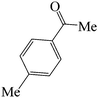 |
60 | 81 | 86.54 | 153–155 [156–157.5]38e (yellow solid) |
| 10 | 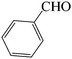 |
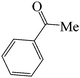 |
60 | 81 | 86.54 | 136–138 [134–135]26 (white solid) |
According to the previously reported method, a reasonable mechanism for the preparation of 2,4,6-triarylpyridines using [SiO2–VO(OH)2] as a catalyst was suggested (Scheme 3).39 Firstly, acetophenone 3 using SVA as a catalyst is converted into its enol form 10 (via tautomerization), which affords nucleophilic addition on the intermediate 8 (SVA as a catalyst activates the carbonyl group of the aromatic aldehyde 2 to give intermediate 8) to give chalcone product 11 (via aldol condensation). Then second molecule of enol form 10 undergoes Michael addition reaction with 11 to form the 1,5-diketone intermediate 12. This 1,5-diketone 12 on reaction with ammonium acetate followed by cyclization gives intermediate 13 and its dehydration affords intermediate 14. Previously reported studies have been proposed aerobic auto oxidation of intermediate (15) to its corresponding 2,4,6-triarylpyridines (4). In contrast to the previously reported mechanistic description for the final step of the above described organic synthesis,28–31,38,39 we believed that, this step might be progress through uncommon hydride transfer as well as Cannizzaro reaction (Scheme 4)40 and H2 releasing from tricyclic orthoamide which presented in Scheme 5.41 Very recently, we proposed an anomeric based oxidation for the final step in mechanistic pathways of 1,4-dihydropyrano-[2,3-c]-pyrazole derivatives synthesis (Scheme 6).42 For improving of this idea, reaction was occurred under nitrogen atmosphere and in the absence of any molecular oxygen. It was detected that, the reaction progressed under atmosphere of nitrogen as well as normal reaction condition using oxygen. By considering the above-mentioned evidence, conversion of intermediate (15) to its corresponding 2,4,6-triarylpyridines (4) might be happened through uncommon hydride transfer and releasing of molecular hydrogen (H2). The C–H bond is so weakend via electron donation from the nitrogen lone pairs into the anti-bonding of C–H (σ*C–H orbital) which it can be broken by reaction with a proton to give molecular hydrogen. As we know, this phenomenon has been named as anomeric effect. As it can be seen in the proposed mechanism, the chalcone intermediate (11) is performed in the route of reaction, which is stable and considered as a by-product. Excess ammonium acetate is used to convert chalcone into 2,4,6-triarylpyridine derivatives (4) and to improve reaction yields. Also, because of high temperature of reaction (considering conversion of ammonium acetate in the route of reaction to ammonia and acetic acid) it is used to prevent evaporation and elimination of ammonia to improve reaction yields.
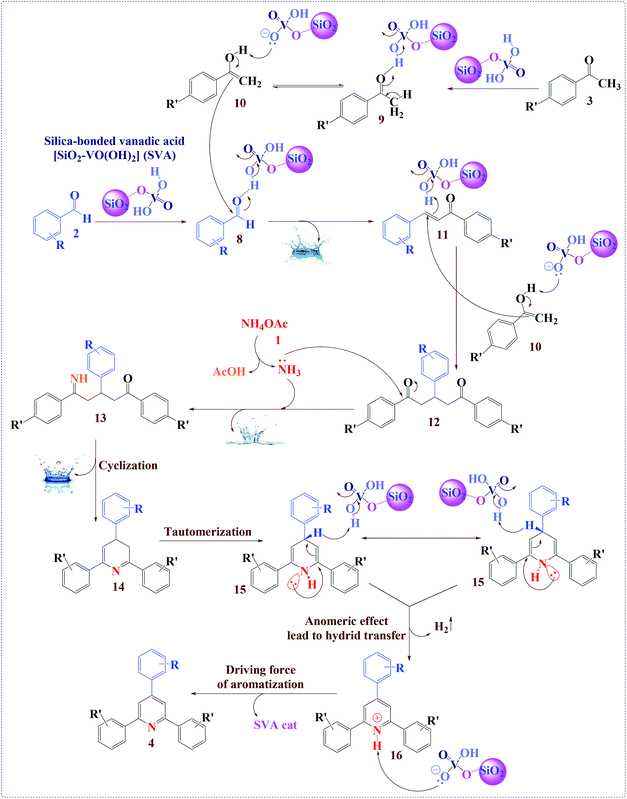 | ||
| Scheme 3 The proposed mechanism for the synthesis of 2,4,6-triarylpyridines using [SiO2–VO(OH)2] as a catalyst. | ||
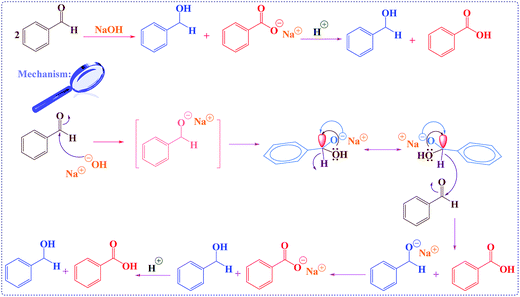 | ||
| Scheme 4 The proposed mechanism for the in situ oxidation-reduction in Cannizzaro reaction through unusual hydride transfer via anomeric effect.40 | ||
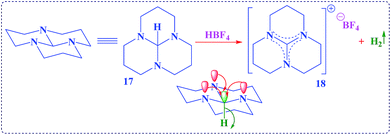 | ||
| Scheme 5 A striking example which had been observed for an unusual hydride transfer from tricyclic orthoamide (16) through anomeric effect.41 | ||
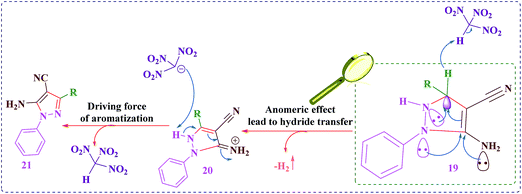 | ||
| Scheme 6 An anomeric based oxidation for the final step in mechanistic pathways of 1,4-dihydropyrano-[2,3-c]-pyrazole derivatives synthesis.42 | ||
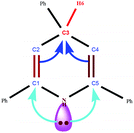
Recently, Taherpour et al. reported theoretical studies on the rotamers and dynamic behaviors dihydropyridines.43 Herein, in order to prove the above suggested mechanism we performed a DFT calculation. Starting from intermediate 15, different orientations of two H atoms attached to N1 and C4 atoms on 2,4,6-triarylpyridine ring with respect to each other give cis- and trans-like isomers. Our calculations show that the trans-like isomer is about 0.14 kcal mol−1 more stable than cis one. As illustrated in Fig. 1, by addition of SVA catalyst and through the formation of transition structures, TS-1 and TS-2, and then removing the molecular hydrogen (H2) the zwitterionic intermediate 16 will be formed. According to values of calculated Gibbs free energies, this reaction is about −13.18 kcal mol−1 exothermic for trans isomer. There are two pathways that lead to the formation of intermediate 16. Fig. 1 indicates that these to be centered on the stereo orientation of SVA catalyst with intermediate 15, which in turn leads to formation of TS-1 and TS-2 structures. The TS-1 pathway independent of orientation of substituents of 15 are plausible for both cis and trans isomers (ΔG‡ is 39.59 and 39.73 kcal mol−1 for cis and trans isomers, respectively). The weak hydrogen bond between the oxygen atom of SVA catalyst and the H atom attached to atom N1 of triarylpyridine ring in TS-2 (see Fig. 2) allows a low-energy pathway to complete removal of H2, starting just from cis isomer. So TS-2 pathway by ΔG‡ = 36.50 kcal mol−1 is more favorable pathway. The important step of this newly proposed mechanism is the direct elimination of H2 molecule from 15 and SVA catalyst via both TS-1 and TS-2 (Fig. 1) in which the C–H bond in compound 15 is so weakened due to the delocalization of π electron of adjacent C–C double bond to σ* C–H bond (the anomeric effect) and therefore the activation energy is relatively small. The NBO analysis of donor–acceptor interactions identified that the anomeric effect due to delocalization of π electron of C–C double bond to σ* C–H bond for intermediate 15 is 6.56 and 4.84 kcal mol−1 for cis and trans isomers, respectively.
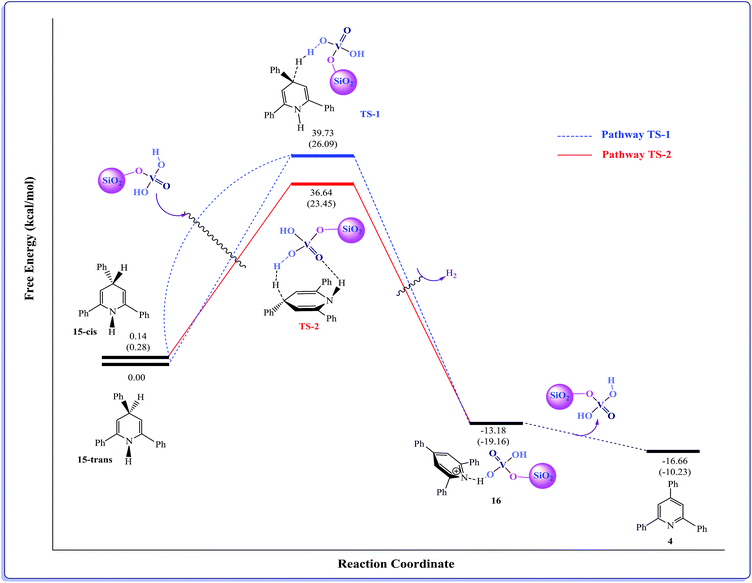 | ||
| Fig. 1 Energy profile calculated for synthesis of 2,4,6-triarylpyridine (4) beginning from compound 15 (see Scheme 3). The relative Gibbs free energies and the total electronic energies (Eel + ZPE, figures in parentheses) obtained from the B3LYP/SVP calculations both are given in kcal mol−1. In the calculations, we used Si(OH)3 group as a model for silica moiety. | ||
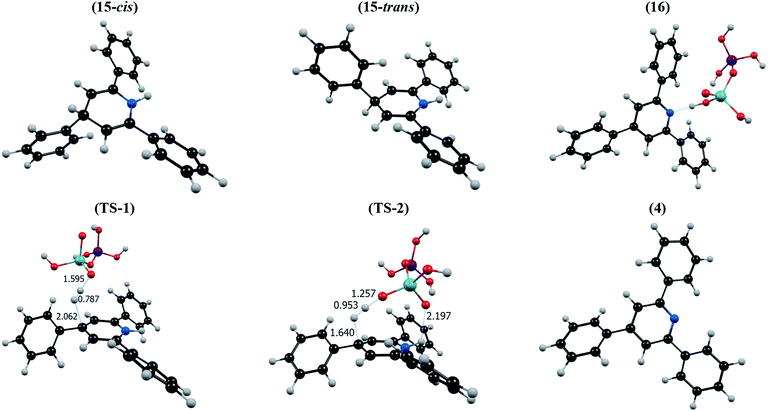 | ||
| Fig. 2 The optimized structure of all compounds involved in conversion of 15 to 4 according to the mechanism suggested in Fig. 1. | ||
The summation of the different delocalizations from the lone pair of nitrogen atom to σ* and π* of C–C double bond is 57.78 and 57.60 kcal mol−1 for cis and trans isomers, respectively. The main donor–acceptor interaction of 2,4,6-triarylpyridine ring for both cis and trans isomers of intermediate 15 is shown in Table 4.
In the final step of the reaction the zwitterionic intermediate 16 converts to 2,4,6-triarylpyridine (4) through an exothermic process (ΔG = −3.48 kcal mol−1). In conclusion, the whole process of conversion of 15 to 4 through the releasing molecular hydrogen is exothermic (ΔG is −16.80 and −16.66 kcal mol−1 for cis and trans isomers, respectively). Thus the above theoretical studies, similar to our previous work,42 support our suggested mechanism and shows that releasing molecular hydrogen (H2) is quite possible in such systems. The optimized structures of all compounds involved in our suggested mechanism are shown in Fig. 2.
It should be noted that in the above calculations, the solvent effect was not investigated, because the reaction has been performed under solvent-free conditions.
Furthermore, reusability of the [SiO2–VO(OH)2] as a catalyst was confirmed via its recycling and reusing in condensation of 4-chlorobenzaldehyde, 4-methoxyacetophenone and ammonium acetate. At the end of the reaction, diethyl ether was added to the reaction mixture to extract product from SVA catalyst. Also, the catalyst was filtered and washed with CH3CN. It has been known that the catalytic activity of the catalyst was restored within the limits of the experimental errors for seven continuous runs (Fig. 3). Deactivation of the SVA catalyst is low, while reactant was anticipated. The reaction was scaled up to 10 mmol of 4-chlorobenzaldehyde, 4-methoxyacetophenone and ammonium acetate in the presence of 20 mg of SVA catalyst at 130 °C. The yield of the reaction was 88% in 45 minutes and 79% after the seventh run. The results were summarized in Fig. 3.
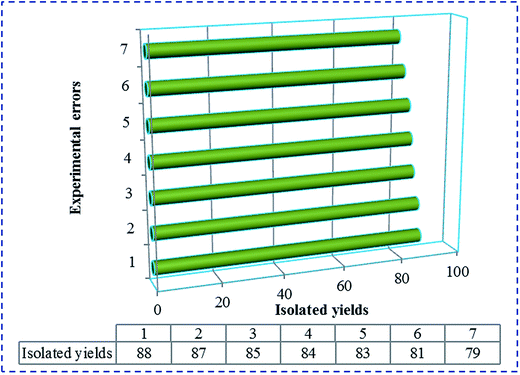 | ||
| Fig. 3 Reusability of [SiO2–VO(OH)2] as a catalyst in the synthesis of 2,4,6-triarylpyridine in 45 minutes. | ||
Subsequently the synthesis of 2,4,6-triarylpyridines, in the additional study for the synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one derivatives, to optimize the reaction conditions, the condensation reaction of 4-chlorobenzaldehyde, β-naphthol and urea was chose as a typical reaction and different amounts of [SiO2–VO(OH)2] catalyst at temperature range of 25–150 °C were used under solvent-free conditions (Table 5). As exposed in Table 5, the best results were attained when the reaction was achieved using 2 mg of SVA catalyst at 130 °C (Table 5, entry 10). No improvement was recognized in the yield of reaction via increasing the amount of the SVA catalyst and the temperature (Table 5, entries 11–14). Table 5 clearly exhibits that in the absence of catalyst, the product was not formed (Table 5, entries 1 and 2). A slight excess of the urea was recognized to be acceptable and hence the molar ratio of aromatic aldehyde and β-naphthol to urea was studied at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2.
1.2.
| Entry | Catalyst amount (mg) | Reaction temperature (°C) | Reaction time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 4-chlorobenzaldehyde (1 mmol), β-naphthol (1 mmol), urea (1.2 mmol).b Isolated yield. | ||||
| 1 | — | 100 | 120 | — |
| 2 | — | 130 | 120 | — |
| 3 | 1 | 100 | 120 | 31 |
| 4 | 1 | 130 | 120 | 49 |
| 5 | 2 | r.t. | 120 | — |
| 6 | 2 | 50 | 120 | — |
| 7 | 2 | 75 | 120 | — |
| 8 | 2 | 100 | 120 | 42 |
| 9 | 2 | 125 | 60 | 87 |
| 10 | 2 | 130 | 45 | 88 |
| 11 | 2 | 150 | 45 | 88 |
| 12 | 5 | 100 | 120 | 42 |
| 13 | 5 | 130 | 45 | 88 |
| 14 | 5 | 150 | 45 | 88 |
To compare the proficiency of the solution versus solvent-free conditions, a mixture of 4-chlorobenzaldehyde, β-naphthol and urea as model reaction, using 2 mg of SVA as a catalyst in various solvents for example H2O, C2H5OH, CH3CN, CH3CO2Et and CH2Cl2 was tested under reflux conditions. Also reaction was done under solvent-free condition at 130 °C. The results are summarized in Table 6. As it can be recognized in Table 6, solvent-free condition was the best conditions in this reaction.
Consequently optimization of the reaction conditions, to consider the efficiency and the scope of the presented procedure, many 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one derivatives were prepared via the one-pot three-component condensation reaction between aromatic aldehydes with β-naphthol and urea using catalytic amounts of [SiO2–VO(OH)2] as a catalyst under solvent-free reaction conditions. The results have been shown in Table 7. The effect of substituents on the aromatic ring was assessed strong effects in terms of yields under described reaction conditions. Both class of aromatic aldehydes containing electron-releasing, electron-withdrawing substituents on their aromatic ring gained the suitable products with high yields. The reaction times of aromatic aldehydes having electron withdrawing groups were rather faster than electron donating groups.
| Entry | Aldehyde | Time (min) | Yieldb (%) | TOF | Mp (°C) [lit.]Ref. |
|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (1 mmol), β-naphthol (1 mmol), urea (1.2 mmol), [SiO2–VO(OH)2] as a catalyst (2 mg), solvent-free, 130 °C.b Isolated yields. | |||||
| 1 | 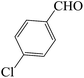 |
45 | 88 | 125.36 | 208–210 [209–212]44a (yellow solid) |
| 2 | 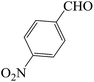 |
45 | 88 | 125.36 | 169–171 [172–174]44b (yellow solid) |
| 3 | 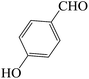 |
60 | 81 | 86.54 | 181–183 [180]44c (red solid) |
| 4 | 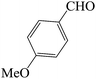 |
60 | 83 | 88.68 | 182–184 [185–187]44a (yellow solid) |
| 5 | 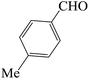 |
60 | 82 | 87.61 | 169–171 [170–172]44b (white solid) |
| 6 | 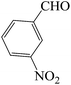 |
45 | 84 | 89.74 | 224–226 [226–227]44d (brown solid) |
| 7 | 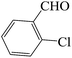 |
45 | 82 | 116.81 | 253–255 [251–253]44e (yellow solid) |
| 8 | 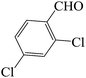 |
45 | 83 | 118.23 | 211–213 [214–217]44a (yellow solid) |
| 9 | 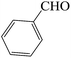 |
60 | 82 | 116.81 | 217–219 [218–220]44c (yellow solid) |
| 10 | 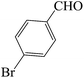 |
45 | 87 | 123.93 | 218–220 [220–223]44c (white solid) |
It is obvious that the SVA catalyst activates the carbonyl group of the aromatic aldehyde 2 to give intermediate 8. Therefore, the reaction firstly includes the creation of ‘fitted ion pair complex’ intermediate 8 between SVA catalyst (because of proton releasing of SVA) and non-bonded electrons on the oxygen of the carbonyl group of the aldehydes 2. Consequently, carbon of the carbonyl group becomes more electrophilic toward nucleophile for the attack via π electrons of β-naphthol 5. Later, the attack of π electrons of β-naphthol 5 happens more easily to form adduct 22 which loses one water molecule and results in intermediate o-QMs: 23 which further involvements nucleophilic attack of NH2 of urea to form adduct 24. After again the ‘ion pair complex’ made because of the coordination of proton and oxygen of the urea carbonyl favors the nucleophilic attack of electrons on the phenolic OH group and results into the creation of the analogous compounds 7, which was represented in Scheme 7.44a,45
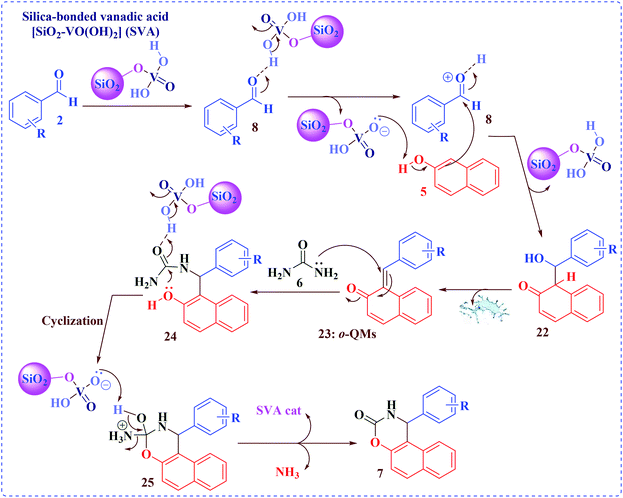 | ||
| Scheme 7 The plausible mechanism for the synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one derivatives using [SiO2–VO(OH)2] as a catalyst. | ||
Additionally, reusability of the SVA catalyst was studied in the condensation of 4-chlorobenzaldehyde, β-naphthol and urea as a typical reaction. At the end of the reaction, acetonitrile was added to the reaction mixture and heated to extract product from SVA catalyst. Also, the catalyst was filtered and washed with CH3CN. It has been identified that the catalytic activity of the catalyst was restored within the limits of the experimental errors and negligible loosing catalytic activities for seven continuous runs (Fig. 4). Deactivation of the SVA catalyst is low, while reactant was anticipated. The reaction was scaled up to 10 mmol of 4-chlorobenzaldehyde, β-naphthol and urea using 20 mg of SVA catalyst at 130 °C. The yield of the reaction was 88% in 45 minutes and 79% after the seventh run. The results were summarized in Fig. 4.
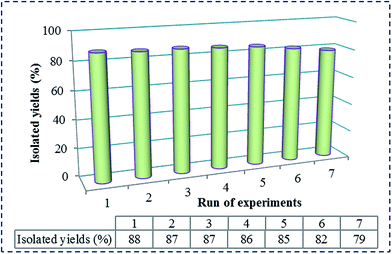 | ||
| Fig. 4 Reusability of [SiO2–VO(OH)2] as a catalyst on the synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one in 45 minutes. | ||
In continuation of our consider into the use of SVA catalyst in the synthesis of 2,4,6-triarylpyridine derivatives, we considered the efficiency of SVA catalyst (an agreement of proposed mechanism) is comparable with numerous catalysts. To optimize the reaction conditions, the reaction between 4-nitrobenzaldehyde, 4-methoxyacetophenone and ammonium acetate under N2 atmosphere at 130 °C was applied as a model (Table 8).
| Entry | Catalyst | Catalyst loading | Time (min) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 4-nitrobenzaldehyde (1 mmol), 4-methoxyacetophenone (2 mmol), ammonium acetate (5 mmol), under N2 atmosphere, 130 °C.b Isolate yield. | ||||
| 1 | SVA | 2 mg | 45 | 88 |
| 2 | HBF4 | 10 mol% | 60 | 77 |
| 3 | Fe(HSO4)3 | 12 mol% | 90 | 41 |
| 4 | Ca(HSO4)2 | 15 mol% | 90 | 27 |
| 5 | Zn(HSO4)2 | 10 mol% | 90 | 43 |
| 6 | Oxone | 5 mol% | 90 | 35 |
| 7 | Ce(HSO4)3·7H2O | 10 mol% | 90 | 44 |
| 8 | Al(HSO4)3 | 12 mol% | 90 | 45 |
| 9 | Bi(HSO4)3 | 12 mol% | 90 | 25 |
| 10 | CeO2 | 10 mol% | 90 | 35 |
| 11 | PbO2 | 10 mol% | 90 | 20 |
| 12 | Silica sulfuric acid | 2 mg | 60 | 85 |
| 13 | [MIMPS]3PW12O40 | 1 mol% | 60 | 88 |
| 14 | Nano-ZnO | 1 mol% | 60 | 83 |
| 15 | [MSIM]Cl | 1 mol% | 60 | 83 |
| 16 | NH2SO3H | 10 mol% | 60 | 73 |
| 17 | ZrOCl2 | 10 mol% | 90 | 25 |
| 18 | H3PW12O40 | 1 mol% | 60 | 78 |
To compare the efficiency of our catalyst with some reported catalysts for the synthesis of 2,4,6-triarylpyridines and 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one derivatives, the results of using these catalysts in the condensation of benzaldehyde, acetophenone and ammonium acetate (in the synthesis of 2,4,6-triarylpyridine) and 4-chlorobenzaldehyde with β-naphthol and urea (in the synthesis of 2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one) are presented in Table 9. As it can be seen, the SVA catalyst has remarkably improved the synthesis of products in different terms.
| Entry | Reaction condition | Catalyst loading | Time (min) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | SVA cat, solvent-free, 130 °C | 2 mg | 60 | 81 | This work |
| 2 | DPAT, solvent-free, 120 °C | 6 mg | 240 | 96 | 46 |
| 3 | H14[NaP5W30O110], solvent-free, 120 °C | 100 mg | 210 | 98 | 30 |
| 4 | Bi(OTf)3, neat, 120 °C | 5 mol% | 120 | 89 | 39 |
| 5 | [HO3S(CH2)4MIM][HSO4], solvent-free, 120 °C | 20 mol% | 180 | 88 | 47 |
| 6 | Nanocrystalline MgAl2O4, solvent-free, 120 °C | 7 mol% | 180 | 85 | 48 |
| 7 | SVA cat, solvent-free, 130 °C | 2 mg | 45 | 88 | This work |
| 8 | H3Mo12O40P, DMF, 100 °C | 1 mmol | 180 | 89 | 49 |
| 9 | HClO4–SiO2, solvent-free, 150 °C | 40 mg | 60 | 88 | 17 |
| 10 | RuCl2(PPh3)3, toluene, reflux | 40 mg | 600 | 75 | 50 |
| 11 | ZnO NPs, solvent-free, 150 °C | 0.3 eq | 60 | 90 | 51 |
| 12 | Cellulose sulphuric acid, water, SDS, 80 °C | 50 mg | 150 | 88 | 44a |
Conclusion
In conclusion, a mild, recyclable and reusable silica vanadic acid [SiO2–VO(OH)2] (SVA) catalyst was applied in the synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one derivatives under solvent-free conditions via three-component condensation reaction of various aromatic aldehydes, β-naphthol and urea. Subsequently, 2,4,6-triarylpyridines were also prepared by one-pot three-component condensation reaction between several aromatic aldehydes, acetophenone derivatives and ammonium acetate under similar reaction conditions. A new anomeric based oxidation was proposed for the final step of the 2,4,6-triarylpyridine derivatives synthesis which was supported by theoretical studies. So the described new mechanistic approach can open up a new and promising insight in the course of rational design, synthesis and applications of novel hydride releasing compounds. The suggested mechanisms exposed that the dual acidic ability of SVA (Lewis and Brönsted acid), probably plays the important roles in the defined reactions. Lastly, noteworthy advantages of the described procedure and study are reasonably simple work-up, cleaner reaction profile, recycle and reusability of the SVA catalyst which makes it in close impact with the green chemistry disciplines.Experimental
General procedure for the preparation of silica-bonded vanadic acid [SiO2–VO(OH)2] (SVA)
The silica-bonded vanadic acid [SiO2–VO(OH)2] (SVA) as a catalyst was synthesized and prepared according to our previously reported process with a slightly modification.37 In a suction flask (100 mL), n-hexane (25 mL) and silica gel (4.0 g) were stirred for 10 min. It was equipped with a constant-pressure dropping funnel containing of vanadium oxytrichloride (I) (4.0 g) in n-hexane (25 mL) and gas inlet tube for conducting HCl gas over an adsorbing solution i.e. water. Then it was added drop wise to silica gel suspension (15–20 min). Subsequently addition of vanadiumoxytrichloride, the red-brown mixture was stirred for 10 h. Formerly, solvent was evaporated and the residue (the red-brown powder (II) attained after the grafting procedure) was filtered, washed with dry n-hexane numerous times to remove unreacted vanadiumoxytrichloride, dried under vacuum, and stirred in the air for 72 h to promote the hydrolysis of V–Cl bonds. At the moment, the red-brown powder transformed to dark green powder (III) gradually (Scheme 2). Additional amounts of HCl produced in the synthesis of SVA catalyst is neutralize in the presence of a certain concentration of sodium hydroxide. The SVA catalyst was characterized by FT-IR, thermal gravimetric (TG), derivative thermal gravimetric (DTG), X-ray diffraction patterns (XRD), scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) analysis (Fig. S13 to S17†).General procedure for the synthesis of 2,4,6-triarylpyridines
To the mixture of aromatic aldehydes (1 mmol), acetophenone derivatives (2 mmol) and ammonium acetate (5 mmol) in a round bottom flask, was added SVA (2 mg, 0.0156 mol% of V) as a catalyst and the resulting mixture was firstly stirred magnetically under solvent-free conditions at 130 °C in an oil bath for the suitable time (Table 3). After completion of the reaction, as checked via TLC n-hexane/ethyl acetate (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), mass was cooled to room temperature and then diethyl ether was added to the reaction mixture to extract product from SVA catalyst. Also, the catalyst was filtered and washed with CH3CN. The remained catalyst was applied for alternative reaction. The solvent of organic layer was evaporated and the crude product was purified by recrystallization from ethanol. More purification can be happened by recrystallization in n-hexane. In this work, SVA catalyst was recycled and reused for seven times without noteworthy loss of its catalytic activity.
1), mass was cooled to room temperature and then diethyl ether was added to the reaction mixture to extract product from SVA catalyst. Also, the catalyst was filtered and washed with CH3CN. The remained catalyst was applied for alternative reaction. The solvent of organic layer was evaporated and the crude product was purified by recrystallization from ethanol. More purification can be happened by recrystallization in n-hexane. In this work, SVA catalyst was recycled and reused for seven times without noteworthy loss of its catalytic activity.
General procedure for the synthesis of 1,2-dihydro-1-aryl-3H-naphth[1,2-e][1,3]oxazin-3-one derivatives
To the mixture of aromatic aldehydes (1 mmol), β-naphthol (1 mmol) and urea (1.2 mmol) in a round bottom flask, was added SVA (2 mg, 0.0156 mol% of V) as a catalyst and the resulting mixture was firstly stirred magnetically under solvent-free conditions at 130 °C in an oil bath for the appropriate time (Table 7). After completion of the reaction, as known by TLC n-hexane/ethyl acetate (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), mass was cooled to room temperature and then acetonitrile was added to the reaction mixture and heated to extract product from SVA catalyst. Also, the catalyst was filtered and washed with CH3CN. The remained catalyst was used for alternative reaction. The solvent of organic layer was evaporated and the crude product was purified by recrystallization from ethanol/water (10
2), mass was cooled to room temperature and then acetonitrile was added to the reaction mixture and heated to extract product from SVA catalyst. Also, the catalyst was filtered and washed with CH3CN. The remained catalyst was used for alternative reaction. The solvent of organic layer was evaporated and the crude product was purified by recrystallization from ethanol/water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In this work, SVA catalyst was recycled and reused for seven times without significant loss of its catalytic activity.
1). In this work, SVA catalyst was recycled and reused for seven times without significant loss of its catalytic activity.
Spectral data analysis for compounds should be added
2,6-Bis(4-methoxyphenyl)-4-(p-tolyl)pyridine (Table 3, entry 6)
Yellow solid; Mp: 153–155 °C; yield: 82%; IR (KBr): ν 3033, 1671, 1599, 1575, 1402, 1183, 809 cm−1; 1H NMR (400 MHz, DMSO-d6): δppm 2.40 (s, 9H, –CH3), 7.39 (d, 6H, J = 8.4 Hz, ArH), 7.95 (d, 2H, J = 8.0 Hz, ArH), 8.10 (s, 2H, ArH), 8.23 (d, 4H, J = 8.0 Hz, ArH); 13C NMR (100 MHz, DMSO-d6): δppm 20.8, 20.9, 115.5, 126.8, 127.1, 129.3, 129.6, 134.8, 136.1, 138.7, 138.9, 149.2, 156.3.4-(4-Nitrophenyl)-2,6-di-p-tolylpyridine (Table 3, entry 8)
Green solid; Mp: 233–235 °C; yield: 85%; IR (KBr): ν 3031, 1669, 1598, 1513, 1341, 1175, 832 cm−1; 1H NMR (400 MHz, DMSO-d6): δppm 2.39 (s, 6H, –CH3), 7.36 (d, 4H, J = 8.4 Hz, ArH), 8.18 (s, 2H, ArH), 8.23 (d, 4H, J = 8.4 Hz, ArH), 8.30 (d, 2H, J = 8.8 Hz, ArH), 8.36 (d, 2H, J = 8.8 Hz, ArH); 13C NMR (100 MHz, DMSO-d6): δppm 20.9, 21.0, 116.1, 123.9, 126.8, 128.7, 129.3, 135.7, 138.9, 144.2, 147.0, 147.7, 156.6.Computational details
All of the calculations were performed with the Gaussian 09 program.52 Full geometry optimization was carried out with the B3LYP hybrid density functional and the SVP basis set for all atoms. Frequency calculations at the same level of theory have also been performed to identify all of the stationary points as minima (zero imaginary frequencies) or transition structures (one imaginary frequency). In addition, the nature of transition structures was confirmed by intrinsic reaction coordinate (IRC).53 In order to investigate the mechanism of the synthesis of 2,4,6-triarylpyridine derivatives in the presence of silica vanadic acid [SiO2–VO(OH)2] (SVA) catalyst we used the total electronic energies (Eel + ZPE) and the Gibbs free energies. To make the calculations possible and less expensive the Si(OH)3 group was used as a model for silica moiety. Natural bond orbital (NBO)54 analysis was employed to evaluate the intramolecular interactions.Acknowledgements
The authors gratefully acknowledge the Bu-Ali Sina and Nahavand universities, University Research Councils, and also Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS), for providing support to this work.Notes and references
- (a) P. Gupta and S. Paul, Catal. Today, 2014, 236, 153 CrossRef CAS; (b) M. Kaur, S. Sharma and P. M. S. Bedi, Chin. J. Catal., 2015, 36, 520 CrossRef CAS.
- M. M. Heravi and Z. Faghihi, J. Iran. Chem. Soc., 2014, 11, 209 CrossRef CAS.
- K. Zeng, Z. Huang, J. Yang and Y. Gu, Chin. J. Catal., 2015, 36, 1606 CrossRef CAS.
- S. Rostamnia and E. Doustkhaha, Synlett, 2015, 26, 1345 CrossRef CAS.
- (a) X. Pan, M. Li and Y. Gu, Chem.–Asian J., 2014, 9, 268 CrossRef CAS PubMed; (b) M. Li, A. Taheri, M. Liu, S. Sun and Y. Gu, Adv. Synth. Catal., 2014, 356, 537 CrossRef CAS.
- A. S. Girgis, Pharmazie, 2000, 55, 426 CAS.
- Y. Wang, X. Li and K. Ding, Tetrahedron: Asymmetry, 2002, 13, 1291 CrossRef CAS.
- M. Adib, E. Sheibani, M. Mosto, K. Ghanbary and H. R. Bijanzadeh, Tetrahedron, 2006, 62, 3435 CrossRef CAS.
- T. Kurz, Tetrahedron, 2005, 61, 3091 CrossRef CAS.
- P. Zhang, E. A. Terefenko, A. Fensome, J. Wrobel, R. Winneker and Z. Zhang, Bioorg. Med. Chem. Lett., 2003, 13, 1313 CrossRef CAS PubMed.
- H. Poel, G. Guilaument and M. Viaud-Massuard, Tetrahedron Lett., 2002, 43, 1205 CrossRef.
- J. N. Joyce, S. Presgraves, L. Renish, S. Borwege, D. H. Osredkar, M. Replogle, M. Pazsoldan and M. J. Millan, Exp. Neurol., 2003, 184, 393 CrossRef CAS PubMed.
- F. A. J. Kerdesky, Tetrahedron Lett., 2005, 46, 1711 CrossRef CAS.
- C. Cimarelli, A. Mazzanti, G. Palmieri and E. Volpini, J. Org. Chem., 2001, 66, 4759 CrossRef CAS PubMed.
- C. Cimarelli, G. Palmieri and E. Volpini, Tetrahedron, 2001, 57, 6089 CrossRef CAS.
- Y. Dong, J. Sun, X. Wang, X. Xu, L. Cao and Y. Hu, Tetrahedron: Asymmetry, 2004, 15, 1667 CrossRef CAS.
- S. B. Sapkal, K. F. Shelke, B. B. Shingate and M. S. Shingare, J. Korean Chem. Soc., 2010, 54, 437 CrossRef CAS.
- H. A. Ahangar, G. H. Mahdavinia, K. Marjani and A. Hafezian, J. Iran. Chem. Soc., 2010, 7, 770 CrossRef CAS.
- Z. Turgut, E. Pelit and A. Koycu, Molecules, 2007, 12, 345 CrossRef CAS PubMed.
- M. Balasubramanian and J. G. Keay, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Rees and E. V. F. Scriven, Pergamon Press, London, 1996, vol. 5, p. 245, and references cited therein Search PubMed.
- (a) V. Klimesova, M. Svoboda, K. Waisser, M. Pour and J. Kaustova, Il Farmaco, 1999, 54, 666 CrossRef CAS PubMed; (b) I. J. Enyedy, S. Sakamuri, W. A. Zaman, K. M. Johnson and S. Wang, Bioorg. Med. Chem. Lett., 2003, 13, 513 CrossRef CAS PubMed; (c) A. D. Pillai, P. D. Rathod, P. X. Franklin, M. Patel, M. Nivsarkar, K. K. Vasu, H. Padh and V. Sudarsanam, Biochem. Biophys. Res. Commun., 2003, 301, 183 CrossRef CAS PubMed; (d) B. Y. Kim, J. B. Ahn, H. W. Lee, S. K. Kang, J. H. Lee, J. S. Shin, S. K. Ahn, C. I. Hong and S. S. Yoon, Eur. J. Med. Chem., 2004, 39, 433 CrossRef CAS PubMed.
- (a) S. Kelch and M. Rehahn, Macromolecules, 1999, 32, 5818 CrossRef CAS; (b) B. G. G. Lohmeijer and U. S. Schubert, Angew. Chem., Int. Ed., 2002, 41, 3825 CrossRef CAS; (c) B. G. G. Lohmeijer and U. S. J. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 1413 CrossRef CAS; (d) P. R. Andres and U. S. Schubert, Adv. Mater., 2004, 16, 1043 CrossRef CAS.
- F. Krohnke and W. Zecher, Angew. Chem., Int. Ed., 1962, 1, 626 CrossRef.
- F. Krohnke, Synthesis, 1976, 24 Search PubMed.
- K. T. Potts, M. J. Cipullo, P. Ralli and G. Theodoridis, J. Am. Chem. Soc., 1981, 103, 3584 CrossRef CAS.
- M. Adib, H. Tahermansouri, S. A. Koloogani, B. Mohammadi and H. R. Bijanzadeh, Tetrahedron Lett., 2006, 47, 5957 CrossRef CAS.
- T. Kobayashi, H. Kakiuchi and H. Kato, Bull. Chem. Soc. Jpn., 1991, 64, 392 CrossRef CAS.
- Y. M. Ren and C. Cai, Monatsh. Chem., 2009, 140, 49 CrossRef CAS.
- L. Nagarapu, A. R. Peddiraju and S. Apuri, Catal. Commun., 2007, 8, 1973 CrossRef CAS.
- M. M. Heravi, K. Bakhtiari, Z. Daroogheha and F. F. Bamoharram, Catal. Commun., 2007, 8, 1991 CrossRef CAS.
- S. Tu, T. Li, F. Shi, F. Fang, S. Zhu, X. Wei and Z. Zong, Chem. Lett., 2005, 34, 732 CrossRef CAS.
- See our review: (a) P. Salehi, M. A. Zolfigol, F. Shirini and M. Baghbanzadeh, Curr. Org. Chem., 2006, 10, 2171 CrossRef CAS; (b) M. Daraei, M. A. Zolfigol, F. Derakhshan-Panah, M. Shiri, H. G. Kruger and M. Mokhlesi, J. Iran. Chem. Soc., 2015, 12, 855 CrossRef CAS; (c) D. Azarifar, S. M. Khatami, M. A. Zolfigol and R. Nejat-Yami, J. Iran. Chem. Soc., 2014, 11, 1223 CrossRef CAS; (d) A. Khazaei, M. A. Zolfigol, M. Mokhlesi and R. Rostamian, J. Iran. Chem. Soc., 2013, 10, 1297 CrossRef CAS.
- See our review: F. Shirini, M. A. Zolfigol, P. Salehi and M. Abedini, Curr. Org. Chem., 2008, 12, 183 CrossRef CAS.
- (a) A. R. Moosavi-Zare, M. A. Zolfigol, M. Zarei, A. Zare and J. Afsar, Appl. Catal., A, 2015, 505, 224 CrossRef CAS; (b) A. R. Moosavi-Zare, M. A. Zolfigol, M. Zarei, A. Zare and V. Khakyzadeh, J. Mol. Liq., 2015, 211, 373 CrossRef CAS; (c) A. Zare, M. Merajoddin, A. R. Moosavi-Zare, M. Zarei, M. H. Beyzavi and M. A. Zolfigol, Res. Chem. Intermed., 2015, 1–14, DOI:10.1007/s11164-015-2154-7.
- (a) M. A. Zolfigol, A. Khazaeia, A. R. Moosavi-Zare and A. Zare, Org. Prep. Proced. Int., 2010, 42, 95 CrossRef CAS; (b) A. Khazaei, M. A. Zolfigol, A. R. Moosavi-Zare and A. Zare, Sci. Iran., 2010, 17, 31 CAS; (c) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare and V. Khakyzadeh, Appl. Catal., A, 2011, 400, 70 CrossRef CAS; (d) M. A. Zolfigol, V. Khakyzadeh, A. R. Moosavi-Zare, A. Zare, S. B. Azimi, Z. Asgari and A. Hasaninejad, C. R. Chim., 2012, 15, 719 CrossRef CAS; (e) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare, H. G. Kruger, Z. Asgari, V. Khakyzadeh and M. Kazem-Rostami, J. Org. Chem., 2012, 77, 3640 CrossRef CAS PubMed; (f) A. Zare, A. R. Moosavi-Zare, M. Merajoddin, M. A. Zolfigol, T. Hekmat-Zadeh, A. Hasaninejad, A. Khazaei, M. Mokhlesi, V. Khakyzadeh, F. Derakhshan-Panah, M. H. Beyzavi, E. Rostami, A. Arghoon and R. Roohandeh, J. Mol. Liq., 2012, 167, 69 CrossRef CAS; (g) M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare, Z. Asgari, V. Khakyzadeh and A. Hasaninejad, J. Ind. Eng. Chem., 2013, 19, 721 CrossRef CAS; (h) A. R. Moosavi-Zare, M. A. Zolfigol, M. Zarei, A. Zare and V. Khakyzadeh, J. Mol. Liq., 2013, 186, 63 CrossRef CAS; (i) A. R. Moosavi-Zare, M. A. Zolfigol, M. Zarei, A. Zare, V. Khakyzadeh and A. Hasaninejad, Appl. Catal., A, 2013, 467, 61 CrossRef CAS; (j) M. A. Zolfigol, H. Vahedi, S. Azimi and A. R. Moosavi-Zare, Synlett, 2013, 24, 1113 CrossRef CAS; (k) A. R. Moosavi-Zare, M. A. Zolfigol, O. Khaledian, V. Khakyzadeh, M. D. Farahani and H. G. Kruger, New J. Chem., 2014, 38, 2342 RSC; (l) A. R. Moosavi-Zare, M. A. Zolfigol and M. Daraei, Synlett, 2014, 25, 1173 CrossRef; (m) M. A. Zolfigol, A. R. Moosavi-Zare and M. Zarei, C. R. Chim., 2014, 17, 1264 CrossRef CAS; (n) A. R. Moosavi-Zare, M. A. Zolfigol, V. Khakyzadeh, C. Böttcher, M. H. Beyzavi, A. Zare, A. Hasaninejad and R. Luque, J. Mater. Chem. A, 2014, 2, 770 RSC; (o) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare and S. M. Vahdat, RSC Adv., 2015, 5, 32933–32940 RSC; (p) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare, S. M. Vahdat, H. Alinezhad and M. Norouzi, RSC Adv., 2015, 5, 45027 RSC; (q) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare and S. M. Vahdat, J. Mol. Catal. A: Chem., 2015, 409, 216 CrossRef CAS; (r) M. A. Zolfigol, R. Ayazi-Nasrabadi and S. Baghery, RSC Adv., 2015, 5, 71942 RSC; (s) M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare, S. M. Vahdat, H. Alinezhad and M. Norouzi, RSC Adv., 2014, 4, 57662 RSC.
- (a) M. Dabiri, P. Salehi, M. Baghbanzadeh, M. A. Zolfigol, M. Agheb and S. Heydari, Catal. Commun., 2008, 9, 785 CrossRef CAS; (b) A. R. Moosavi-Zare, M. A. Zolfigol, S. Farahmand, A. Zare, A. R. Pourali and R. Ayazi-Nasrabadi, Synlett, 2014, 25, 193 CrossRef CAS; (c) P. Salehi, M. Dabiri, M. A. Zolfigol and M. Baghbanzadeh, Synlett, 2005, 1155 CrossRef CAS; (d) P. Salehi, M. Dabiri, M. A. Zolfigol and M. Baghbanzadeh, Tetrahedron Lett., 2005, 46, 7051 CrossRef CAS.
- (a) M. Safaiee, M. A. Zolfigol, M. Tavasoli and M. Mokhlesi, J. Iran. Chem. Soc., 2014, 11, 1593 CrossRef CAS; (b) M. A. Zolfigol, A. Khazaei, M. Safaiee, M. Mokhlesi, R. Rostamian, M. Bagheri, M. Shiri and H. G. Kruger, J. Mol. Catal. A: Chem., 2013, 370, 80 CrossRef CAS; (c) A. Khazaei, M. A. Zolfigol, M. Safaiee, M. Mokhlesi, E. Donyadari, M. Shiri and H. G. Kruger, Catal. Commun., 2012, 26, 34 CrossRef CAS.
- (a) M. Borthakur, M. Dutta, G. Shyamalee and R. C. Boruah, Synlett, 2008, 3125 CAS; (b) S. Tu, R. Jia, B. Jiang, J. Zhang, Y. Zhang, C. Yao and S. Ji, Tetrahedron, 2007, 63, 381 CrossRef CAS; (c) X. Q. Huang, H. X. Li, J. X. Wang and X. F. Jia, Chin. Chem. Lett., 2005, 16, 607 CAS; (d) M. R. M. Shafiee, R. Moloudi and M. Ghashang, APCBEE Proc., 2012, 1, 221 CrossRef; (e) R. Lombard and J. P. Stephan, Bull. Soc. Chim. Fr., 1958, 1458 CAS.
- P. V. Shinde, V. B. Labade, J. B. Gujar, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2012, 53, 1523 CrossRef CAS.
- M. A. Zolfigol, H. Gholami and V. Khakyzadeh, Principles of organic synthesis with a new approach, Bu-Ali Sina University Publishers, Hamedan, Iran, 3rd edn, 2014, p. 26 Search PubMed.
- (a) J. M. Erhardt and J. D. Wuest, J. Am. Chem. Soc., 1980, 102, 6363 CrossRef CAS; (b) T. J. Atkins, J. Am. Chem. Soc., 1980, 102, 6364 CrossRef CAS; (c) J. M. Erhardt, E. R. Grover and J. D. Wuest, J. Am. Chem. Soc., 1980, 102, 6365 CrossRef CAS.
- M. A. Zolfigol, F. Afsharnadery, S. Baghery, S. Salehzadeh and F. Maleki, RSC Adv., 2015, 5, 75555 RSC.
- M. Abdoli-Senejani, A. A. Taherpour, H. R. Memarian and M. Khosravani, Struct. Chem., 2013, 24, 191 CrossRef CAS.
- (a) A. Kumar, M. K. Gupta and M. Kumar, RSC Adv., 2012, 2, 7371 RSC; (b) N. Basavegowda, K. B. S. Magar, K. Mishra and Y. R. Lee, New J. Chem., 2014, 38, 5415 RSC; (c) M. Dabiri, A. S. Delbari and A. Bazgir, Synlett, 2007, 821 CAS; (d) F. Nemati and A. Beyzai, J. Chem., 2013, 365281, DOI:10.1155/2013/365281; (e) M. Dabiri, A. S. Delbari and A. Bazgir, Heterocycles, 2007, 71, 543 CrossRef CAS.
- R. Hunnur, R. Kamble, A. Dorababu, B. S. Kumar and C. Bathula, Arabian J. Chem. DOI:10.1016/j.arabjc.2013.06.028.
- J. Li, P. He and C. Yu, Tetrahedron, 2012, 68, 4138 CrossRef CAS.
- A. Davoodnia, M. Bakavoli, R. Moloudi, N. Tavakoli-Hoseini and M. Khashi, Monatsh. Chem., 2010, 141, 867 CrossRef CAS.
- J. Safari, Z. Zarnegar and M. Borjian-Borujeni, Chem. Pap., 2013, 67, 688 CAS.
- A. Chaskari, V. Vyavhare, V. Padalkar, K. Phatangare and H. Deokar, J. Serb. Chem. Soc., 2011, 76, 21 CrossRef.
- X. Zhu and Y. R. Lee, Bull. Korean Chem. Soc., 2012, 33, 3831 CrossRef CAS.
- G. B. D. Rao, M. P. Kaushik and A. K. Halve, Tetrahedron Lett., 2012, 53, 2741 CrossRef.
- M. J. Frisch, et al., Gaussian 09, revision A.02, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- (a) K. J. Fukui, J. Phys. Chem., 1970, 74, 4161 CrossRef CAS; (b) K. Fukui, Acc. Chem. Res., 1981, 14, 363 CrossRef CAS.
- E. D. Glendening, A. E. Reed, J. A. Carpenter and F. Weinhold, NBO Version 3.1 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra21392d |
| This journal is © The Royal Society of Chemistry 2015 |