Significant enhancement of the electroactive β-phase of PVDF by incorporating hydrothermally synthesized copper oxide nanoparticles
Biplab Dutta,
Epsita Kar,
Navonil Bose and
Sampad Mukherjee*
Department of Physics, Indian Institute of Engineering Science and Technology, Shibpur, Howrah-711103, India. E-mail: smukherjee.besu@gmail.com; Tel: +91 9433579392
First published on 23rd November 2015
Abstract
The influence of copper oxide nanoparticles (CONPs) on the polymorphism of poly(vinylidene fluoride) (PVDF) was systematically investigated in this work. Copper oxide nanoparticles having an average diameter of 60 nm were synthesized by a simple, cost effective, environmentally benign modified hydrothermal method. Then a series of copper oxide nanoparticles incorporating flexible, self-standing PVDF films were prepared by the solution casting technique. The impact of the CONP loading on the structural and morphological properties of PVDF were studied by X-ray diffraction, scanning electron microscopy and Fourier transform infrared spectroscopy techniques. The thermal properties of the sample were investigated by differential thermal analysis, thermogravimetric analysis and differential scanning calorimetry techniques. The incorporation of CONPs leads to faster crystallization and thereby promotes the formation of electroactive β-phase enriched PVDF films. Strong interfacial interactions between the negatively charged nanoparticle surface and positively charged –CH2 dipoles of the PVDF lead to a significant enhancement of the electroactive β-phase. The 5 wt% CONP–PVDF composite film exhibits a maximum β-phase fraction of 90% due to having the highest interfacial area between the well-dispersed nanoparticles’ surfaces and the polymer.
1. Introduction
Recently, electroactive polymers have attracted much attention due to their various potential applications in energy harvesting, sensors, actuators and in the biomedical field.1–4 Of the few polymers showing piezoelectric, pyroelectric and ferroelectric properties, such as Nylon-11, poly(lactic-co-glycolic acid) (PLGA) and polylactic acid (PLLA),5–9 poly(vinylidene fluoride) (PVDF) and its copolymers have the most versatile electroactive properties. Therefore PVDF and its copolymers such as poly(vinylidene fluoride–trifluoroethylene) [P(VDF–TrFE)] and poly(vinylidene fluoride–hexafluoropropylene) [P(VDF–HFP)] are emerging as the popular polymers of choice for a growing number of potential applications.10–13 Poly(vinylidene fluoride) ([–CH2–CF2–]n), a flexible, cost-effective fluoropolymer, has received much interest due to its wide range of significant applications in piezoelectric nanogenerators, high charge storage capacitors, electro-striction for artificial muscles, magneto-striction, non-volatile memories in microelectronics, thin film transistors and pulsed lasers.1,10–13 PVDF is a semicrystalline polymer having five different crystalline phases, i.e., α, β, γ, δ and ε.14 Among all of these phases, the non-polar α-phase is the most common and thermodynamically stable state at ambient temperature and pressure. On the other hand the β- and γ-phases are the polar phases of PVDF. The α-phase possesses a monoclinic unit cell structure with a TGTG′ (T – trans, G – gauche+, G′ – gauche) dihedral conformation, whereas the β- and γ-phases contain orthorhombic unit cell structures with TTT (all trans) and 3TG3TG′ conformations respectively.14–17 However the β-phase has become more important than the other phases due to its better piezoelectric, ferroelectric and pyroelectric properties.8,9,11,24 Thus, many research works regarding the enhancement of the electroactive β-phase content in PVDF have been reported for various applications of this particular polymer.8,9,14,18,19 Different techniques, including the uniaxial or biaxial drawing of α-PVDF films, simultaneous poling, stretching and quenching the α-PVDF films, melting crystallization of α-PVDF under high pressure, the supercritical carbon dioxide technique and the electro-spinning method, are used to achieve a higher β-phase content in PVDF.9 The enhancement of the electroactive β-phase fraction in the PVDF matrix can also be achieved by the incorporation of various sub-micron or nanosized filler materials including metals, metal oxides, clays, ceramics, semiconductor oxides, carbon nanotubes, graphene, inorganic salts and ferrites.8,9,11,18–26 Since the sizes of fillers are in the nano-dimension, the interfacial interactions between the nanofillers and the polymer matrices increase significantly due to the high surface-to-volume ratio of the nanofillers. Thus the addition of a small amount of nanofiller improves the physicochemical properties of the polymer without compromising the polymer flexibility. Gold,18 silver,19 iron oxide and cobalt oxide24 nanomaterials are used to enhance the electroactive β-phase content in PVDF. It is well-known that the use of metal and metal oxide nanoparticles can also significantly improve the mechanical, electrical and magnetic properties of polymeric composite films without losing the flexibility of the composite films.18,20,21,25 Of the different metal oxide nanoparticle fillers, copper oxide nanoparticles are of paramount importance due to their key role in the enhancement of the electrical properties of PVDF.20,25 A significant enhancement of the dielectric constant (ε′ ∼ 103) of copper oxide–PVDF nanocomposite films has been previously reported.20 It will be interesting to study the effect of the incorporation of comparatively cost-effective copper oxide nanoparticles (CONPs) on the nucleation of the electroactive β-phase in PVDF, as well as to study the structural and thermal properties of the copper oxide–PVDF nanocomposite films, which have not been reported till date.This present work deals with the synthesis of copper oxide nanoparticles by a modified hydrothermal method. Hydrothermally synthesized copper oxide nanoparticles are incorporated into the PVDF matrix. Detailed analyses of the structural and thermal properties of the CONP loaded PVDF films have been included in this study. The effect of the copper oxide nanoparticles on the nucleation of the electroactive β-phase in PVDF, along with the reason behind the significant improvement of the β-phase fraction, has also been discussed from the physicochemical point of view.
2. Experimental
2.1 Materials
Poly(vinylidene fluoride) (PVDF) pellets (Mw = 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1) were obtained from Sigma Aldrich, USA. Dry N,N-dimethylformamide (DMF; Merck, India), copper(II) sulphate pentahydrate (CuSO4·5H2O, Mw = 249.685 g mol−1; Merck, India), sodium hydroxide (NaOH; Merck, India) and ethyl alcohol (C2H6O; Merck, India) were used in this work. All the materials were used in the experiments without further purification.
500 g mol−1) were obtained from Sigma Aldrich, USA. Dry N,N-dimethylformamide (DMF; Merck, India), copper(II) sulphate pentahydrate (CuSO4·5H2O, Mw = 249.685 g mol−1; Merck, India), sodium hydroxide (NaOH; Merck, India) and ethyl alcohol (C2H6O; Merck, India) were used in this work. All the materials were used in the experiments without further purification.
2.2 Synthesis of copper oxide nanoparticles
The copper oxide nanoparticles were synthesized by a modified hydrothermal method.27 In this typical procedure, initially a 0.1 M solution of CuSO4·5H2O was prepared in distilled water. A 1 M NaOH solution was then added drop-by-drop to the solution of CuSO4·5H2O with vigorous stirring until the pH of the resultant solution reached 13. After that, the solution was transferred to a stainless steel autoclave and fully sealed. The autoclave was heated in a furnace at a temperature of 110 °C for 18 hours. The autoclave was then set for natural cooling. After cooling to room temperature (30 °C), the obtained solution was centrifuged (4000 rpm) for 30 minutes. The as-obtained product was thoroughly washed several times using distilled water and ethyl alcohol to remove the residual impurities. Finally, the solution was dried to afford a powder and the powder sample was then kept in a vacuum desiccator for the complete removal of the residual solvent contained in the powder.2.3 Synthesis of copper oxide loaded PVDF nanocomposite films
The copper oxide nanoparticle loaded PVDF films were synthesized by the simple solution casting method. In this typical synthesis procedure, 500 mg of PVDF was dissolved in 20 ml DMF with vigorous stirring at 60 °C and the complete dissolution of PVDF in DMF was achieved. Then a certain weight percent (1–5 wt%) of the as-synthesized copper oxide nanoparticles were added to the solution of PVDF and vigorously stirred for 16 hours followed by 30 min sonication to obtain a homogeneous mixture. The nanocomposite films were prepared by casting the mixture in a thoroughly cleaned and dried Petri dish, and the solvent was evaporated at 90 °C. Neat PVDF films were also prepared following the same procedure along with the loaded PVDF films. 1 wt%, 3 wt% and 5 wt% copper oxide nanoparticle loaded PVDF films were prepared in this work and are named PCO1, PCO3 and PCO5 respectively. The prepared neat PVDF is named P0.2.4 Characterization methods
The structural properties of the as-synthesized CONPs and composite films were studied by X-ray diffractometry (Bruker-D8) with Cu-Kα radiation (wavelength 1.541 Å) using Bragg–Brentano goniometer geometry and the θ–2θ mechanism. The XRD patterns of all the samples are recorded with a scan speed of 0.3 s per step and under an operating voltage of 40 kV with 2θ varying from 10 to 50°.Absorption spectra of the as-synthesized CONPs and CONP loaded PVDF films were recorded by UV-visible spectrophotometry (Jasco V-630 Spectrophotometer) in the wavelength range 200–1100 nm.
The surface morphology of the nanoparticles and the as-synthesized copper oxide nanoparticle doped PVDF films were studied by field emission scanning electron microscopy (FESEM) (Quanta, FEG 250). Energy dispersive X-ray spectroscopy (EDAX) of the samples was also carried out in the same FESEM instrument. The structure, shape and size distribution of the as-synthesized copper oxide nanoparticles were studied by transmission electron microscopy (TEM) using an FEI.TECHNAI.T-20 G2 SUPERTWIN (200 kV) instrument.
Vibrational spectra for all the samples were recorded at room temperature by Fourier transform infrared (FTIR) spectroscopy (Jasco FT/IR-460 PLUS) with a resolution of 1 cm−1. The fraction of β-phase F(β) in the as-synthesized nanocomposite films was calculated from the FTIR spectra using the Beer–Lambert Law as given by,14
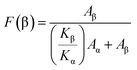 | (1) |
Differential thermal analysis (DTA) and thermogravimetric analysis (TGA) were performed using TGA/SDTA851 Mettler Toledo apparatus in an air atmosphere at a heating rate of 10 °C min−1. The crystallization and melting behavior of the pure and copper oxide nanoparticle loaded PVDF films were analyzed using a differential scanning calorimeter (DSC-60, Shimadzu (Asia Pacific) Pte. Ltd, Singapore). All the samples were heated from 30 °C to 200 °C at a rate of 10 °C min−1 in a nitrogen gas atmosphere. The degree of crystallinity (Xc) of the samples was calculated using the following equation:28
| Xc = ΔHc/ΔH100% | (2) |
3. Results and discussion
3.1 Copper oxide nanoparticles
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11), (111), (020), (
11), (111), (020), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 13), (
13), (![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 11), (113) and (311) planes of the crystalline phase of monoclinic cupric oxide (CuO) in accordance with JCPDS no. 41-0254 (a = 4.685 Å, b = 3.423 Å, c = 5.132 Å, space group: C12/c1). The peaks positioned at 2θ values 13.7°, 16.8°, 21.3°, 27.6°, 32.3° are assigned to the (110), (111), (220) and (311) planes of the crystalline phase of cubic cuprous oxide (Cu2O) (JCPDS no. 34-1354, a = 4.217 Å, space group: Pn
11), (113) and (311) planes of the crystalline phase of monoclinic cupric oxide (CuO) in accordance with JCPDS no. 41-0254 (a = 4.685 Å, b = 3.423 Å, c = 5.132 Å, space group: C12/c1). The peaks positioned at 2θ values 13.7°, 16.8°, 21.3°, 27.6°, 32.3° are assigned to the (110), (111), (220) and (311) planes of the crystalline phase of cubic cuprous oxide (Cu2O) (JCPDS no. 34-1354, a = 4.217 Å, space group: Pn![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m). The XRD analysis indicates that the as-synthesized copper oxide nanoparticles are in the mixed crystalline phases of CuO and Cu2O with a dominating fraction of the CuO phase.
m). The XRD analysis indicates that the as-synthesized copper oxide nanoparticles are in the mixed crystalline phases of CuO and Cu2O with a dominating fraction of the CuO phase.
3.2 Copper oxide loaded PVDF nanocomposite films
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 11) can be readily indexed to the monoclinic CuO phase (JCPDS-41-0254) of the CONPs, whereas peaks at 2θ values 13.57° (110), 22.54° (200) and 33.17° (311) can be assigned to the cubic Cu2O phase (JCPDS-34-1354) of CONPs. The occurrence of these peaks attributed to the different phases of CONPs indicates the successful incorporation of CONPs in the PVDF matrix. The XRD patterns also show that with the increasing loading fraction of the nanoparticles, the intensity of the peaks corresponding to the α-phase of PVDF (positioned at 17.5°, 18.2°, 19.8° and 26.6°) decreases gradually. However, a new peak positioned around 2θ = 20.6° ((020), (101)), which is the characteristic peak of β-phase PVDF, appears in the copper oxide nanoparticle loaded PVDF films.14,23 A closer observation of the XRD patterns reveals that for sample PCO5, the intensity of the peak positioned at 26.6° corresponding to the non-polar α-phase of PVDF is almost completely diminished, whereas the relative intensity of the peak positioned at 2θ = 20.6° (characteristic peak of β-phase PVDF) becomes maximized. It can be also seen from the XRD pattern that a peak positioned at 2θ = 38.9° (211) corresponding to the γ-phase of PVDF14,23 is present for the neat as well as the loaded PVDF films. The ratio I20.6°/I18.2° is a measurement of the α- and β-phase content in the neat PVDF and CONP incorporating nanocomposite films, where I20.6° and I18.2° are the intensities of the peaks positioned at 20.6° (characteristic peak for the β-phase) and 18.2° (characteristic peak for the α-phase) respectively. Fig. 4b represents the content dependence of the ratio (I20.6°/I18.2°). For the neat PVDF film the ratio is about 0.93 and this ratio increased with the increasing weight fraction of the CONPs in the PVDF matrix. The maximum value of the ratio is ca. 12.05, obtained for sample PCO5 (5 wt%). Thus, as a whole, the XRD patterns of the loaded PVDF films confirm that the incorporation of the CONPs results in a phase transformation from the α-phase to the electroactive β-phase of PVDF.
11) can be readily indexed to the monoclinic CuO phase (JCPDS-41-0254) of the CONPs, whereas peaks at 2θ values 13.57° (110), 22.54° (200) and 33.17° (311) can be assigned to the cubic Cu2O phase (JCPDS-34-1354) of CONPs. The occurrence of these peaks attributed to the different phases of CONPs indicates the successful incorporation of CONPs in the PVDF matrix. The XRD patterns also show that with the increasing loading fraction of the nanoparticles, the intensity of the peaks corresponding to the α-phase of PVDF (positioned at 17.5°, 18.2°, 19.8° and 26.6°) decreases gradually. However, a new peak positioned around 2θ = 20.6° ((020), (101)), which is the characteristic peak of β-phase PVDF, appears in the copper oxide nanoparticle loaded PVDF films.14,23 A closer observation of the XRD patterns reveals that for sample PCO5, the intensity of the peak positioned at 26.6° corresponding to the non-polar α-phase of PVDF is almost completely diminished, whereas the relative intensity of the peak positioned at 2θ = 20.6° (characteristic peak of β-phase PVDF) becomes maximized. It can be also seen from the XRD pattern that a peak positioned at 2θ = 38.9° (211) corresponding to the γ-phase of PVDF14,23 is present for the neat as well as the loaded PVDF films. The ratio I20.6°/I18.2° is a measurement of the α- and β-phase content in the neat PVDF and CONP incorporating nanocomposite films, where I20.6° and I18.2° are the intensities of the peaks positioned at 20.6° (characteristic peak for the β-phase) and 18.2° (characteristic peak for the α-phase) respectively. Fig. 4b represents the content dependence of the ratio (I20.6°/I18.2°). For the neat PVDF film the ratio is about 0.93 and this ratio increased with the increasing weight fraction of the CONPs in the PVDF matrix. The maximum value of the ratio is ca. 12.05, obtained for sample PCO5 (5 wt%). Thus, as a whole, the XRD patterns of the loaded PVDF films confirm that the incorporation of the CONPs results in a phase transformation from the α-phase to the electroactive β-phase of PVDF.
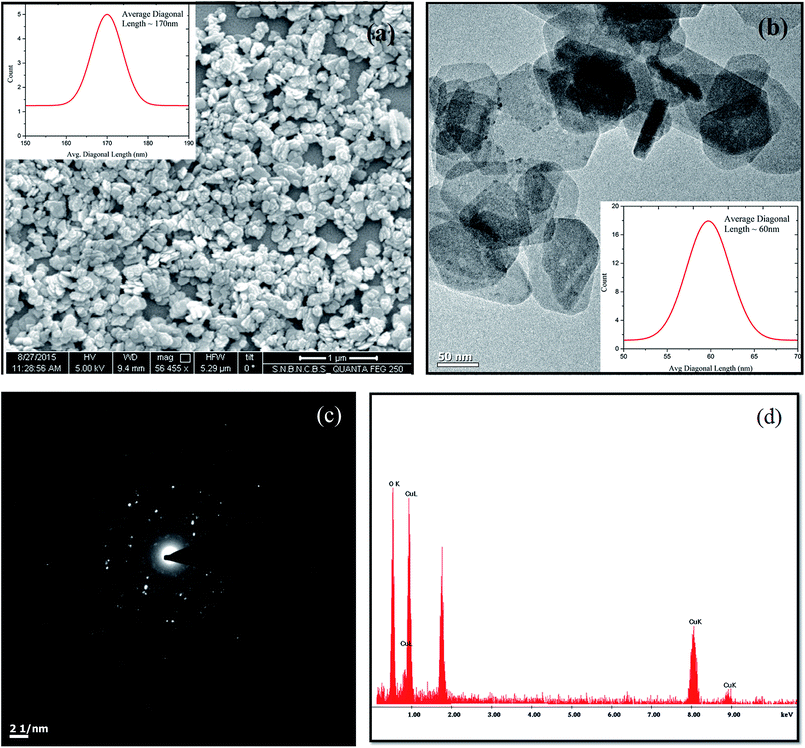
The uniform distribution of the spherulites confirms the good, homogeneous dispersion of the copper oxide nanoparticles in the PVDF matrix. The smaller size of the spherulites for sample PCO5 in comparison to that of the neat PVDF represents the faster nucleation kinetics in the CONP–PVDF composite films28 due to the presence of additional CONP nucleation centers, and the enhancement of the β-phase fraction may be achieved in the composite film due to the existence of the large number of favorable nucleation centers.28 Again the presence of the added CONPs in the PVDF film can be clearly seen from the high magnification FESEM micrograph of sample PCO5 in Fig. 6b. It is evident from the figure that the surface of the PVDF film is completely modified with the addition of CONPs. Impinging spherulites (marked by straight red lines) are observed in the figure due to the faster kinetics in the presence of CONPs. Thus the FESEM images for both the neat and composite films support the enhancement of the electroactive β-phase due to the incorporation of the CONPs which is consistent with the result obtained from the X-ray diffraction patterns of the samples. Fig. 6c represents the EDAX result of the CONP loaded PVDF film (PCO5). The figure shows the presence of characteristic peaks corresponding to Cu, O, C and F elements where C and F are the polymeric components of the PVDF matrix, and Cu and O are the components of the CONPs as previously shown in Fig. 2d. Hence the presence of copper oxide in PVDF is further confirmed by the EDAX result.
To determine the kinetic parameters for the thermal degradation of the nanocomposite films the Coats–Redfern equation31 is usually used, which is as follows:
 | (3) |
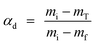 | (4) |
| Name of the sample | Maximum degradation temperature (Tmax) (°C) | Activation energy at the major degradation region (Ed) (kJ mol−1) | Regression factor (R2) |
|---|---|---|---|
| P0 | 478.7 | 545.64 | 0.9998 |
| PCO1 | 480.9 | 628.41 | 0.9997 |
| PCO5 | 477.7 | 654.22 | 0.9995 |
The interface between the polymer and nanofillers plays a key role in determining the final properties of the nanocomposite films. From the TGA results, the nanofiller–polymer interface region (mI) of the CONP–PVDF composite was obtained by using the following equation,37
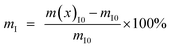 | (5) |
The thermal properties and polymorphism of the composites were also studied using DSC. Fig. 12a–c show the DSC heating cycles of the samples P0, PCO1 and PCO5 respectively. To get a deeper insight into the coexistence of different PVDF phases in the composite films, the DSC curves were fitted with multiple Gaussian functions corresponding to α-, β- and γ-phase PVDF. The multi-peak fitted DSC heating curves reveal the presence of three distinguishable peaks, indicating the coexistence of α-, β- and γ-phases in the neat as well as the PVDF nanocomposite films. The melting temperature for all the samples corresponding to the three different phases are shown in Table 2. The figures show that the relative intensity and the area under the peak related to the melting temperature of the β-phase increase with the increasing filler concentration of the CONPs. On the other hand, the intensity and the area under the curve assigned to α-PVDF diminishes significantly with the increasing loading fraction of the CONPs in the PVDF matrix. This observation can be explained by the increment in the β-PVDF nucleation with the expense of a reduction in the α-PVDF fraction in the composite films. The transformation from the α to β polymorph occurs due to the incorporation of CONPs. It can be seen from Fig. 12c that for sample PCO5 the relative intensity of the peak related to the β-phase of PVDF is greatest, while the peak related to α-phase PVDF is almost completely diminished. It is worthwhile to mention here that the XRD and FTIR analyses of the neat and PVDF nanocomposite films also validate the presence of the maximum β-phase fraction in sample PCO5. The DSC cooling cycles for neat PVDF and copper oxide nanoparticle loaded PVDF films are shown in Fig. 13. Though multiple endothermic peaks can be seen from the heating cycle of the neat and CONP loaded PVDF films, the cooling cycles of the samples show a single exothermic peak. This indicates the existence of polymorphs in the films. However, a single exothermic peak is obtained for the cooling cycle as, after the first heating cycle in DSC, the processing history is erased. The single exothermic peak present in the DSC cooling cycle is attributed to the crystallization temperature (Tc) of the neat and loaded PVDF films. The crystallization temperature for the CONP incorporated PVDF films is higher than that of neat PVDF as observed from Fig. 13 and it increases with the increasing content of CONPs in the PVDF matrix. This observation is expected as the incorporated copper oxide nanoparticles act as the nucleating agent in the PVDF matrix, thereby influencing the crystallization temperature. For sample PCO5 the crystallization temperature is increased by ∼2 °C in comparison to that of the neat PVDF. This indicates that the addition of well-dispersed CONPs as the nucleating agent lowers the free energy barrier of nucleation, thus accelerating the crystallization of PVDF, which manifests in a higher crystallization temperature for the nanocomposite films.28 The faster nucleation kinetics is also evident from the FESEM micrograph of PCO5 (Fig. 6a) where smaller spherulites are observed in comparison to neat PVDF. DSC thermographs were also used to obtain the enthalpy of crystallization (ΔHc J g−1) and the degree of crystallinity (Xc) of the neat PVDF as well as the CONP–PVDF composite films. The degree of crystallinity of the samples was calculated using eqn (2). The crystallization temperatures (Tc), enthalpy of crystallization (ΔHc), and the degree of crystallinity (Xc) for samples P0, PCO1 and PCO5 are reported in Table 3. The crystallinity decreases with the addition of CONPs to the PVDF matrix and PCO5 shows the minimum value of Xc. As is evident from the XRD and FTIR results, neat PVDF contains three phases, α, β and γ, but for sample PCO5 the α-phase of PVDF is almost completely diminished and the only present dominant crystalline phase is β-PVDF, resulting in a reduction in the overall crystallinity of the composite films. This indicates the intimate interaction of the CONPs with PVDF, and thus the strong influence of the nanoparticles on the structural modification of PVDF matrix.
 | ||
| Fig. 12 DSC heating cycles for samples (a) P0; (b) PCO1; and (c) PCO5. The DSC curves are fitted with multiple Gaussian functions corresponding to α-, β- and γ-phase PVDF. | ||
| Sample name | Melting temperature of α-PVDF (Tm)α (°C) | Melting temperature of β-PVDF (Tm)β (°C) | Melting temperature of γ-PVDF (Tm)γ (°C) |
|---|---|---|---|
| P0 | 156.0 | 161.5 | 164.6 |
| PCO1 | 158.3 | 162.8 | 166.7 |
| PCO5 | 153.5 | 162.4 | 166.9 |
| Sample name | Crystallization temperature (Tc) (°C) | Enthalpy (ΔHc) | Crystallinity (Xc) (%) |
|---|---|---|---|
| P0 | 134.6 | 36.9 | 35 |
| PCO1 | 136.0 | 34.8 | 33 |
| PCO5 | 136.8 | 26.7 | 26 |
3.3 Mechanism of electroactive β-phase nucleation
The results obtained from the XRD, FTIR, and thermal studies of the nanocomposite films confirm that the incorporation of copper oxide nanoparticles in the PVDF matrix leads to an increase in electroactive β-phase nucleation. Neat PVDF films primarily contain α-phase PVDF and the transformation from the α to the electroactive β polymorph in PVDF is possible due to the incorporation of CONPs as well as the homogeneous dispersion of these CONPs in the PVDF matrix. Therefore, in this context it is meaningful to analyze the interaction between the copper oxide nanoparticles and the polymer, which promotes the formation of the electroactive β-phase. The improvement of the electroactive β-phase fraction may occur due to the strong interactions between the PVDF matrix and embedded nanoparticles.8,9,24 This type of ion–dipole interaction between the PVDF matrix and the loaded ferrite nanoparticles to form the electroactive β-phase has also been reported by Martins et al.32 During the synthesis of the copper oxide nanoparticles we maintained the pH of the copper precursor solution at 13 by the use of NaOH solution. The variation of the zeta potential of the copper oxide nanoparticles with the pH of the respective precursor solution has been reported by Hassan et al.33 This variation of zeta potential confirms that the surface of the as-synthesized copper oxide nanoparticles are negatively charged. When the negatively charged nanoparticles are added to the PVDF matrix in the solution phase, the nanoparticles will act as substrates for the formation of the electroactive β-phase. The partially positive –CH2 dipoles of the PVDF chains experience strong electrostatic interactions with the negatively charged nanoparticle surfaces. The interaction leads to the alignment of the stabilized PVDF chains on the surface of the nanoparticles in a longer ‘all-trans’ (TTT) conformation resulting in the formation of the electroactive β-phase. Thus the surface of the nanoparticles acts as the nucleation center for the formation of the electroactive β-phase. Fig. 14 depicts the schematic of the ion–dipole interaction mechanism between the nanoparticles and the polymer chains leading to the formation of the electroactive β-phase. It is worthwhile to mention here that our experimental observations show the presence of a small fraction of the electroactive γ-phase of PVDF for the loaded PVDF films. Similar results have also been reported by Lopes et al. for aluminosilicate–PVDF composites.34–36 This γ-phase formation may occur due to the gauge effect which may develop due to the easier local internal chain rotation.4. Conclusion
In this work, a series of flexible CONP–PVDF composite films were synthesized by a simple solution casting technique and the effect of the CONPs on the polymorphism of PVDF was rigorously studied. The hexagonal copper oxide nanoparticles with an average size of 60 nm were synthesized by the modified hydrothermal method. Our study confirms the successful inclusion of well-dispersed copper oxide nanoparticles in the PVDF matrix. XRD, FESEM, FTIR and thermal analyses of the composite films confirm that the loading of copper oxide nanoparticles in PVDF leads to the successful transformation from the α to the electroactive β polymorph in PVDF. The β-phase fraction in PVDF increases with the increasing CONP loading in the polymer matrix. PCO5 shows the highest β-phase fraction of ca. 90%. The enhancement in the β-phase fraction of PVDF is attributed to the interactions of the negatively charged nanoparticle surfaces and the positive –CH2 groups of the PVDF polymer chains, which leads to the alignment of the stabilized, longer ‘all-trans’ (TTT) conformation of the PVDF chains on the surface of the nanoparticles, resulting in the nucleation of the electroactive β-phase. The calculated nanoparticle–polymer interface is also maximal for PCO5. The highest rate of enhancement (with nanofiller loading) in the β-phase fraction and nanoparticle–polymer interface values is observed for the 1 wt% CONP loaded PVDF composite film. However, the values are further enhanced with increasing CONP filler content in the PVDF matrix. This indicates that the CONPs are nicely dispersed in the PVDF matrix up to 5 wt% loading. The FESEM micrographs show that the size of the spherulites is decreased for the CONP loaded PVDF films in comparison to the neat PVDF, which is facilitated due to the faster nucleation kinetics in PVDF due the presence of the homogeneously dispersed CONP nucleating agent. This phenomenon is also supported by the DSC results. The faster nucleation kinetics help to enhance the β polymorph in the nanoparticle loaded PVDF. The thermal stability of the composite films is not degraded with the CONP loading. Rather, the melting temperature is increased by ∼3 °C for PCO5 in comparison to neat PVDF. In this context it should be noted that the copper oxide nanoparticles are synthesized by a simple, cost effective and environmentally benign hydrothermal method. Thus the as-synthesized copper oxide nanoparticle loaded PVDF films with a significantly enhanced β-phase fraction may be used in future cost effective, efficient piezoelectric devices.Acknowledgements
The authors wants to acknowledge DST INSPIRE, Government of India (IF140204, IF140209) for the financial support and Dr Sukhen Das for providing different characterization facilities.References
- Q. M. Zhang, V. Bharti and X. Zhao, Science, 1998, 280(5372), 2101–2104 CrossRef CAS.
- S. Bauer, J. Appl. Phys., 1996, 80(10), 5531–5558 CrossRef CAS.
- Q. M. Zhang, H. F. Li, M. Poh, H. S. Xu, Z.-Y. Cheng, F. Xia and C. Huang, Nature, 2002, 419, 284–287 CrossRef CAS PubMed.
- S. Nambiar and J. T. W. Yeow, Biosens. Bioelectron., 2011, 26, 1825–1832 CrossRef CAS PubMed.
- S. C. Mathur, J. I. Scheinbeim and B. A. Newman, J. Appl. Phys., 1984, 56, 2419–2425 CrossRef CAS.
- D. J. Bryan, J. B. Tang, S. A. Doherty, D. D. Hile, D. J. Trantolo, D. L. Wise and I. C. Sum-merhayes, J. Neural Eng., 2004, 1, 91–98 CrossRef PubMed.
- L. Huang, X. Zhuang, J. Hu, L. Lang, P. Zhang, Y. Wang, X. Chen, Y. Wei and X. Jing, Biomacromolecules, 2008, 9, 850–858 CrossRef CAS PubMed.
- E. Kar, N. Bose, S. Das, N. Mukherjee and S. Mukherjee, Phys. Chem. Chem. Phys., 2015, 17, 22784–22798 RSC.
- P. Thakur, A. Kool, B. Bagchi, N. A. Hoque, S. Das and P. Nandy, RSC Adv., 2015, 5, 62819–62827 RSC.
- P. Xu, K. Ye and M. Du, et al., RSC Adv., 2015, 5, 36656–36664 RSC.
- P. Martins, M. Silva and S. L. Mendez, Nanoscale, 2015, 7, 9457–9461 RSC.
- H. Fang, Q. Li, W. He, J. Li, Q. Xue and C. Xu, et al., Nanoscale, 2015, 7, 17306–17311, 10.1039/c5nr05098g.
- Y. Yuan, Z. Xiao, B. Yang and J. Huang, J. Mater. Chem. A, 2014, 2, 6027–6041 CAS.
- P. Martins, A. C. Lopes and S. Lanceros-Mendez, Prog. Polym. Sci., 2014, 39, 683–706 CrossRef CAS.
- Y. Lu, J. Claude, B. Neese, Q. Zhang and Q. Wang, J. Am. Chem. Soc., 2006, 128, 8120–8121 CrossRef CAS.
- N. Karawasa and W. A. Goddard III, Macromolecules, 1992, 25, 7268–7281 CrossRef.
- V. Tomer, E. Manias and C. A. Randall, J. Appl. Phys., 2011, 110, 044107–044110 CrossRef.
- D. Mandal, K. Henkel and D. Schmeißer, Mater. Lett., 2012, 73, 123–125 CrossRef CAS.
- D. Mandal, K. Henkel and D. Schmeisser, J. Phys. Chem. B, 2011, 115, 10567–10569 CrossRef CAS.
- A. B. da Silva, M. Arjmand, U. Sundararaj and R. E. S. Bretas, Polymer, 2014, 55, 226–234 CrossRef.
- S. M. Levedev, O. S. Gefle and S. N. Tkachenko, J. Electrost., 2010, 68, 122–127 CrossRef.
- C. Putson, L. Lebrun, D. Guyomar, N. Muensit, P. J. Cottinet, L. Seveyrat and B. Guiffard, J. Appl. Phys., 2011, 109, 024104–024108 CrossRef.
- P. Thakur, A. Kool, B. Bagchi, S. Das and P. Nandy, Appl. Clay Sci., 2014, 99, 149–159 CrossRef CAS.
- P. Thakur, A. Kool, B. Bagchi, S. Das and P. Nandy, Phys. Chem. Chem. Phys., 2015, 17, 1368–1378 RSC.
- D. Bhadra, M. G. Masud, S. K. Deand and B. K. Chaudhuri, J. Phys. D: Appl. Phys., 2012, 45, 485002 CrossRef.
- J. S. Lee, K.-Y. Shin, C. Kim and J. Jang, Chem. Commun., 2013, 49, 11047–11049 RSC.
- N. Bose, M. Basu and S. Mukherjee, Mater. Res. Bull., 2012, 47, 1368–1373 CrossRef CAS.
- M. Sharma, G. Madras and S. Bose, Cryst. Growth Des., 2015, 15, 3345–3355 CAS.
- Y. Abboud, T. Saffaj, A. Chagraoui, A. E. Bouari, K. Brouzi, O. Tanane and B. Ihssane, Appl. Nanosci., 2014, 4, 571–576 CAS.
- S.-H. Park and W.-J. Lee, Sci. Rep., 2015, 5, 09754 CrossRef CAS PubMed.
- A. Chafidz, M. Kaavessina, S. Al-Zahrani and M. N. Al-Otaibi, J. Polym. Res., 2014, 21, 483 CrossRef.
- P. Martins, C. M. Costa, M. Benelmekki, G. Botelhob and S. L. Mendez, CrystEngComm, 2012, 14, 2807 RSC.
- H. B. Hassan and Z. Abdel Hamid, Int. J. Electrochem. Sci., 2011, 6, 5741–5758 CAS.
- A. Catarina Lopes, I. Correia Neves and S. Lanceros Mendez, J. Phys. Chem. C, 2015, 119(9), 5211–5217 Search PubMed.
- A. C. Lopes, C. M. Costa, C. J. Tavares, I. C. Neves and S. Lanceros-Mendez, J. Phys. Chem. C, 2011, 115(37), 18076–18082 CAS.
- A. C. Lopes, C. Caparros, S. Ferdov and S. Lanceros-Mendez, J. Mater. Sci., 2013, 48, 2199–2206 CrossRef CAS.
- P. Martins, C. M. Costa, M. Benelmekki, G. Botelho and S. Lanceros-Méndez, J. Mater. Sci., 2013, 48, 2681–2689 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2015 |