Synthesis and structure–property investigation of multi-arm oligothiophenes†
Choong Ping Sen and
Suresh Valiyaveettil*
Department of Chemistry, 3 Science Drive 3, National University of Singapore, Singapore-117543. E-mail: chmsv@nus.edu.sg
First published on 7th December 2015
Abstract
Oligothiophenes are used in many applications owing to their easy accessibility in high purity, tunable electron density, high chemical stability and desired processability in solution and in the solid state. In this work, a series of soluble branched oligothiophenes have been synthesized and fully characterized. The target molecules (SCT-1 to SCT-4) are soluble in common organic solvents. Full characterization of optical and electrochemical properties indicated that the extended π-conjugation led to red-shifts in the absorption maximum (λmax) from 360 nm (SCT-1) to 440 nm (SCT-4). Fluorescence quantum yields were in the range of 6–45% for the SCTs. The observed properties of the target molecules are better than the corresponding linear oligothiophenes. Electron deficient Hg(II) cations interact with electron rich SCT-4 to form a charge-transfer complex which led to significant quenching of photoluminescence in solution. Moreover, an electron deficient molecule, 7,7,8,8-tetracyanoquinodimethane (TCNQ), also interacts with electron rich SCTs to form a TCNQ dianion (TCNQ2−). Such conjugated optical materials could be used for developing potential applications in the near future.
Introduction
One-dimensional or multidimensional oligothiophenes have been synthesized and used in optoelectronic applications such as organic solar cells (OSCs),1,2 light emitting diodes (OLEDs),3 sensors4 and field effect transistors (OFETs)5–8 owing to their excellent chemical stability, high hole mobility, tunable optoelectronic properties and accessibility of high purity materials as compared to their polymeric counterparts.9 High crystallinity of oligothiophenes results in improved performance of devices owing to enhanced charge transport,10 but leads to poor solubility and low processability. Solubilizing groups such as alkyl chains are commonly attached on oligothiophenes for enhancing the solubility in common organic solvents.11,12 In addition, bulky groups or multidimensional architectures hinder the formation of face to face π-dimers, which is useful for understanding the charge transport mechanism.13 Multi-dimensional oligothiophenes with star-,14 H-shaped,15 spiro-,16 and dendrimeric architectures17 have been synthesized and applied in various applications. The increased dimensionality of the conjugated oligothiophene is found to exhibit isotropic charge transport as compared to that in linear oligothiophenes,18 which could be advantageous in order to obtain a higher charge carrier mobility in devices. Recently, 3D swivel cruciform oligothiophene dimers have shown an improved solubility in organic solvent due to high degree of rotational freedom between two linear oligothiophenes.19,20 Design and synthesis of oligothiophenes with interesting architectures are needed to improve intrinsic electronic and transport properties.21,22Herein, we design and synthesize a series of new multi-branched oligothiophenes (SCTs) based on a quaterthiophene backbone with extended conjugation (Scheme 1). The correlation between the structures of SCTs with observed optical properties is investigated using absorption and photoluminescence spectroscopy. These highly branched SCTs are easy to prepare and structurally defined. In addition, high solubility and processability are expected in SCTs as compared to their linear oligothiophene analogues. The conformations of the molecules are established using optical studies in solution and in solid state.
Results and discussion
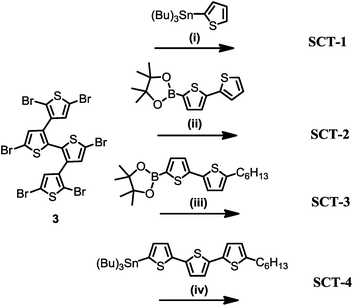
Target molecules, SCT-1 to SCT-4 (Fig. 1) were synthesized using the routes given in Schemes 2 and 3 and fully characterized. | ||
| Scheme 2 Synthesis of quaterthiophene trimer backbone; (i) (a) LDA, THF, −78 °C, 1 h; (b) CuCl2, −78 °C, 10 min; (ii) Pd(PPh3)4, K2CO3, THF/H2O, 70 °C, 24 h; (iii) NBS, CHCl3/AcOH, 80 °C, 4 h. | ||
Synthesis and characterization
Synthesis of the hexabrominated central quaterthiophene is given in Scheme 2. 3,3′-Dibromo-2,2′-bithiophene (1) was prepared by lithiating commercially available 3-bromothiophene with lithium diisopropylamide (LDA) followed by CuCl2-oxidative homocoupling to obtain the product in 57% yield.23 The palladium-catalyzed Suzuki–Miyaura cross-coupling between 1 and 2.2 equivalents of 3-theinyl boronic acid afforded 3,3′:2′,2′′:3′′,3′′′-quaterthiophene 2 in 45% yield.24 Bromination of 2 with 12 equivalents of NBS in CHCl3/AcOH at 80 °C afforded 2,2′′′,5,5′,5′′,5′′′-hexabromo-3,3′:2′,2′′:3′′,3′′′-quaterthiophene 3 in 56% yield, which was used as a central building block for synthesizing the target molecules. SCT-1, SCT-2, SCT-3 and SCT-4 were synthesized using Stille or Suzuki–Miyaura palladium-catalyzed cross-coupling reactions with the corresponding stanylated or borylated oligothiophenes in good yields (Scheme 3). Alkyl chains are introduced in larger molecules to enhance the solubility.Solubility of SCT-2 is poor in THF and chloroform, hence six hexyl groups were introduced at the terminal α-positions of the SCT-3 to improve the solubility. As expected, both SCT-1 (small molecule with sufficient flexibility) and SCT-3 are highly soluble in common organic solvents. This allowed us to fully characterize the molecular structures of intermediates and SCTs using 1H and 13C NMR spectroscopy, mass spectrometry and elemental analysis (ESI, Fig. S1–S18†).
Thermal stabilities of SCTs
In order to understand the thermal stability of the compounds, thermogravimetric analyses (TGA) and differential scanning calorimetry (DSC) of all SCTs were conducted under nitrogen atmosphere. The observed TGA traces indicated high thermal stability with only 5% weight loss before 430 °C, 500 °C, 400 °C and 380 °C for SCT-1, SCT-2, SCT-3 and SCT-4, respectively (Fig. 2).SCT-2 has higher melting point (270 °C) and decomposition temperature (492 °C) than SCT-1 owing to the better π-stacking as oligothiophene length increases. In contrast to SCT-1, which showed a reversible melting and crystallization (215 °C), SCT-2 did not crystallize upon cooling from the melt. However, an exothermic peak at 235 °C was observed during second heating, which corresponded to recrystallization of SCT-2. Both melting point (155 °C) and decomposition temperature (405 °C) of alkylated SCT-3 are lower than unsubstituted SCT-2. This is explained by disruption of intermolecular interaction in SCT-3 with six hexyl groups. SCT-3 showed a slow crystallization at 60 °C during cooling process. A higher melting point (250 °C) was observed in SCT-4 with longer oligothiophene length as compared to SCT-3 (155 °C). On the other hand, the decomposition temperature of SCT-4 (390 °C) is not significantly different from SCT-3 (405 °C), which could be due to a more rigid conformation of SCT-4. All thermal properties of SCTs are summarized in Table 1.
| Decomposition temperaturea, °C | Melting pointb, °C | |
|---|---|---|
| a Decomposition temperature was measured at 5% weight degradation of SCTs under nitrogen atmosphere using TGA.b Melting point was determined at the maximum of endothermic peak observed in DSC thermograms. | ||
| SCT-1 | 430 | 230 |
| SCT-2 | 492 | 270 |
| SCT-3 | 406 | 155 |
| SCT-4 | 390 | 250 |
Photophysical properties of SCTs
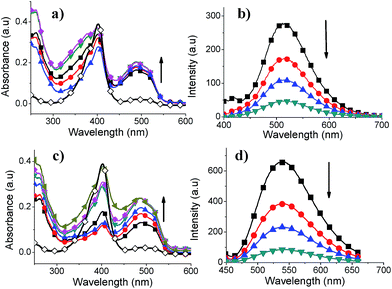
Absorption spectra of SCTs were recorded in THF at room temperature (Fig. 3). The maximum absorption wavelengths were red-shifted as number of thiophene moieties increases in the structure of SCT-1 to SCT-4. Such trend was also observed for linear oligothiophenes with progressive extension in π-conjugation.25 A red-shift (5 nm) in absorption maximum was observed for SCT-1 (360 nm) as compared to linear unsubstituted terthiophene analogues (355 nm, CHCl3)26 and a blue shift in absorption maximum as compared to linear quaterthiophene analogue (390 nm, CHCl3).26 This is expected due to the twisted conformation and disruption in π-conjugation. The absorption maxima of SCTs are influenced by the number of thiophene moieties present on the side chains. In the case of SCT-2 and SCT-3, the absorption maxima are similar to the linear pentathiophenes (410 nm, CHCl3).27 The absorption maximum of SCT-4 (440 nm) is comparable to the α-connected linear heptathiophenes.28 In addition, the absorption band edge has shifted to longer wavelength from SCT-1 (453 nm) to SCT-4 (533 nm) in THF, indicating an extended π-conjugated system in SCT-4. The emission wavelengths for SCT-1, SCT-2, SCT-3 and SCT-4 are 516 nm, 542 nm, 542 nm and 550 nm, respectively. The largest stoke shift observed in SCT-1 suggests a significant conformational change between ground and excited state (Table 2). As the number of thiophene rings increases in SCT-1 to SCT-4, the torsional motion becomes smaller owing to the extended oligothiophene length in the system. Thus a smaller Stoke shift is expected in a more rigid SCT-4. In addition, enhanced photoluminescence was observed from larger SCTs with more extended conjugation. The fluorescence quantum yields of SCT-1, SCT-2, SCT-3 and SCT-4 in THF were determined as 6%, 11.1%, 11.4% and 46.6%, respectively, with reference to fluorescein (0.1 M NaOH, quantum yield of 0.85). Moreover, higher molar extinction coefficient was observed with increasing conjugation length from SCT-1 to SCT-4. The absorption spectra of SCTs films were recorded to investigate the changes in absorption maximum and absorption edge from solution to solid state. The SCT films were prepared by spin casting the solutions of SCTs in THF at room temperature. Red shifts in absorption maxima were observed for SCT-1 (20 nm), SCT-2 (28 nm), and SCT-3 (17 nm) after comparing the values from solution spectra with those obtained from solid state spectra (Fig. 4). Similarly, red shifts in absorption edges for SCT-1 (31 nm), SCT-2 (45 nm), and SCT-3 (50 nm) were also observed after comparing the solution spectra with solid state spectra. | ||
Fig. 3 UV-vis absorption spectra (a) and photoluminescence spectra (b) of SCT-1 ( ), SCT-2 ( ), SCT-2 ( ), SCT-3 ( ), SCT-3 ( ) and SCT-4 ( ) and SCT-4 ( ) in THF. ) in THF. | ||
| λmaxa, nm | εb × 105 cm−1 M−1 | λonsetc, nm | λmaxd, nm | λonset c, nm | Ege, eV | λemf, nm | Φfg, % | |
|---|---|---|---|---|---|---|---|---|
| a Recorded in THF.b ε extinction coefficient was calculated by dividing absorbance with concentration and cuvette path length.c λonset was calculated from the intersection of the tangent lines drawn to the lowest energy absorption edge to the baseline.d λmax was measured from spin-casted thin film on quart plate.e Eg = 1240/λonset thin film.f Steady state photoluminescence recorded in THF.g Φf, fluorescence quantum yields were determined in THF using fluorescein (0.1 M NaOH, quantum yield of 0.85) as reference. | ||||||||
| SCT-1 | 360 | 0.49 | 453 | 380 | 484 | 2.56 | 516 | 6.0 |
| SCT-2 | 410 | 1.01 | 500 | 438 | 545 | 2.28 | 542 | 11.1 |
| SCT-3 | 413 | 1.03 | 505 | 430 | 555 | 2.23 | 542 | 11.4 |
| SCT-4 | 440 | 1.44 | 533 | 413 | 545 | 2.27 | 515, 550 | 46.6 |
 | ||
Fig. 4 Comparison of absorption spectra of (a) SCT-1, (b) SCT-2, (c) SCT-3 and (d) SCT-4 in THF ( ) and spin casted films ( ) and spin casted films ( ) on quartz plate at room temperature. ) on quartz plate at room temperature. | ||
In contrast, absorption maximum of SCT-4 thin film shows a blue-shift (27 nm) and a broad peak. Even though, the absorption edge of SCT-4 in solid state is red-shifted to longer wavelength, the magnitude of red-shift (12 nm) is significantly smaller as compared to other SCTs, and linear oligothiophenes. Therefore it is conceivable that SCT-4 does not adapt a planar conformation in solid state owing to the steric hindrance from side arms. The band gap energies were calculated to be 2.56 eV, 2.28 eV, 2.23 eV, and 2.27 eV for SCT-1, SCT-2, SCT-3, and SCT-4, respectively. All photophysical properties are summarized in Table 2.
All SCT molecules are photochemically stable under ambient conditions, both in solution and thin film. The photochemical stability of SCTs molecules was evaluated based on changes in absorption and emission profile after photoirradiation. No changes in absorption or emission maxima and intensities were observed for all SCTs molecules, both in solution and in thin film. To further test the photochemical stability of SCTs, high intensity light source (50 Watt white LED light, 15 cm distance between light source and substrates) was used for continuous irradiation on SCT molecules in dilute THF at ambient conditions. SCT-1 showed no changes in absorption and emission intensities after 24 hours of irradiation (ESI, Fig. S31 and 32†). In contrast, the absorption maximum of SCT-2 had shifted to shorter wavelength with 13% loss of emission intensity after 24 hours. Similarly, the absorption maximum of SCT-3 also shifted to shorter wavelength with 10% loss of emission intensity after 24 hours. In the case of SCT-4, the absorbance and emission intensity were decreased by 5% and 7%, respectively. The spin-casted films of SCTs were tested under exact conditions (ESI, Fig. S33 and 34†). SCT-1 showed 23% and 56% decrease in absorbance and emission intensity, respectively. The absorbance and emission intensities of SCT-2 thin film were decreased by 23% and 43%, respectively after 24 hours. In contrast, SCT-3 showed a blue shift in absorption maximum and 88% loss in emission intensity. On the other hand, SCT-4 showed 53% and 70% decreases in absorbance and emission intensities, respectively. This suggested that the SCT-3 and SCT-4 are more photochemically unstable owing to their high HOMO energy level.
Electrochemical properties and theoretical computation of SCTs
Cyclic voltammetry (CV) was used to probe the redox behaviors of SCTs in thin films and in dichloromethane solution (Fig. 5). The CV scans of thin film were recorded in 0.1 M n-Bu4NPF6 solution in anhydrous acetonitrile and SCTs dissolved in dichloromethane solution with 0.1 M n-Bu4NPF6 solution using a scan rate of 100 mV s−1 and a standard calomel electrode (SCE) as reference electrode. The HOMO energy levels of SCTs were estimated from the onset oxidation and calibrated with ferrocene/ferrocenium (Fc/Fc+) redox couple as internal reference. In thin film, SCT-1 shows one partial reversible oxidation peak Eox,1 at 0.65 V corresponding to the formation of oligothiophene cation radical of terthiophene.26 Absence of quaterthiophene oxidation/reduction peaks in CV indicates minimal electronic interaction between oligothiophene arms of the same molecule. In the more extended conjugated SCT-2, there are two oxidation peaks Eox,1 and Eox,2 at 0.53 and 0.91 V owing to the formation of monocation and dication. The first oxidation peak in SCT-2 has shifted to a lower positive potential owing to the formation of cation on an extended oligothiophene unit. Similarly, SCT-3 showed two oxidation peaks Eox,1 and Eox,2 at 0.57 V and 0.76 V. The CV traces of SCT-4 show three oxidation processes at 0.38 V, 0.61 V and 0.91 V. However, formation of a radical trication on single oligothiophene of SCT-4 would be unstable. Therefore it is conceivable that the three oxidation processes correspond to the formation of three different radical cations on three independent oligothiophene arms of SCT-4.In comparison to CV traces in thin films, the SCTs in dichloromethane showed a more pronounced reversibility of the oxidation processes. SCT-3 and SCT-4 showed multiple oxidation processes with partial reversibility (Fig. 5c–f). All HOMO and LUMO energy levels of SCTs are calculated and summarized in Table 3.
| SCT- | Eox,1 film | Eox,2 film | Eox,3 film | HOMOafilm (eV) | Eox,1 dcm | Eox,2 dcm | Eox,3 dcm | HOMObdcm (eV) |
|---|---|---|---|---|---|---|---|---|
| a Electrochemical HOMO of SCTs thin films = −(Eox + 4.8) eV, where Eox was determined from the onset potentials of first oxidation peak calibrated with ferrocene/ferrocenium ion redox couple.b Electrochemical HOMO of SCTs in dichloromethane = −(Eox + 4.8) eV, where Eox was determined from the onset potentials of first oxidation peak calibrated with ferrocene/ferrocenium ion redox couple. | ||||||||
| 1 | 0.65 | — | — | −5.28 | 0.70 | — | — | −5.32 |
| 2 | 0.53 | 0.91 | — | −5.26 | 0.52 | — | — | −5.20 |
| 3 | 0.57 | 0.76 | — | −5.22 | 0.26 | 0.63 | 0.77 | −5.05 |
| 4 | 0.38 | 0.61 | 0.91 | −5.07 | 0.31 | 0.50 | 0.79 | −5.03 |
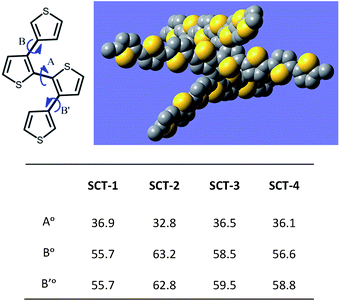
The optimized structures and Frontier orbital energy levels of SCTs were calculated using density functional theory (DFT) at B3LYP/6-31G(d) level (ESI, Fig. S21–30†). For example in SCT-4, the dihedral angle between the planes involving end thiophene and center bithiophene moiety (A) is 36.1° while the dihedral angles between side thiophene and center bithiophene (B and B′) are 56.6° and 58.8°, respectively (Fig. 6). All SCT molecules have a swivel-type conformation where three oligothiophene chains are pointing in different directions. Moreover, the out-of-plane conformation of all three branches led to inefficient orbital overlapping as compared to that found in linear oligothiophenes, which is in good agreement with the results obtained from optical and electrochemical studies. The theoretical HOMO energy levels of SCTs are higher than the one estimated using the data from CV in solid state or solution state (ESI, see Table S1†). This could be due to the twisted structural conformation, which leads to inefficient orbital overlapping. Nevertheless, SCTs follow the same trend, where the HOMO energy levels are increased with decreased band gaps. In addition, the HOMO and LUMO electron densities are distributed evenly in all three oligothiophene branches of SCTs.
Morphology of SCTs
Precipitation of SCTs was carried out using slow evaporation of dilute solution in THF–H2O mixture over two days. The resulting suspension was dropcasted on a glass substrate and dried at ambient condition. Field effect scanning electron microscopy (SEM) was used to examine the morphology of solid precipitate obtained from all SCTs. SEM micrographs of SCT-1 showed formation of microspheres with a diameter in the range of 0.5 μm to 2 μm. Slow evaporation of THF solution allows SCT-2 to form crystalline needle-like rods with an average width of 2 μm and up to a few millimeters in length. In contrast, SCT-3 with solubilizing hexyl groups formed nanosized spherical particles with an average diameter of 180 nm, while SCT-4 formed random shapes under similar conditions (Fig. 7).Interaction of SCTs with Hg(II) cations
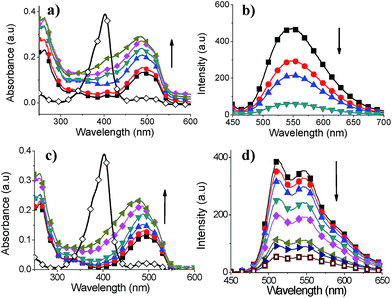
It is well-known that Pb(II), Cd(II) and Hg(II) cations show strong affinity for sulfur containing groups such as thiol. Even though, thiophene–metal ion interactions are expected to be weak, thiophene groups from adjacent chains are able to interact with metal ions owing to the structural rigidity and orientation of S atoms of thiophene rings in SCTs. The complexation between thiophene derivatives and Hg(II) cation for sensor application has been reported.29,30 Our study was focused towards understanding the interaction between thienyl sulfur atoms of SCTs and heavy metal ions for potential application such as sensor. The interaction of heavy metal ions with SCTs was investigated using absorption and photoluminescence spectroscopy. It is found that Pb(II) and Cd(II) cations did not change absorption and emission profiles of SCTs (ESI, Fig. S35†). On the other hand, Hg(II) ions showed significant changes in the absorption and emission maxima of SCTs. Both absorbance and emission intensities were decreased upon addition of Hg(II) ions to the solution of SCTs in THF (Fig. 8 and 9). Particularly, formation of yellow precipitate was observed when excess Hg(II) ions was added into SCT-4 in THF, resulting in disappearance of absorption and quenching of emission intensity of the solution. This indicates a strong interaction of SCT-4 with Hg(II) ions. | ||
Fig. 8 Absorption spectra of (a) SCT-1, (b) SCT-2, (c) SCT-3 and (d) SCT-4 in THF before ( ) and after ( ) and after ( ) addition of aqueous Hg(OAc)2 solution (1 × 10−3 M). ) addition of aqueous Hg(OAc)2 solution (1 × 10−3 M). | ||
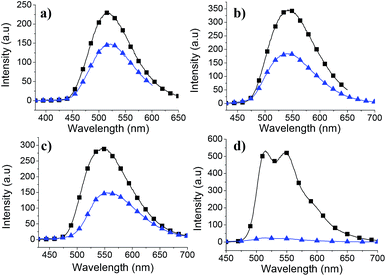 | ||
Fig. 9 Photoluminescence spectra of (a) SCT-1, (b) SCT-2, (c) SCT-3 and (d) SCT-4 in THF before ( ) and after ( ) and after ( ) addition of aqueous Hg(OAc)2 solution (1 × 10−3 M). ) addition of aqueous Hg(OAc)2 solution (1 × 10−3 M). | ||
The concentration dependent study was carried out by titrating the SCT-4 solution in THF with Hg(II) ion (Fig. 10a) to understand the absorption changes with respect to the Hg(II) ion concentration. The absorption maximum of SCT-4 was shifted from 440 nm to 458 nm upon increase in concentration of Hg(II) ions. Such observation could be attributed to the formation of SCT-4/Hg complexes.31 Increasing the concentration of Hg(II) ions further led to precipitation of SCT-4/Hg complex from solution, resulting the disappearance of low energy absorption band. Solid state absorption spectra were recorded using the precipitated solids on a quartz plate (Fig. 10b). All Hg(II)–SCTs compounds showed a small red shift (2 to 10 nm) in absorption maximum with respect to the pristine SCTs thin films. No significant differences in absorption maximum were observed for SCT-2/Hg and SCT-3/Hg complex. In addition, absorption spectra of SCT-4 were recorded at different pHs to exclude the effect of possible interaction of H+ with SCT-4. It is found that absorption of SCT-4 is independent of changes in pH from 1–7 (ESI, Fig. S36†). The SEM micrograph on the SCT-4/Hg complex (ESI, Fig. S37†) shows a micron-sized solid cluster that stacks up in layers.
Charge transfer complex between SCTs and TCNQ
Formation of donor–acceptor complexes through charge transfer has been well established from electron rich p-type and electron deficient n-type materials.32–34 Such strong donor–acceptor charge transfer complexes with ordered nano/microstructures are known for high electrical conductivity and used as additives in organic electronics.35–37 In this work, the interaction of SCTs with a strong acceptor, 7,7,8,8-tetracyanoquinodimethane (TCNQ) was studied using spectroscopic techniques. Formation of TCNQ dianion (TCNQ2−) was observed at 500 nm upon mixing of SCTs and TCNQ in THF with quenching of neutral TCNQ band at 400 nm (Fig. 11).38,39 Linear terthiophene40 and quaterthiophenes41 were known to form weak charge transfer complexes with TCNQ, resulting in low absorbance at 750 and 840 nm (TCNQ monoanion, TCNQ1−). Recently, formation of sexithiophene–TCNQ charge transfer complex with a small net charge transfer was reported.42,43 In contrast, the highly branched SCTs showed full conversion of TCNQ to TCNQ2−, which suggested strong charge transfer from SCTs to TCNQ. Optical titration studies were carried out to investigate the concentration effect of SCTs on formation of TCNQ dianion (TCNQ2−) in THF (Fig. 12 and 13). As shown in Fig. 13c, low concentration of SCT-4 (3 meq.) shows immediate quenching of neutral TCNQ absorption band. In contrast, presence of neutral TCNQ band was observed in SCT-1 and SCT-2 even at high concentration (Fig. 12a and c). This suggests SCT-4 is strongly electron-donating and the charge transfer to TCNQ is more efficient. The observed quenching of emission from SCTs upon titration with TCNQ were explained due to the formation of ground state charge transfer complex of SCTs and TCNQ. | ||
Fig. 11 Solution state absorption spectra of pristine SCTs ( ) and TCNQ ( ) and TCNQ ( , 6.0 × 10−5 M), and after mixing in THF ( , 6.0 × 10−5 M), and after mixing in THF ( ). ). | ||
A higher equivalent of TCNQ was required for quenching of emission from SCT-4 as compared to that observed in other SCTs (Fig. 13d). This is consistent to the absorption studies which indicate a smaller amount of SCT-4 was sufficient to quench the neutral TCNQ absorption peak. Both absorption and photoluminescence studies indicate that SCTs have strong affinity towards TCNQ in the formation of charge-transfer complex.
Morphology of SCTs/TCNQ
The surface morphology of SCTs/TCNQ complex was studied using SEM. The solutions of SCTs/TCNQ in THF were dropcasted on a glass substrates and slow evaporation of the solvent gave nanosized needle or rod-shaped crystals (ESI, Fig. S38†). These needles were morphologically different from the pristine flat crystals of TCNQ. The high magnification SEM micrographs of the SCT-4/TCNQ showed needles with a diameter in the range of 140–180 nm. Similar organization of molecules was also reported in other TCNQ charge-transfer complexes such as CuTCNQ,44 Ni[TCNQ]2(H2O)2,45 and [TTF][TCNQ]46 (TTF: tetrathiafulvalene).Conclusions
In summary, a series of multi-dimensional branched oligothiophenes with different conjugation lengths was synthesized and characterized using different techniques. All compounds were soluble in common organic solvents. SCTs showed a red-shift in absorption maxima as number of thiophene units increases from SCT-1 (λmax = 360 nm) to SCT-2 (λmax = 410 nm), SCT-3 (λmax = 413 nm), and SCT-4 (λmax = 440 nm) in THF. Both optical and electrochemical studies suggest a non-planar conformation for structurally rigid SCTs. Electron deficient molecules such as TCNQ and heavy metal cations interact strongly with electron rich oligothiophenes. Hg(II) cations completely quenched the photoluminescence intensity of SCT-4 through charge-transfer complex formation. Optical studies suggest that the formation of ground state SCTs–TCNQ charge transfer complex is favored. Such materials with good solubility, high thermal stability, controlled micro/nanostructures and strong electron donating property will be useful in various applications.Experimental
Materials
All chemicals and reagents were purchased from commercial sources (Sigma Aldrich, Alfa Aesar and Merck) and used without further purification. Reactions were monitored by thin-layer chromatography (TLC) carried out on silica gel plates. Preparative separations were performed by column chromatography using silica gel grade 60 (0.040–0.063 mm) from Silicycle.Instrumentation
1H NMR and 13C NMR spectra were recorded on Bruker Avance AV300 (300 MHz) and Bruker Avance AV500 (500 MHz) NMR instruments using appropriate deuterated solvents from Cambridge Isotope Laboratories. The chemical shifts were reported in part per million or ppm and referenced to the residual solvent peak: s = singlet, d = doublet, t = triplet, m = multiplet, and br = broad. Electron impact mass spectroscopy (EI-MS) high resolution mass spectra were obtained on a Finnigan TSQ7000. Elemental analysis was carried out on Elementar Vario Micro Cube. Matrix assisted laser desorption ionization-time of flight (MALDI-TOF) mass spectra were recorded on a Bruker Autoflex III TOF/TOF with reflectron analyzers in positive ionization mode. UV-visible spectra were measured on a UV-1800 Shimadzu UV-VIS spectrophotometer with an optical filter that is calibrated at a bandwidth of 1 nm. The emission spectra were measured on a RF-5301PC Shimadzu spectrofluorophotometer with appropriate analytical grade solvents. The cyclic voltammograms were recorded with a computer controlled CHI electrochemical analyzer at a constant scan rate of 100 mV s−1. The potentials were calibrated using ferrocene/ferrocenium ion redox couple as internal reference. The onset of oxidation (Eonsetox) and reduction (Eonsetred) were used to estimate HOMO (EHOMO) and LUMO (ELUMO) energy levels of polymers using the equation EHOMO = −(4.8 + Eonsetox) and ELUMO = −(4.8 + Eonsetred). Structural optimization and frontier orbital energy levels were calculated using density functional (DFT) level at B3LYP/6-31G(d) level. The LUMO and HOMO electron density plots were generated from the optimized structures using Gaussview 5. Thermogravimetric analysis (TGA) was recorded under nitrogen atmosphere using a heating rate of 10 °C min−1 on a TA Instruments 2960. Differential Scanning Calorimetry (DSC) data were recorded under nitrogen atmosphere using Mettler Toledo DSC1. All samples (3 mg) were dried at 50 °C under vacuum for 24 hours to remove residual moisture prior to thermal analysis. Scanning electron micrographs were recorded using a JEOL JSM-6701F Field Emission Scanning Electron Microscope (SEM).Synthesis of materials and sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) mixture to yield a bright orange solid (0.13 g, yield 60%). 1H NMR (300 MHz, THF-d8, δ ppm) 7.33 (dd, 2H, J = 5.2, 1.1 Hz), 7.30 (dd, 2H, J = 5.2, 1.2 Hz), 7.23 (dd, 2H, J = 5.1, 1.1 Hz), 7.22 (dd, 2H, J = 3.6, 1.1 Hz), 7.20 (dd, 2H, J = 3.6, 1.1 Hz), 7.18 (s, 2H), 7.11 (dd, 2H, J = 3.6, 1.1 Hz), 7.08 (d, 2H, J = 3.8 Hz), 7.04 (d, 2H, J = 3.4 Hz), 7.01–6.97 (4H), 6.94 (d, 2H, J = 3.8 Hz), 6.92–6.87 (6H), 6.86 (s, 2H), 6.71 (d, 2H, J = 3.8 Hz). 13C NMR (126 MHz, THF-d8, δ ppm) δ 139.06, 138.93, 137.76, 137.66, 137.63, 137.44, 137.00, 136.40, 136.14, 136.12, 136.06, 135.14, 134.11, 131.97, 128.52, 128.46, 128.37, 127.63, 126.57, 126.55, 125.41, 125.32, 125.23, 125.19, 125.02, 125.00, 124.90, 124.63, 124.48, 124.45, 124.34. MALDI-TOF: (C64H34S16) calculated m/z = 1316.0, found m/z = 1316.2. Anal. calcd for C64H34S16: C, 58.41; H, 2.60; S, 38.98, found: C, 58.41; H, 2.50; S, 38.78%.
20) mixture to yield a bright orange solid (0.13 g, yield 60%). 1H NMR (300 MHz, THF-d8, δ ppm) 7.33 (dd, 2H, J = 5.2, 1.1 Hz), 7.30 (dd, 2H, J = 5.2, 1.2 Hz), 7.23 (dd, 2H, J = 5.1, 1.1 Hz), 7.22 (dd, 2H, J = 3.6, 1.1 Hz), 7.20 (dd, 2H, J = 3.6, 1.1 Hz), 7.18 (s, 2H), 7.11 (dd, 2H, J = 3.6, 1.1 Hz), 7.08 (d, 2H, J = 3.8 Hz), 7.04 (d, 2H, J = 3.4 Hz), 7.01–6.97 (4H), 6.94 (d, 2H, J = 3.8 Hz), 6.92–6.87 (6H), 6.86 (s, 2H), 6.71 (d, 2H, J = 3.8 Hz). 13C NMR (126 MHz, THF-d8, δ ppm) δ 139.06, 138.93, 137.76, 137.66, 137.63, 137.44, 137.00, 136.40, 136.14, 136.12, 136.06, 135.14, 134.11, 131.97, 128.52, 128.46, 128.37, 127.63, 126.57, 126.55, 125.41, 125.32, 125.23, 125.19, 125.02, 125.00, 124.90, 124.63, 124.48, 124.45, 124.34. MALDI-TOF: (C64H34S16) calculated m/z = 1316.0, found m/z = 1316.2. Anal. calcd for C64H34S16: C, 58.41; H, 2.60; S, 38.98, found: C, 58.41; H, 2.50; S, 38.78%.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) mixture to yield an orange solid (0.22 g, yield 47%). Due to the poor solubility of SCT-4 in common solvents, we were unable to get a good 13C NMR spectrum. 1H NMR (300 MHz, THF-d8, δ ppm) 7.25–7.12 (m, 5H), 7.11–6.76 (m, 26H), 6.75–6.50 (m, 9H), 2.78 (m, 12H), 1.63 (m, 12H), 1.32 (m, 36H), 0.90 (m, 18H). MALDI-TOF: (C124H118S22) calculated m/z = 2312.3, found m/z = 2312.8. Anal. calcd for C124H118S22: C, 64.37; H, 5.14; S, 30.49, found: C, 64.14; H, 5.18; S, 30.21%.
20) mixture to yield an orange solid (0.22 g, yield 47%). Due to the poor solubility of SCT-4 in common solvents, we were unable to get a good 13C NMR spectrum. 1H NMR (300 MHz, THF-d8, δ ppm) 7.25–7.12 (m, 5H), 7.11–6.76 (m, 26H), 6.75–6.50 (m, 9H), 2.78 (m, 12H), 1.63 (m, 12H), 1.32 (m, 36H), 0.90 (m, 18H). MALDI-TOF: (C124H118S22) calculated m/z = 2312.3, found m/z = 2312.8. Anal. calcd for C124H118S22: C, 64.37; H, 5.14; S, 30.49, found: C, 64.14; H, 5.18; S, 30.21%.Preparation of SCTs stock solutions
Dilute solutions of SCTs were prepared by dissolving appropriate amounts of SCTs in HPLC grade THF. The solution was sonicated for 30 minutes to make sure all solids were fully dissolved and used for further studies. The concentrations for SCT-1, SCT-2, SCT-3 and SCT-4 were 1.3 × 10−4 M, 6.0 × 10−5 M, 4.0 × 10−5 M, and 2.6 × 10−5 M, respectively.Sample preparations for morphology studies of SCTs
Appropriate amount of stock solution of SCTs (100 μL) was taken and diluted to 1.0 mL with THF, which was added slowly to 1.0 mL of H2O in a glass vial with screw cap. The above solution was left at room temperature for two days. The resulting solid was transferred to a glass substrate and dried under ambient condition before examination by FESEM.Sample preparations for photophysical studies of SCTs/Hg(II)
To SCTs solution (200 μL), appropriate amount of mercury(II) acetate dissolved in deionized water was added, and the resulting solution was diluted with THF to make up a total volume of 1.0 mL prior to spectroscopic studies. For example, 200 μL of the SCT-4 was added with 20 μL of mercury(II) acetate in deionized water (0.05 M), and the solution was diluted with THF to a final volume of 1.0 mL.Sample preparations for photophysical studies of SCTs/TCNQ
Stock solution (6.0 × 10−5 M) of TCNQ was prepared in THF and appropriate volume (300 μL) of solution was mixed with desired amount of SCTs and diluted with THF to make up a total volume of 2.0 mL prior to spectroscopic studies.Acknowledgements
We would like to thank Department of Chemistry, National University of Singapore (NUS) for funding and technical supports, and NUS high performance computing centre for computations.Notes and references
- J. L. Segura, N. Martin and D. M. Guldi, Chem. Soc. Rev., 2005, 34, 31–47 RSC.
- M. T. Dang, L. Hirsch and G. Wantz, Adv. Mater., 2011, 23, 3597–3602 CrossRef CAS PubMed.
- G. Gigli, O. Inganäs, M. Anni, M. de Vittorio, R. Cingolani, G. Barbarella and L. Favaretto, Appl. Phys. Lett., 2001, 78, 1493–1495 CrossRef CAS.
- M. Melucci, M. Zambianchi, L. Favaretto, V. Palermo, E. Treossi, M. Montalti, S. Bonacchi and M. Cavallini, Chem. Commun., 2011, 47, 1689–1691 RSC.
- G. Horowitz and M. E. Hajlaoui, Adv. Mater., 2000, 12, 1046–1050 CrossRef CAS.
- J. G. Mei, K. R. Graham, R. Stalder, S. P. Tiwari, H. Cheun, J. Shim, M. Yoshio, C. Nuckolls, B. Kippelen, R. K. Castellano and J. R. Reynolds, Chem. Mater., 2011, 23, 2285–2288 CrossRef CAS.
- R. P. Ortiz, J. Casado, V. Hernández, J. T. L. Navarrete, J. A. Letizia, M. A. Ratner, A. Facchetti and T. J. Marks, Chem.–Eur. J., 2009, 15, 5023–5039 CrossRef PubMed.
- M. Halik, H. Klauk, U. Zschieschang, G. Schmid, S. Ponomarenko, S. Kirchmeyer and W. Weber, Adv. Mater., 2003, 15, 917–922 CrossRef CAS.
- F. Laquai, D. Andrienko, R. Mauer and P. W. M. Blom, Macromol. Rapid Commun., 2015, 36, 1001–1025 CrossRef CAS.
- A. Mishra, C. Q. Ma and P. Bäuerle, Chem. Rev., 2009, 109, 1141–1276 CrossRef CAS.
- Z. Li, J. Bian, Y. Wang, F. Jiang, G. Liang, P. He, Q. Hou, J. Tong, Y. Liang, Z. Zhong, Y. Zhou and W. Tian, Sol. Energy Mater. Sol. Cells, 2014, 130, 336–346 CrossRef CAS.
- S. C. Lan, C. K. Chang, Y. H. Lu, S. W. Lin, A. K. Y. Jen and K. H. Wei, RSC Adv., 2015, 5, 67718–67726 RSC.
- R. Shoumura, K. Sugiyasu, T. Yasuda, A. Sato and M. Takeuchi, Macromolecules, 2012, 45, 3759–3771 CrossRef.
- L. Guan and P. Liu, J. Nanosci. Nanotechnol., 2015, 15, 3029–3034 CrossRef CAS.
- J. Luo, K. W. Huang, H. M. Qu, X. J. Zhang, L. J. Zhu, H. S. O. Chan and C. Y. Chi, Org. Lett., 2010, 12, 5660–5663 CrossRef CAS PubMed.
- V. Jeux, C. Dalinot, M. Allain, L. Sanguinet and P. Leriche, Tetrahedron Lett., 2015, 56, 1383–1387 CrossRef CAS.
- C. J. Xia, X. W. Fan, J. Locklin and R. C. Advincula, Org. Lett., 2002, 4, 2067–2070 CrossRef CAS PubMed.
- F. Huang, K. S. Chen, H. L. Yip, S. K. Hau, O. Acton, Y. Zhang, J. D. Luo and A. K. Y. Jen, J. Am. Chem. Soc., 2009, 131, 13886–13887 CrossRef CAS PubMed.
- A. Bilge, A. Zen, M. Forster, H. Li, F. Galbrecht, B. S. Nehls, T. Farrell, D. Neher and U. Scherf, J. Mater. Chem., 2006, 16, 3177–3182 RSC.
- A. Zen, A. Bilge, F. Galbrecht, R. Alle, K. Meerholz, J. Grenzer, D. Neher, U. Scherf and T. Farrell, J. Am. Chem. Soc., 2006, 128, 3914–3915 CrossRef CAS.
- M. Tateno, M. Takase, M. Iyoda, K. Komatsu and T. Nishinaga, Chem.–Eur. J., 2013, 19, 5457–5467 CrossRef CAS PubMed.
- S. Klod, K. Haubner, E. Jähne and L. Dunsch, Chem. Sci., 2010, 1, 743–750 RSC.
- S. Gronowitz, Acta Chem. Scand., 1961, 15, 1393–1395 CrossRef CAS.
- N. Jayasuriya, J. Kagan, D. Huang and B. Teo, Heterocycles, 1988, 27, 1391–1394 CrossRef CAS.
- C. Taliani and W. Gebauer in Handbook of Oligo- and Polythiophenes, Wiley-VCH Verlag GmbH, 2007, pp. 361–404 Search PubMed.
- D. D. Cunningham, L. Laguren-Davidson, H. B. Mark, C. van Pham and H. Zimmer, J. Chem. Soc., Chem. Commun., 1987, 1021–1023 RSC.
- F. Charra, D. Fichou, J. M. Nunzi and N. Pfeffer, Chem. Phys. Lett., 1992, 192, 566–570 CrossRef CAS.
- J. P. Parakka, J. A. Jeevarajan, A. S. Jeevarajan, L. D. Kispert and M. P. Cava, Adv. Mater., 1996, 8, 54–59 CrossRef CAS.
- A. F. D. de Namor and I. Abbas, J. Phys. Chem. B, 2007, 111, 5803–5810 CrossRef PubMed.
- A. Pal and B. Bag, J. Photochem. Photobiol., A, 2012, 240, 42–49 CrossRef CAS.
- R. Castañeda, V. N. Khrustalev, A. Fonari, J. L. Bredas, Y. A. Getmanenko and T. V. Timofeeva, J. Mol. Struct., 2015, 1100, 506–512 CrossRef.
- N. S. Sariciftci, L. Smilowitz, A. J. Heeger and F. Wudl, Science, 1992, 258, 1474–1476 CAS.
- K. Kim, J. Liu, M. A. G. Namboothiry and D. L. Carroll, Appl. Phys. Lett., 2007, 90, 163511 CrossRef.
- X. Han, Z. Wu and B. Sun, Org. Electron., 2013, 14, 1116–1121 CrossRef CAS.
- J. K. Jeszka, J. Ulański and M. Kryszewski, Nature, 1981, 289, 390–391 CrossRef CAS.
- H. Kobayashi and J. Nakayama, Bull. Chem. Soc. Jpn., 1981, 54, 2408–2411 CrossRef CAS.
- T. Sumimoto, K. Kudo, T. Nagashima, S. Kuniyoshi and K. Tanaka, Synth. Met., 1995, 70, 1251–1252 CrossRef CAS.
- H. T. Jonkman and J. Kommandeur, Chem. Phys. Lett., 1972, 15, 496–499 CrossRef CAS.
- Y. H. Kim, S. D. Jung, M. A. Chung, K. D. Song and D. W. Cho, Bull. Korean Chem. Soc., 2008, 29, 948–952 CrossRef CAS.
- B. Xu, D. Fichou, G. Horowitz and F. Garnier, Synth. Met., 1991, 42, 2319–2322 CrossRef CAS.
- S. Hotta and K. Waragai, Synth. Met., 1989, 32, 395–397 CrossRef CAS.
- F. Jäckel, U. G. E. Perera, V. Iancu, K. F. Braun, N. Koch, J. P. Rabe and S. W. Hla, Phys. Rev. Lett., 2008, 100, 126102 CrossRef.
- K. F. Braun and S. W. Hla, J. Chem. Phys., 2008, 129, 064707 CrossRef PubMed.
- S. Pal, S. Maiti, U. N. Maiti and K. K. Chattopadhyay, J. Mater. Chem. C, 2014, 2, 4005–4011 RSC.
- J. Song, Z. Ji, Q. Nie and W. Hu, Nanoscale, 2014, 6, 2573–2576 RSC.
- J. P. Savy, D. de Caro, C. Faulmann, L. Valade, M. Almeida, T. Koike, H. Fujiwara, T. Sugimoto, J. Fraxedas, T. Ondarcuhu and C. Pasquier, New J. Chem., 2007, 31, 519–527 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H and 13C NMR spectra, mass spectra, absorption and photoluminescence spectra, DFT optimized geometries and SEM images of all compounds. See DOI: 10.1039/c5ra21089e |
| This journal is © The Royal Society of Chemistry 2015 |