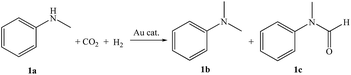
Direct methylation of N-methylaniline with CO2/H2 catalyzed by gold nanoparticles supported on alumina†
Gao Tanga,
Hong-Liang Baob,
Chan Jinb,
Xin-Hua Zhong*a and
Xian-Long Du*b
aKey Laboratory for Advanced Materials, Institute of Applied Chemistry, East China University of Science and Technology, Shanghai 200237, P.R. China. E-mail: zhongxh@ecust.edu.cn
bKey Laboratory of Interfacial Physics and Technology, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Jialuo Road 2019, Shanghai 201800, P.R. China. E-mail: duxianlong@sinap.ac.cn
First published on 13th November 2015
Abstract
Small gold nanoparticles (∼3 nm) loaded onto various supports have been prepared by a deposition–precipitation method and studied for direct methylation of N-methylaniline with CO2/H2. Among the catalysts examined, an acid–base bifunctional support γ-alumina supported gold catalyst (Au/γ-Al2O3) exhibits the best catalytic performance. Au/γ-Al2O3 catalysts with controlled mean Au particle sizes (1.8–8.3 nm) have also been successfully prepared by regulating the concentration of HAuCl4 in solution, aging time, aging temperature and mole ratio of urea to gold in the process of deposition–precipitation with urea. The turnover frequency (TOF) values for direct methylation of N-methylaniline with CO2/H2 increase on decreasing the mean size of Au nanoparticles (from 8.3 to 1.8 nm), showing that methylation of N-methylaniline with CO2/H2 is a structure-sensitive reaction. A fast increase in TOF occurs when the mean Au particle size becomes smaller than 3 nm. Through TEM (transmission electron microscope), gold L3-edge XAFS (X-ray absorption fine structure) and CO2- and NH3-TPD (temperature programmed desorption) analysis, we can conclude the Au particle sizes, oxidation state of the gold species and acid–base properties of the supports are responsible for the high catalytic activity of direct methylation of N-methylaniline with CO2/H2.
Introduction
Carbon dioxide (CO2) is a greenhouse gas and the accumulation of a huge amount of CO2 has caused significant and negative effects on the global environment.1 It is crucial, therefore, to take effective measures to reduce CO2 emission and utilize CO2 resources. In this regard, carbon dioxide has been used as an inexpensive and nontoxic C1 feedstock for producing commodity chemicals, fuels and various materials in the last few decades.2–4 Owing to its thermodynamic stability, highly efficient activation of CO2 and a strong thermodynamic driving force are required for efficient transformation of CO2. In this respect, only a handful of industrial processes have been realized for CO2 utilization, such as the production of urea, salicylic acid, and carbonates.5 Additionally, the catalytic hydrogenation of CO2 to methanol, formic acid or its derivatives have been reported by using various catalysts.6,7 Carbon dioxide can also be converted into cyclic carbonates,8 dimethyl carbonate (DMC),9 formamides10 and carboxylic acids,11 activated by relevant reactive substrates such as epoxides, alcohols, amines and olefins respectively.Methylamines, including dimethylamine, trimethylamine, methylamine and dimethylformamide (DMF), have been usually used as platform chemicals for preparing fertilizers, fungicides, synthetic leathers or polymers.12 Hazardous alkylating agents, such as methyl iodide, dimethyl sulfate, and dimethyl carbonate, are traditionally preferred for the preparation of methylamines.13 Meanwhile, dimethylsulfoxide and formic acid have also been found to be the available carbon sources for methylation of amines by catalysts.14 Great efforts on the other hand have been made to develop new catalytic process for preparation of N-methylated compounds using CO2 as a C1 building block in the absence of the toxic alkylating agents. In early 2013, two catalytic systems were independently reported by two research groups for the methylation of amines with CO2 and silanes by homogeneous catalysts, producing siloxane as a by-product.15 Shortly afterwards, a green method for methylation of amines with CO2/H2 has been reported using homogeneous Ru-based catalysts by the groups of Klankermayer and Beller, generating water as the only by-product.16 Lately, two new metal-free homogeneous catalysts for methylation of amines using CO2 as a C1 source, were developed by the groups of Dyson and Cantat.17 Despite the significant progress that has been made in the area of amine methylation using CO2/H2 with homogeneous systems, there are still few available relevant reports on the heterogeneously catalyzed methylation of amines with CO2/H2. Recently, N-methylation of amines with CO2/H2 was realized by heterogeneous catalysts such as CuAlOx,18 Pt–MoOx/TiO2 (ref. 19) and Pd/CuZrOx (ref. 20) respectively. These heterogeneous systems however are limited by their low turnover frequencies (TOF, <3.3 h−1) and prolonged reaction times (24–48 h) for achieving the high product yields (>75%).
Catalysts based on supported gold nanoparticles (NPs) have been found to be versatile catalysts for many chemical transformations in various mild chemical processes, since Haruta and his associates discovered gold particles (<5 nm) supported on transition metal oxides were very active for CO oxidation at low temperature.21 It is generally accepted that the mean size of gold NPs, supports and preparation methods markedly influence the catalytic performances of supported gold catalysts.22 Bus et al. reported that small gold particles (size < 2 nm) loaded on γ-Al2O3 were active for selective hydrogenation of cinnamaldehyde to cinnamyl alcohol.23 It was previously reported that both size and support of supported gold catalyst affected the reaction rates for the water–gas shift reaction.24 Sun et al. showed that basic resin R201 supported Au catalyst displayed good performance in the one-pot synthesis of styrene carbonate from styrene and CO2.25 Recently, Liu et al. has reported the effective synthesis of benzimidazoles from 2-nitroanilines and CO2/H2 using supported gold catalyst.26 Besides, as reported by the group of Manzoli, gold sites were essential for hydrogen dissociation on metal oxide supports, which could activate hydrogen.27 These established knowledge provide inspiration for our current study of methylation of N-methylaniline using CO2/H2 with supported gold catalysts.
We previously reported that Au/Al2O3-VS (very small gold NPs loaded on γ-Al2O3) catalyzed direct methylation of aniline using CO2/H2 with total conversion of aniline and excellent selectivity towards N,N-dimethylaniline (92%) at 140 °C for 7 h.28 To the best of our knowledge, it is the highest TOF value ever reported to date for methylation of aniline with CO2/H2 in this catalysis system based total gold atoms (26 h−1). Small Au NPs (∼2 nm) supported on alumina is critical for high activity for methylation of amines using CO2/H2, in comparison with Au NPs supported on other supports.28 We herein extend the previous work, and systematically study the effects of the mean size of Au NPs, gold species and acid–base properties of supports on catalytic activity of Au/Al2O3 catalyst for direct methylation of N-methylaniline with CO2/H2. We have also examined the mechanism of methylation of N-methylaniline in the presence of CO2/H2 catalyzed by Au/Al2O3 catalyst.
Experimental
Chemicals
Commercially available organic and inorganic compounds were used without further purification. Gold catalysts including 1 wt% Au/TiO2 (catalogue number 79–0165), 1 wt% Au/ZnO (catalogue number 79–0170) were supplied by Mintek.Synthesis of Al2O3, Mn2O3 and Co3O4 supports
Al2O3 powders were prepared by a conventional precipitation method. 45.0 g Al(NO3)3·9H2O was dissolved in 500 mL deionized water at room temperature, the pH was adjusted to 9.0 by dropwise addition of NH4OH (2.5 M). The resultant hydro gel was washed with deionized water until free of NO3− ions. The precipitate was then dried at 110 °C overnight and calcined at 500 °C for 4 h in air. Then the particles were passed a 200-mesh sieve to get the qualities of particles with diameters of less than 0.074 millimeter. The crystal structure of Al2O3 was gamma phase (based on XRD analysis, see Fig. S1†).Mn2O3 powders were prepared by calcination of MnCO3 sample (AR) at 500 °C in flowing air (1 atm) for 4 h and then cooled.29
Co3O4 powders were prepared by a conventional precipitation method. 10.0 g Co(NO3)2·6H2O was dissolved in 200 mL deionized water at room temperature, the pH was adjusted to 9.0 by dropwise addition of NH4OH (2.5 M). The resultant hydro gel was washed with deionized water and dried at 110 °C overnight. The product was calcined at 400 °C for 4 h in air and then cooled.
Catalyst synthesis
The theoretical Au loading for each catalyst used in this work was 1 wt%, unless otherwise stated. Au/SiO2 was prepared according to sonication-aided impregnation method reported by Wang et al.30 The calculated amount of SiO2 (Degussa, Aerosil 380, specific surface area: 380 m2 g−1) was suspended in an aqueous solution of HAuCl4, and the suspension was placed into the sonication bath with irradiation at 40 kHz and 200 W output power. After sonication, the water was removed by evaporation at 80 °C. The solid powder was finally reduced in H2 atmosphere at 350 °C for 2 h. The deposition–precipitation with urea method was used for the preparation of Au/Al2O3, Au/MgO, Au/Co3O4, Au/Mn2O3 and Au/CeO2 catalysts. In a typical procedure, urea was dissolved in HAuCl4 solution at room temperature. The support was then added to this clear solution, and the temperature of the resulting slurry was increased gradually to a fixed temperature. The temperature was maintained for a certain time. After cooling to room temperature, the solid was recovered by filtering and washing several times with distilled water. The sample was dried under vacuum at 50 °C for 12 h, followed by reduction with a stream of 5 vol% H2/Ar at 350 °C for 2 h. The standard conditions were as follows: [HAuCl4] = 0.48 mmol L−1; aging time = 6 h; aging temperature = 80 °C; mole ratio of urea to Au = 200. The concentration of gold by weight was confirmed by ICP-AES.Characterization
Au loadings in all of the catalysts were measured by ICP-AES with an ICAP 6300 instrument when the sample was dissolved in aqua regia and diluted with distilled water to the proper concentration. The crystal structures of the prepared catalysts were characterized with powder X-ray diffraction (XRD) on a Bruker D8 Advance X-ray diffractometer using the Ni-filtered Cu Kα radiation source at 40 kV and 40 mA. TEM images for supported gold catalysts were taken with a Tecnai G2 electron microscope operating at 200 kV. Before being transferred into the TEM chamber, the samples dispersed with ethanol were deposited onto a carbon-coated copper grid and then quickly moved into the vacuum evaporator. The size distribution of the metal nanoclusters was determined by measuring about 200 random particles on the images. NH3-TPD and CO2-TPD were performed on an AutoChem 2950 HP instrument. Typically, the sample loaded in a quartz reactor was pretreated with high purity He at 300 °C for 1 h. After cooling the sample to 50 °C, NH3 adsorption was performed by switching the He flow to a NH3–He (10 vol% NH3) gas mixture and then maintaining the sample at 50 °C for 1 h. The gas-phase (and/or weakly adsorbed) NH3, was purged by high-purity He at the same temperature. NH3-TPD was then performed in the He flow by raising the temperature to 700 °C at a rate of 10 °C min−1. The desorbed NH3 molecules were detected by using a MKS Cirrus 2 mass spectrometer at the signal of m/z 17. CO2-TPD was performed by using a similar procedure. The X-ray absorption data at the Au L-edge of the samples were recorded at room temperature in transmission mode using ion chambers at beam line BL14W1 of the Shanghai Synchrotron Radiation Facility (SSRF), China. The synchrotron radiation was monochromatized with a Si (111) double crystal monochromator, and mirrors were used to eliminate higher harmonics. The incident and transmitted beam intensities were monitored using ionization chambers filled with pure N2. During the measurement, the synchrotron was operated at energy of 3.5 GeV and a current of 250 mA. Data processing was performed using the program ATHENA and ARTEMIS. The X-ray absorption near-edge structure (XANES) was normalized with edge height and the first-order derivatives was taken to compare variation of absorption edge energies.Catalytic testing
A mixture of solvent (10 mL), N-methylaniline (1.0 mmol), and supported Au catalysts (Au 2.7–5.0 μmol) were charged into a 50 mL Hastelloy-C high pressure Parr reactor. The reactor was then exchanged with H2, followed by introducing 1.0 MPa CO2 and 3.0 MPa H2. The reaction was reacted at desired temperature for given reaction time under magnetic stirring. Subsequently, the autoclave was cooled to room temperature, and n-octane (1 mmol, internal standard) was added for quantitative analysis by GC-FID (Agilent 7820A).Results and discussion
Solvent and support effects
In the course of identifying and optimizing key reaction parameters for N-methylaniline (1a) with CO2/H2, several solvents were investigated. The Au/γ-Al2O3 catalyst with very small Au NPs (2.0 nm) was used as the standard catalyst. The highest yield of N,N-dimethylaniline (1b) was obtained when cyclohexane was used as the reaction medium (Table 1, entry 1). In contrast, the reaction in hexane, toluene, 1,4-dioxane, CH2Cl2 and THF (Table 1, entries 2–6) gave moderate to good yield (24–61%) with excellent selectivity towards 1b (>90%), and in ethanol (Table 1, entry 7) obtained the lowest yield (2%) with poor selectivity towards 1b (18%). Based on above results, we empirically concluded that the use of apolar solvents for direct methylation of 1a with CO2/H2 gave better conversion than the use of polar solvents, which shows that the polarity of solvents has a remarkable influence on the conversion of 1a with CO2/H2 in the presence of Au/Al2O3 catalyst.| Entry | Solvent | Catalyst | Au Sizeb (nm) | Au contentc (wt%) | Conv.d (%) | Sel.e (%) |
|---|---|---|---|---|---|---|
| a Reaction conditions: 0.5 mol% Au, N-methylaniline (1 mmol), CO2 (1 MPa), H2 (3 MPa), solvent (10 mL), T = 140 °C, t = 5 h, n. r. = no reaction.b Obtained from TEM.c Measured by ICP-AES.d Conversation and selectivity were determined by GC with n-octane as the internal standard.e N-methylformanilide is the only other product. | ||||||
| 1 | Cyclohexane | Au/Al2O3 | 2.0 | 0.73 | 76 | >99 |
| 2 | Hexane | Au/Al2O3 | 2.0 | 0.73 | 61 | >99 |
| 3 | Toluene | Au/Al2O3 | 2.0 | 0.73 | 52 | >99 |
| 4 | 1,4-Dioxane | Au/Al2O3 | 2.0 | 0.73 | 25 | 96 |
| 5 | CH2Cl2 | Au/Al2O3 | 2.0 | 0.73 | 34 | >99 |
| 6 | THF | Au/Al2O3 | 2.0 | 0.73 | 29 | 91 |
| 7 | Ethanol | Au/Al2O3 | 2.0 | 0.73 | 12 | 18 |
| 8 | Cyclohexane | Au/SiO2 | 3.7 | 0.97 | 1 | >99 |
| 9 | Cyclohexane | Au/α-Mn2O3 | 2.4 | 0.95 | 1 | >99 |
| 10 | Cyclohexane | Au/CeO2 | 3.7 | 0.74 | 3 | >99 |
| 11 | Cyclohexane | Au/Co3O4 | 4.4 | 0.96 | 7 | 42 |
| 12 | Cyclohexane | Au/TiO2 | 2.8 | 1.00 | 15 | >99 |
| 13 | Cyclohexane | Au/MgO | 2.6 | 0.91 | n.r. | — |
| 14 | Cyclohexane | Au/ZnO | 2.8 | 1.00 | n.r. | — |
| 15 | Cyclohexane | Au/C | 4.0 | 1.00 | n.r. | — |
| 16 | Cyclohexane | Au powder | 150 | 100 | n.r. | — |
Subsequently, the effects of some typical supports on catalytic activity were studied for methylation of 1a with CO2/H2. The mean size of Au NPs loaded on various supports at around 3 nm was successfully obtained by using the DPU method and sonication-aided impregnation techniques. The typical TEM micrographs and corresponding Au particle size distributions for these catalysts are shown in Fig. S2.† Of the different supports tested, Al2O3 (Table 1, entry 1) provided the best result in terms of high conversion (76%) and excellence selectivity (>99%). The use of SiO2, α-Mn2O3, CeO2 and Co3O4 resulted in low yields of 1b (<4%) under similar reaction conditions (Table 1, entries 8–11). Moderate yield of 1b (15%) could be obtained when Au/TiO2 catalyst was used (Table 1, entry 12). However, 1b was not detected when MgO, ZnO and C were used as supports (Table 1, entries 13–15). In addition, the use of the Au0 powder, did not promote the reaction at all (Table 1, entry 16). It was reported that MgO has strong basic character and SiO2 is an acidic to neutral oxide.30 Al2O3 with moderate electronegativity can be considered to have both acidic and basic surface sites.31 These results may suggest that the acid–base properties of the support can affect the catalytic behavior of the supported Au catalysts for methylation of 1a with CO2/H2.
Next, the acid–base properties of the typical supported Au catalysts were examined by temperature-programmed desorption (TPD) of NH3 and CO2. As shown in Fig. 1, desorption peaks of NH3 are observed from Au/Al2O3, Au/Mn2O3, Au/TiO2, Au/ZnO, Au/CeO2 and Au/SiO2, which indicates that acidic sites exist on these catalysts. From the temperature and intensity of the NH3 desorption peak, we can see that Au/Al2O3 possesses relatively strong acidity. No significant acidity, on the other hand, exists on Au/MgO, Au/C and Au/Co3O4, as no desorption peak of NH3 can be detected from these catalysts. CO2-TPD profiles in Fig. 2 suggest that Au/Al2O3 and Au/MgO both have relatively strong basic sites, whereas no basic sites can be observed on Au/TiO2, Au/Co3O4, Au/C, Au/ZnO and Au/SiO2. Weak desorption peaks of CO2 are detected in Au/Mn2O3 and Au/CeO2, which suggests weak basic sites exist on these catalysts. According to the NH3-TPD and CO2-TPD results, Au/Al2O3 possesses both relatively strong basicity and acidity, while Au/Co3O4 and Au/C have neither acidic nor basic sites. Therefore, we have observed significant support effects in the Au-catalyzed methylation of N-methylaniline with CO2/H2, and our studies suggest that the acid–base properties of the supports play important roles in determining both its activity and the selectivity. The strong basicity of supports may promote the adsorption of CO2 and accelerate departure of product from the surface of catalysts. The strong acidity of supports may be important to anchor the amines (reactive molecules). So the strong acidity and basicity will benefit the methylation reaction.
 | ||
| Fig. 1 NH3-TPD profiles for some typical supported Au catalysts: (a) Au/CeO2, (b) Au/C, (c) Au/ZnO, (d) Au/Co3O4, (e) Au/Mn2O3, (f) Au/SiO2, (g) Au/MgO, (h) Au/TiO2, (i) Au/Al2O3. | ||
 | ||
| Fig. 2 CO2-TPD profiles for some typical supported Au catalysts: (a) Au/C, (b) Au/ZnO, (c) Au/Co3O4, (d) Au/CeO2, (e) Au/Mn2O3, (f) Au/SiO2, (g) Au/MgO, (h) Au/TiO2, (i) Au/Al2O3. | ||
In summary, acidity and basicity of supports in some typical supported gold catalysts were successfully detected in our experiment and had significant effects on conversion of 1a and selectivity towards 1b during direct methylation of 1a with CO2/H2. The Au/Al2O3 catalyst with relatively strong acidity and basicity contributes to high conversion of 1a and excellent selectivity towards 1b, whereas other catalysts with only strong basic (Au/MgO) or acidic to neutral sites (Au/SiO2 and Au/C) are almost inactive. Catalysts with weak acid–base properties (Au/Mn2O3 and Au/ZnO) also have no significant activity. There are moderate activity and excellent selectivity when TiO2 and CeO2 with a few acidic sites are used as a support in Au/TiO2 and Au/CeO2 catalysts. Au/Co3O4 with neutral sites has very low activity and poor selectivity (<45%) towards 1b.
A further study to investigate the effect of reaction parameters was carried out in our experiment. As shown in Fig. 3, the methylation of 1a with CO2 and H2 can be carried out smoothly under relatively mild conditions although the reaction time becomes longer (18 h). It is noteworthy that the selectivity is constant (>99%) during the process, which suggests the reaction has high selectivity towards 1b. Besides, increasing the reaction temperature and pressure respectively can boost the conversion of 1a to a certain extent (see Fig. S3 and Fig. S4 in the ESI†). However, the conversion of 1a and selectivity towards 1b kept nearly the same when the total initial pressure and temperature were higher than 4 MPa and 140 °C respectively. Obviously, 1a could not be fully converted by increasing the total initial pressure or boosting reaction temperature under the present reaction conditions. Nevertheless, 1a could be fully converted with excellent selectivity towards 1b when reaction time was up to 18 h under the standard conditions. Therefore, total initial pressure and temperature both affected the yield of 1b when the reaction time was fixed at 5 h with the yield of 1b lower than 76%. In order to get 1a totally converted, prolonged reaction time is required under proper total initial pressure and at appropriate temperature.
Preparation and characterization of Au/γ-Al2O3 with controlled mean Au particle sizes
There are still challenges to prepare supported Au catalysts with controlled Au sizes. It was found that aging temperature, aging time and the mole ratio of urea to Au used in DPU process played key roles to obtain controlled Au particle sizes. Louis et al. reported that the pH increased with time due to urea decomposition and most gold was deposited within the first hour in the preparation of Au/TiO2 by DPU method.32 Urea begins to decompose when aging temperature is over 60 °C. Compared with Au/TiO2 prepared by DPU, we found that by regulating aging temperature from 20 to 80 °C in the DPU process could markedly decrease the mean size of Au NPs from 8.3 to 2.0 nm of Au/Al2O3 catalysts (Table 2, entries 1–2 and 5). These results clarified that aging temperature used during the DPU process could be an important factor in determining the mean size of Au NPs supported on alumina. As is well known, the rates of nucleation and growth determine the size of a particle formed in the solution. Increasing the aging temperature from 20 to 80 °C can increase the nucleation rates and the pH values in solution because of urea decomposition. At 80 °C, when the mole ratio of urea to Au decreased to 50, the pH value could not reach at 8 as too little urea was added in solution. So a large Au particle size (5.7 nm) was formed (Table 2, entry 3). When aging time was 1 h instead of 6 h, large mean Au NPs size (3.6 nm) was obtained (Table 2, entry 4), indicating that less aging time caused the formation of large Au particle. Alternatively, prolonged aging time favored the formation of small Au particles in the DPU process.32 However, pH value was over 8 when urea added in solution was enough, which produced the smallest mean Au NPs size (1.8 nm) (Table 2, entry 6). Fig. 4 shows the TEM micrographs and the Au particle size distributions for these Au/Al2O3 samples. Grisel et al. reported the Au loading depended strongly on the final pH of the precipitation solution and a sharp decrease in Au loading was observed with pH value over 8.5.33 Low Au loadings (yields: <50%) due to incomplete decomposition of urea in solution were obtained when aging temperatures were below 80 °C (Table 2, entries 1–2). Most Au was loaded on alumina (yield: >70%) when the mole ratio of urea to gold was less than or equal to 200 at 80 °C (Table 2, entries 3–5). Too much urea added in solution on the other hand caused low Au loading (yield: 40%) because of high pH value over 8.5 (Table 2, entry 6). Thus, regulating pH of the solution during DPU process contributed to the formation of small Au particle and could cause the alteration of Au loadings.| Entry | Aging temperature (°C) | Aging time (h) | Mole ratio of urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au (mol Au (mol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mol) mol) |
Au loadingb (wt%) | Mean diameterc (nm) |
|---|---|---|---|---|---|
| a If not mentioned, preparation conditions were: [HAuCl4] = 0.48 mmoL L−1, aging time = 6 h, aging temperature = 80 °C, mole ratio of urea to Au = 200, theoretical Au loading = 1 wt%.b Measured by ICP-AES.c Obtained from TEM. | |||||
| 1 | 20 | 6 | 200 | 0.47 | 8.3 |
| 2 | 50 | 6 | 200 | 0.46 | 7.2 |
| 3 | 80 | 6 | 50 | 0.71 | 5.7 |
| 4 | 80 | 1 | 200 | 0.70 | 3.6 |
| 5 | 80 | 6 | 200 | 0.73 | 2.0 |
| 6 | 80 | 6 | 400 | 0.40 | 1.8 |
Catalytic properties of Au/γ-Al2O3 catalysts with various Au NPs sizes for direct methylation of N-methylaniline with CO2/H2
The dependence of catalytic activity on the mean size of Au NPs in Au/Al2O3 catalysts prepared by DPU with various preparation parameters was shown in Table 3. The highest yield (83%) of 1b (Table 3, entry 1) was acquired with the smallest mean Au NPs size (1.8 nm). Small Au NPs (2.0–5.7 nm) could lead to moderate yields (61–76%) and high selectivity (>99%) towards 1b (Table 3, entries 2–7). However, on the other hand, low yields (<8%) of 1b (Table 3, entries 8–9) were obtained due to large mean size of Au NPs (>7.2 nm).| Entry | Preparation conditionsb | Au loading (wt%) | Au sizec (nm) | Conv.d (%) | Sel.e (%) |
|---|---|---|---|---|---|
| a Reaction conditions: catalyst 0.14 g (Au 2.7–5.0 μmol), N-methylaniline (1 mmol), CO2 (1 MPa), H2 (3 MPa), cyclohexane (10 mL), T = 140 °C, t = 5 h.b If not mentioned, preparation conditions were: [HAuCl4] = 0.48 mmol L−1, aging time = 6 h, aging temperature = 80 °C, mole ratio of urea to Au = 200.c Obtained from TEM.d Conversation and selectivity were determined by GC with n-octane as the internal standard.e N-Methylformanilide is the only other product. | |||||
| 1 | Urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 400 Au = 400 |
0.40 | 1.8 | 83 | >99 |
| 2 | Standard condition | 0.73 | 2.0 | 76 | >99 |
| 3 | [HAuCl4] = 0.12 mmol−1 L | 0.70 | 2.4 | 69 | >99 |
| 4 | Aging time = 16 h | 0.65 | 2.4 | 70 | >99 |
| 5 | [HAuCl4] = 1.92 mmol−1 L | 0.60 | 2.6 | 63 | >99 |
| 6 | Aging time = 1 h | 0.70 | 3.6 | 69 | >99 |
| 7 | Urea![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Au = 50 Au = 50 |
0.70 | 5.7 | 61 | >99 |
| 8 | Aging temperature = 50 °C | 0.46 | 7.2 | 8 | 86 |
| 9 | Aging temperature = 20 °C | 0.47 | 8.3 | 6 | 83 |
The conversion of 1a at the initial stages over Au/Al2O3 catalysts was recorded to prove the relations between mean Au NPs size and the intrinsic catalytic reactivity of Au sites. The time course of Au/Al2O3 catalysts was done with different mean Au NPs sizes in the range of 1.8 to 8.3 nm (see Fig. S5 in the ESI†). No conversion of 1a over Al2O3 (Fig. S5†) indicates that the Al2O3 species had no active sites for the methylation reaction with CO2/H2, which shows only the reduced gold species possess active sites for methylation of 1a with CO2/H2. The conversation of 1a increases almost linearly with reaction time over the initial 30 min in Fig. S5.† So we can calculate initial reaction rates by using the slope of the straight line in Fig. S5† for each catalyst. The results are shown in Table S1.† Obviously, the initial reaction rates decrease from 5.86 to 0.36 mmol h−1 g−1 (cat.) with increasing mean size of Au NPs from 1.8 to 8.3 nm.
Because the gold atoms contribute to the catalytic reactions, we have calculated the TOF (the moles of 1a converted per unit time at the initial stage per mole total gold atoms) using the initial reaction rate for each Au/Al2O3 catalyst. The results are shown in Fig. 5, suggesting the intrinsic activity per mole total gold atoms decreases with increasing the mean Au NPs size and the methylation of 1a with CO2/H2 is a structure-sensitive reaction.
The TOF values increase from 114 to 287 h−1 with decreasing the mean size of Au NPs from 3.6 to 1.8 nm. When the mean sizes of Au NPs are larger than 5.7 nm, the TOF values are lower than 52 h−1. The TOF value (about 300 h−1) with the best catalyst (with 1.8 nm gold size) is much higher than those measured with other heterogeneous catalysts such as CuAlOx (0.1 h−1), Pt–MoOx/TiO2 (1.8 h−1) and Pd/CuZrOx (3.3 h−1) for methylation of amines with CO2/H2.18–20
Effects of reduction temperature of Au/Al2O3 catalysts on direct methylation of N-methylaniline with CO2 and H2
The as-prepared supported gold precursors are easily reduced to the zero state by thermal treatments with reducing gases or oxidant gases.34 We previously demonstrated that the very small Au0 NPs supported on alumina treated with a stream of 5 vol% H2/Ar at 350 °C for 2 h showed high catalytic activity for direct methylation of amines with CO2/H2.28 In our experiment, the as-prepared Au/Al2O3 precursor of standard Au/Al2O3 catalyst was treated with a stream of 5 vol% H2/Ar at 150, 250, 350 and 450 °C respectively for 2 h, at a heating rate of 5 °C min−1 to obtain Au/Al2O3 samples. The samples were designated as Au/Al2O3-T, where T denoted the reduction temperature. With these catalysts, the catalytic activity was investigated for direct methylation of amines with CO2/H2. Table 4 shows that selective production of 1b in a 38% yield could be achieved when Au/Al2O3-150 catalyst was used (Table 4, entry 1). An increase in reduction temperature from 150 to 250 °C markedly increased the 1b yield from 38 to 53% (Table 4, entry 2). The highest yield of 1b was 76% as reduction temperature of the as-prepared Au/Al2O3 precursor was 350 °C (Table 4, entry 3). However, a further increase of reduction temperature from 350 to 450 °C led to a slight decrease in the desired product yield from 76 to 67% (Table 4, entries 3–4). The high reduction temperature may lead to decrease of the quantities of active hydroxyl group in alumina surface and a weak Au-support interaction in Au/Al2O3-450 catalyst, which may cause the slight decrease of the catalytic activity.35 Notably, the selectivity towards 1b was almost the same, showing that the selectivity did not depend on reduction temperature of the as-prepared Au/Al2O3 precursor.| Entry | Reduction temperature (°C) | Au sizeb (nm) | Conv.c (%) | Sel. (%) |
|---|---|---|---|---|
| a Reaction conditions: 0.5 mol% Au, N-methylaniline (1 mmol), CO2 (1 MPa), H2 (3 MPa), cyclohexane 10 mL, T = 140 °C, t = 5 h.b Obtained from TEM.c Conversation and selectivity were determined by GC with n-octane as the internal standard. | ||||
| 1 | 150 | 7.1 | 38 | >99 |
| 2 | 250 | 4.5 | 53 | >99 |
| 3 | 350 | 2.0 | 76 | >99 |
| 4 | 450 | 1.6 | 67 | >99 |
The factor, which caused different catalytic activities of the Au/Al2O3 catalysts treated by different reduction temperature, could be the change of Au particle size and valence of Au species. To investigate the structure of Au species supported on alumina after the H2 reduction, Au L3-edge XAFS measurement of Au/Al2O3 samples and Au reference compounds was carried out. Fig. 6 shows Au L3-edge X-ray absorption near-edge structures (XANES) spectra, which are known to be sensitive to the oxidation states of X-ray absorbing atoms. The XANES features of Au/Al2O3 samples (spectra c–f) after the H2 reduction with various temperature from 150 to 450 °C are clearly different from that of Au2O3 (spectrum a) but rather similar to that of Au foil (spectrum g), indicating that gold species in these samples are in a reduced state.
 | ||
| Fig. 6 Au L3 absorption edge XANES spectra: (a) Au2O3, (b) as prepared Au/Al2O3 precursor, Au/Al2O3 samples reduced at different temperatures: (c) 150, (d) 250, (e) 350, (f) 450 °C, and (g) Au foil. | ||
Fig. 7 shows the Fourier transform (FT) of extended X-ray absorption fine structure (EXAFS) data of Au/Al2O3 samples reduced at different temperature (150, 250, 350, 450 °C), the Au/Al2O3 precursor, Au foil and Au2O3. The structural parameters derived from curve-fitting analysis are listed in Table 5. The Au–O coordination number of 3.8 was observed in pure Au2O3. The EXAFS of the as-prepared Au/Al2O3 precursor (spectrum b) showed the Au–O coordination number (CN) and the Au–Au CN were 1.7 and 3.0 respectively. These results, as reported previously by Grünert et al. for Au/TiO2-MCM-48 catalyst,36 confirmed that metallic gold species as a minority phase might exist in the as prepared Au/Al2O3 precursor due to auto reduction. After H2 reduction at various temperature in the range of 150 to 450 °C for 2 h (spectra c–f), the Au–Au CN decreased from 10.7 to 5.6 and the Au–Au bond length decreased from 2.85 to 2.77 Å. It should be noted that there is a significant Au–O path contribution for Au/Al2O3-T, such as Au/Al2O3-450. However, this Au–O path has longer length (2.11 ± 0.06 Å) than the Au–O bond in Au2O3, implying a weak interaction, different from oxidic Au atoms by oxygen, between Au NPs and Al2O3 support. It implies that the oxygen of Au–O bond comes from Al2O3 support. This result can be further suggested from the big uncertainly (±0.06 Å) of the Au–O path length and the absence of Au oxidation determined by above XANES data. The Au–Au CN in these samples were much less than that in Au foil while close to that in Au/Fe–OH or Au/Fe–O catalysts due to the high proportion of surface atoms, suggesting that the small gold NPs supported on alumina were obtained.37 TEM measurement in Fig. S6† showed that the mean gold NPs size remarkably decreased from 7.1 to 1.6 nm with increasing reduction temperature from 150 to 450 °C. So the TEM result is in good agreement with the XAFS conclusion.
| Samplesa | Shell | Nb | Rc (Å) | σ2d (×10−3 Å2) | ΔE0 (eV) |
|---|---|---|---|---|---|
| a Reduction temperature of catalysts.b Coordination number.c Bond length.d Debye–Waller factor. | |||||
| Au-foil | Au–Au | 11.9 ± 0.5 | 2.86 ± 0.01 | 8.1 ± 0.3 | 3.9 |
| Au/Al2O3-150 | Au–O | 0.5 ± 0.2 | 2.08 ± 0.03 | 3.0 ± 1.0 | −7.0 |
| Au–Au | 10.7 ± 0.9 | 2.85 ± 0.01 | 8.8 ± 0.6 | 3.8 | |
| Au/Al2O3-250 | Au–O | 0.5 ± 0.2 | 2.08 ± 0.03 | 3.0 ± 1.0 | −6.9 |
| Au–Au | 10.0 ± 1.2 | 2.83 ± 0.01 | 10.1 ± 1.2 | 3.5 | |
| Au/Al2O3-350 | Au–O | 0.8 ± 0.3 | 2.09 ± 0.03 | 3.0 ± 1.5 | −3.4 |
| Au–Au | 7.7 ± 0.8 | 2.82 ± 0.01 | 10.0 ± 1.3 | 4.3 | |
| Au/Al2O3-450 | Au–O | 0.9 ± 0.3 | 2.11 ± 0.06 | 3.5 ± 1.2 | −2.0 |
| Au–Au | 5.6 ± 1.4 | 2.77 ± 0.01 | 8.9 ± 1.5 | 0.5 | |
| As prepared precursor | Au–O | 1.7 ± 0.4 | 2.02 ± 0.01 | 5.0 ± 3.0 | 11.8 |
| Au–Au | 3.0 ± 1.0 | 2.83 ± 0.01 | 5.3 ± 2.0 | 1.8 | |
| Au2O3 | Au–O | 3.8 ± 0.3 | 1.99 ± 0.01 | 2.0 ± 0.7 | 11.0 |
Therefore, the EXAFS and XANES results showed that gold species in the Au/Al2O3 samples were fully reduced after H2 treatment at different temperature (150, 250, 350, 450 °C) for 2 h. The mean Au NPs size decreased with increasing the reduction temperature from 150 to 450 °C.
Reaction mechanism
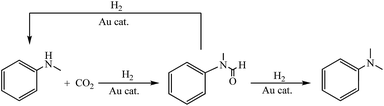
The group of Shi reported that using Pd/CuZrOx under milder conditions or using Pd/ZnZrOx during methylation with CO2/H2, N-formylation mainly processed instead of N-methylation, which indicates the N-formamide could be the intermediate.20 Beller et al. also reported that formamide was the intermediate during methylation of amines with CO2/H2 catalyzed by Ru catalyst and methanol contributed insignificantly to the reaction.15b In order to better understand the mechanism of the methylation reaction catalyzed by the Au/Al2O3 catalyst, control experiments were conducted to confirm the intermediates. Initially, the reduction of the N-methylformanilide (1c) with H2 was investigated in the presence of Au/Al2O3 catalyst. Clearly, 1c was fully converted and 1b was the major products in 61% yield (Scheme 1). Aside from methylation of 1c reduced by H2, 1c was simultaneously reduced by H2 with Au/Al2O3 catalyst to produce 1a in 39% yield. Under standard reaction conditions, trace amount of 1c could be detected. Moreover, we found that increasing the mean size of Au NPs could not only decrease the conversion of 1a, but also decrease the selectivity towards 1b to a certain extent as shown in Fig. S7.† These results suggest that 1c could be the key intermediate and significantly contributed to methylation of 1a with CO2/H2 catalyzed by Au/Al2O3 catalyst.Based on these results, a plausible mechanism is given in Scheme 2. Under our reaction conditions, the formamide 1c first produced due to Au-catalyzed reaction of 1a with CO2/H2. The formamide 1c could be quickly reduced to give 1b (major) or be decarbonylated into 1a (minor). Then, 1a continued to react with CO2/H2 catalyzed by Au/Al2O3 catalyst following a similar hydrogenation and decarbonylation process as described above to produce the corresponding products.
Conclusions
Au NPs with uniform mean sizes (∼3 nm) loaded onto various supports were successfully prepared. These supported Au catalysts were studied in detail to clarify the support and Au particle size effects in the direct methylation reaction of N-methylaniline with CO2/H2. Of the supports tested, Al2O3 with relatively strong acidic and basic surface sites showed the best catalytic performance. Other supports, such as SiO2 with few acidic sites, Mn2O3 and ZnO with weak acid–base properties, Co3O4 and C with neither acidic site nor basic site and MgO with relatively strong basic sites, have little or low catalytic activity. There is moderate catalytic activity when Au/TiO2 and Au/CeO2 with a few acidic sites are used. Supports with relatively strong acid–base bifunctional sites may be necessary for high efficient methylation of N-methylaniline with CO2/H2.By changing preparation parameters during the DPU process, we obtained the Al2O3 supported Au NPs with controlled mean sizes in the range of 1.8 to 8.3 nm. We proved that, during the DPU process, aging time, aging temperature and mole ratio of gold to urea were the main preparation parameters affecting the mean size of Au NPs supported on Al2O3.
The TOF values for direct methylation of N-methylaniline with CO2/H2 increased with decreasing the mean size of Au NPs from 8.3 to 1.8 nm and XAFS results demonstrated that only reduced gold NPs supported on alumina were responsible for high catalytic activity of Au/Al2O3 catalyst. The results suggested that the Au-catalyzed direct methylation of N-methylaniline with CO2/H2 was a structure-sensitive reaction. Then, we proposed that N-methylformanilide could be a key intermediate during methylation of N-methylaniline with CO2/H2.
Acknowledgements
This work was financially supported by the Program of International S&T Cooperation (Grant no. 2014DFG60230), the National Basic Research Program of China (2013CB933104), the National Natural Science Foundation of China (no. 21303246, 11275258, 91127001).Notes and references
- J. T. Houghton, G. J. Jenkins and J. J. Ephraums, Climate Change: The IPCC Scientific Assessment, Cambridge University Press, Cambridge, UK, 1990 Search PubMed.
- H. Arakawa, M. Aresta, J. N. Armor, M. A. Barteau, E. J. Beckman, A. T. Bell, J. E. Bercaw, C. Creutz, E. Dinjus, D. A. Dixon, K. Domen, D. L. DuBois, J. Eckert, E. Fujita, D. H. Gibson, W. A. Goddard, D. W. Goodman, J. Keller, G. J. Kubas, H. H. Kung, J. E. Lyons, L. E. Manzer, T. J. Marks, K. Morokuma, K. M. Nicholas, R. Periana, L. Que, J. R. Nielson, W. M. H. Sachtler, L. D. Schmidt, A. Sen, G. A. Somorjai, P. C. Stair, B. R. Stults and W. Tumas, Chem. Rev., 2001, 101, 953–996 CrossRef CAS PubMed.
- T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365–2387 CrossRef CAS PubMed.
- (a) K. M. Y. Yu, I. Curcic, J. Gabriel and S. C. Edman Tsang, ChemSusChem, 2008, 1, 893–899 CrossRef CAS PubMed; (b) J. Ma, N. N. Sun, X. L. Zhang, N. Zhao, F. K. Xiao, W. Wei and Y. H. Sun, Catal. Today, 2009, 148, 221–231 CrossRef CAS; (c) W. Wang, S. P. Wang, X. B. Ma and J. L. Gong, Chem. Soc. Rev., 2011, 40, 3703–3727 RSC.
- (a) G. Pagani, Hydrocarbon Process., Int. Ed., 1982, 61, 87–91 CAS; (b) S. Walendy, G. Francio, M. Hoelscher and W. Leitner, Exp. Green Sustainable Chem., 2009, 275–277 Search PubMed; (c) S. G. Liang, H. Z. Liu, T. Jiang, J. L. Song, G. Y. Yang and B. X. Han, Chem. Commun., 2011, 47, 2131–2133 RSC; (d) M. Peters, B. Köhler, W. Kuckshinrichs, W. Leitner, P. Markewitz and T. Müller, ChemSusChem, 2011, 4, 1216–1240 CrossRef CAS PubMed.
- (a) S. N. Riduan, Y. Zhang and J. Y. Ying, Angew. Chem., Int. Ed., 2009, 48, 3322–3325 CrossRef CAS PubMed; (b) A. E. Ashley, A. L. Thompson and D. OHare, Angew. Chem., Int. Ed., 2009, 48, 9839–9843 CrossRef CAS PubMed; (c) S. Wesselbaum, T. vom Stein, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2012, 51, 7499–7502 CrossRef CAS PubMed.
- K. Motokura, D. Kashiwame, A. Miyaji and T. Baba, Org. Lett., 2012, 14, 2642–2645 CrossRef CAS PubMed.
- Q. Su, J. Sun, J. Q. Wang, Z. F. Yang, W. Q. Cheng and S. J. Zhang, Catal. Sci. Technol., 2014, 4, 1556–1562 CAS.
- Y. Yoshida, Y. Arai, S. Kado, K. Kunimori and K. Tomishige, Catal. Today, 2006, 115, 95–101 CrossRef CAS.
- L. Schmid, A. Canonica and A. Baiker, Appl. Catal., A, 2003, 255, 23–33 CrossRef CAS.
- T. G. Ostapowicz, M. Schmitz, M. Krystof, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2013, 52, 12119–12123 CrossRef CAS PubMed.
- (a) A. Ricci, Modern Amination Methods, Wiley-VCH, Weinheim, 2000 Search PubMed; (b) R. N. Salvatore, C. H. Yoon and K. W. Jung, Tetrahedron, 2001, 57, 7785 CrossRef CAS; (c) Z. Rapport, The Chemistry of Anilines, Wiley Interscience, 2007 Search PubMed.
- (a) M. F. Ali, B. M. ElAli and J. G. Speight, Handbook of Industrial Chemistry-Organic Chemicals, McGraw-Hill, New York, 2005 Search PubMed; (b) H.-J. Arpe and S. Hawkins, Industrial Organic Chemistry, Wiley-VCH, Weinheim, 1997 Search PubMed; (c) A. Dhakshinamoorthy, M. Alvaro and H. Garcia, Appl. Catal., A, 2010, 378, 19–25 CrossRef CAS.
- (a) X. Jiang, C. Wang, Y. W. Wei, D. Xue, Z. T. Liu and J. L. Xiao, Chem. - Eur. J., 2014, 20, 58–63 CrossRef CAS PubMed; (b) S. Savourey, G. Lefère, J. C. Berthet and T. Cantat, Chem. Commun., 2014, 50, 14033–14036 RSC; (c) I. Sorribes, K. Junge and M. Beller, Chem. - Eur. J., 2014, 20, 7878–7883 CrossRef CAS PubMed.
- (a) O. Jacquet, X. Frogneux, C. D. N. Gomes and T. Cantat, Chem. Sci., 2013, 4, 2127–2131 RSC; (b) Y. Li, X. Fang, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 9568–9571 CrossRef CAS PubMed.
- (a) K. Beydoun, T. vom Stein, J. Klankermayer and W. Leitner, Angew. Chem., Int. Ed., 2013, 52, 9554–9557 CrossRef CAS PubMed; (b) Y. Li, I. Sorribes, T. Yan, K. Junge and M. Beller, Angew. Chem., Int. Ed., 2013, 52, 12156–12160 CrossRef CAS PubMed.
- (a) S. Das, F. D. Bobbink, G. Laurenczy and P. J. Dyson, Angew. Chem., Int. Ed., 2014, 53, 12876–12879 CrossRef CAS PubMed; (b) E. Blondiaux, J. Pouessel and T. Cantat, Angew. Chem., Int. Ed., 2014, 53, 12186–12190 CrossRef CAS PubMed.
- X. J. Cui, X. Dai, Y. Zhang, Y. Deng and F. Shi, Chem. Sci., 2014, 5, 649–655 RSC.
- K. Kon, S. M. A. H. Siddiki, W. Onodera and K. I. Shimizu, Chem. - Eur. J., 2014, 20, 6264–6267 CrossRef CAS PubMed.
- X. Cui, Y. Zhang, Y. Q. Deng and F. Shi, Chem. Commun., 2014, 50, 13521–13524 RSC.
- (a) M. Haruta, Catal. Today, 1997, 36, 153–166 CrossRef CAS; (b) X. Zhang, H. Shi and B. Q. Xu, Angew. Chem., Int. Ed., 2005, 44, 7132–7135 CrossRef CAS PubMed; (c) A. Corma, P. Serna and H. García, J. Am. Chem. Soc., 2007, 129, 6358–6359 CrossRef CAS PubMed; (d) L. He, L. C. Wang, H. Sun, J. Ni, Y. Cao, H. Y. He and K. N. Fan, Angew. Chem., Int. Ed., 2009, 48, 9538–9541 CrossRef CAS PubMed; (e) M. D. Hughes, Y.-J. Xu, P. Jenkins, P. McMorn, P. Landon, D. I. Enache, A. F. Carley, G. A. Attard, G. J. Hutchings, F. King, E. H. Stitt, P. Johnston, K. Griffin and C. J. Kiely, Nature, 2005, 437, 1132 CrossRef CAS PubMed.
- (a) U. Hartfelder, C. Kartusch, M. Makosch, M. Rovezzi, J. Sá and J. A. van Bokhoven, Catal. Sci. Technol., 2013, 3, 454–461 RSC; (b) K. R. Souza, A. F. F. de Lima, F. F. de Sousa and L. G. Appel, Appl. Catal., A, 2008, 340, 133–139 CrossRef CAS; (c) R. Zanella, S. Giorgio, C.-H. Shin, C. R. Henry and C. Louis, J. Catal., 2004, 222, 357–367 CrossRef CAS; (d) H.-S. Oh, J. H. Yang, C. K. Costello, Y. M. Wang, S. R. Bare, H. H. Kung and M. C. Kung, J. Catal., 2002, 210, 375–386 CrossRef CAS; (e) A. Hugon, N. E. Kolli and C. Louis, J. Catal., 2010, 274, 239–250 CrossRef CAS.
- E. Bus, R. Prins and J. A. van Bokhoven, Catal. Commun., 2007, 8, 1397–1402 CrossRef CAS.
- M. Shekhar, J. Wang, W. S. Lee, W. D. Williams, S. M. Kim, E. A. Stach, J. T. Miller, W. N. Delgass and F. H. Ribeiro, J. Am. Chem. Soc., 2012, 134, 4700–4708 CrossRef CAS PubMed.
- D. Xiang, X. F. Liu, J. S. Sun, F. S. Xiao and J. M. Sun, Catal. Today, 2009, 148, 383–388 CrossRef CAS.
- L. D. Hao, Y. F. Zhao, B. Yu, H. Y. Zhang, H. J. Xu and Z. M. Liu, Green Chem., 2014, 16, 3039–3044 RSC.
- M. Manzoli, C. Chiorino, F. Vindigni and F. Boccuzzi, Catal. Today, 2012, 181, 62–67 CrossRef CAS.
- X. L. Du, G. Tang, H. L. Bao, Z. Jiang, X. H. Zhong, D. S. Su and J. Q. Wang, ChemSusChem, 2015, 8, 3489–3496 CrossRef CAS PubMed.
- X. Q. Li, J. Xu, F. Wang, J. Gao, L. P. Zhou and G. Y. Yang, Catal. Lett., 2006, 108, 137–140 CrossRef CAS.
- W. H. Fang, J. S. Chen, Q. H. Zhang, W. P. Deng and Y. Wang, Chem. - Eur. J., 2011, 17, 1247–1256 CrossRef CAS PubMed.
- K. Shimizu, K. Sugino, K. Sawabe and A. Satsuma, Chem. - Eur. J., 2009, 15, 2341–2351 CrossRef CAS PubMed.
- R. Zanella, S. Giorgio, C. R. Henry and C. Louis, J. Phys. Chem. B, 2002, 106, 7634–7642 CrossRef CAS.
- R. J. H. Grisel, P. J. Kooyman and B. E. Nieuwenhuys, J. Catal., 2000, 191, 430–437 CrossRef CAS.
- G. C. Bond, C. Louis and D. T. Tompson, Catalysis by Gold, Imperial College Press, 2006 Search PubMed.
- A. Goguet, R. Burch, Y. Chen, C. Hardacre, P. Hu, R. W. Joyner, F. C. Meunier, B. S. Mun, D. Thompsett and D. Tibiletti, J. Phys. Chem. C, 2007, 111, 16927–16933 CAS.
- M. W. E. van den Berg, A. de Toni, M. Bandyopadhyay, H. Gies and W. Grünert, Appl. Catal., A, 2011, 391, 268–280 CrossRef CAS.
- (a) Y. Guo, D. Gu, Z. Jin, P. P. Du, R. Si, J. Tao, W. Q. Xu, Y. Y. Huang, S. Senanayake, Q. S. Song, C. J. Jia and F. Schüth, Nanoscale, 2015, 7, 4920–4928 RSC; (b) D. P. Akolekar, G. Foran and S. K. Bhargava, J. Synchrotron Radiat., 2004, 11, 284–290 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Details containing figures of TEM, catalysis activity, XRD. See DOI: 10.1039/c5ra20991a |
| This journal is © The Royal Society of Chemistry 2015 |