A NIR-to-NIR upconversion luminescence system for security printing applications†
A. Baridea,
J. M. Merugab,
C. Doumaa,
D. Langermanc,
G. Crawfordb,
J. J. Kellarb,
W. M. Crossb and
P. S. May*a
aDepartment of Chemistry, University of South Dakota, 414 E Clark St., Vermillion, SD 57069, USA. E-mail: Stanley.May@usd.edu
bMaterials and Metallurgical Engineering, South Dakota School of Mines and Technology, 501 E Saint Joseph St., Rapid City, SD 57701, USA
cElectrical and Computer Engineering, South Dakota School of Mines and Technology, 501 E Saint Joseph St., Rapid City, SD 57701, USA
First published on 16th November 2015
Abstract
A covert print-and-read system is demonstrated based on NIR-to-NIR upconversion luminescence. Inks activated with Yb3+/Tm3+ doped β-NaYF4 upconversion nanoparticles were used to print covert features on various substrates, including paper, epoxy resin, and circuit boards. The Yb3+/Tm3+ doping concentrations were optimized to maximize the brightness of 800 nm upconversion emission excited with 980 nm light, while simultaneously minimizing unwanted blue upconversion. Images printed with the NIR-optimized inks are invisible to the naked eye under ambient lighting or under 980 nm excitation. NIR-to-NIR images are easily captured, however, using an inexpensive, modified point-and-shoot CCD camera, even at modest excitation power densities (1.5 W cm−2). It is demonstrated that the latent images can also be read through select hard or soft coatings which are opaque to visible light, such as black inkjet print, or dyed epoxy resin, without significant attenuation of brightness. The ability to protect the printed images with durable, opaque coatings increases the tamper-resistance and the covertness of the system; removes the requirement that the print be invisible on the bare substrate; and blocks any visible emission that might be present, even under very high excitation power densities.
Introduction
Counterfeiting is a major concern for corporate, federal and state organizations. Counterfeits compromise the security, identity and value of products, documents, IDs and currency, thereby exerting a strong negative impact on societal financial and social wellbeing. Counterfeiting of integrated circuits (ICs) is a major concern, because of its potential to compromise the performance of a wide range of critical infrastructure, ranging from healthcare devices to military equipment to space hardware.1,2 Counterfeit ICs are often fraudulent or degraded products that have been either rejected during quality control or recycled from waste. An effective supply-chain management program, supported by strong track-and-trace security features, is an effective deterrence to counterfeiting.1 A strong security feature embeds information in the product and resists tampering, duplication or destruction without damaging product integrity. In this study, we describe a print-and-read system for security applications based on NIR-to-NIR upconversion inks, in which the security feature is covert, and can be protected from tampering by hard opaque coatings.Upconversion phosphors emit at wavelengths shorter than that of the excitation light. Lanthanide-ion-doped β-NaYF4 is recognized for exceptionally efficient near infrared (NIR)-to-visible upconversion upon excitation with 980 nm light.3–7 The concentrations and combinations of lanthanide dopants influence both the wavelength and the intensity of the upconversion emission. Applications of upconversion nanomaterials include sensors,8–12 bio-imaging,13–17 drug delivery18,19 and security/anti-counterfeiting.20–29 Our group has conducted seminal work in using upconversion inks for security printing, including the demonstration of ‘invisible QR codes’ for security printing and anti-counterfeiting applications.20–22 The inks are a formulation of upconversion nanoparticles (UCNPs) dispersed in a polymer base. When used to print text or features (e.g. QR codes) on paper, the upconversion ink is invisible to the eye under ambient conditions or UV excitation, but becomes visible upon excitation with 980 nm light.21 We have recently reported an RGB system for full-color printing using primary color red, green and blue upconversion activated with 10% Er3+/2% Tm3+, 17% Yb3+/3% Er3+ and 25% Yb3+/0.3% Tm3+ nanoparticles, respectively.20
Co-doping solid-state systems using Tm3+ as an activator and Yb3+ as a sensitizer is a common strategy for producing NIR-to-blue upconversion phosphors.20,21,30–36 β-NaYF4 UCNPs doped with Yb3+ and Tm3+ are well known for NIR-to-blue upconversion emission (440–500 nm).20,21,30,31 These ‘blue’ UCNPs, however, also emit 800 nm NIR light, which is invisible to the naked eye, but is also much more intense than the visible blue emission.21 Doping composition has a significant effect on the absolute and relative quantum efficiency of various emission bands. The 25% Yb3+/0.3% Tm3+ doping combination is optimized for blue upconversion emission.37 Zhang et al. studied the effect of Tm3+ concentration on blue and NIR upconversion efficiency in 18 nm 20% Yb3+/(0.3–4% Tm3+) doped β-NaYF4 nanoparticles.38 The group reported that the blue emission is quenched as the Tm concentration increases, but no significant change was observed in 800 nm emission over this doping range. In contrast, Wang et al. reported an increase in 800 nm emission with increasing Tm concentration from 0.2% to 2% Tm3+ in α-NaYF4 UCNPs co-doped with 20% Yb3+.39 To date, however, the optimum doping combination for achieving maximally bright and spectrally pure 800 nm emission has not been reported for β-NaYF4 UCNPs. For our purposes, maximizing the absolute per-particle brightness at 800 nm is of paramount importance.
In this study, we compare the 980-to-800 nm upconversion efficiency for four doping compositions, 25% Yb3+/0.3% Tm3+ (optimized for blue), 25% Yb3+/2% Tm3+, 48% Yb3+/2% Tm3+ and 46% Yb3+/4% Tm3+ and evaluate the optimum doping for the 800 nm NIR emission. Further, we explore the security printing applications of NIR-to-NIR upconversion emission from Yb3+/Tm3+ doped UCNPs and strategies for tamper-resistant security applications of these materials. NIR-to-NIR upconversion is very advantageous for anti-counterfeiting, because most common organic materials are transparent to the 980 nm excitation and 800 nm emission. Consequently, printed features can be excited and read through hard polymer or epoxy coatings. Moreover, these coatings can be completely opaque to visible light, as long as the transmittance is high at 800 nm and 980 nm. A system of markings coated with an opaque epoxy layer would be difficult to detect and quite tamper resistant. In this study, we also demonstrate the excitation and capture of NIR-to-NIR images of UCNPs coated with NIR transparent materials that are either transparent or opaque in the visible range. The NIR luminescent images store information that is not visible to the naked eye, but can be captured with an inexpensive CCD camera.
In addition to the security printing applications discussed here, NIR-to-NIR UCNPs show promise for use in bio-imaging,40–43 because both the excitation (980 nm) and emission (800 nm) wavelengths fall within the biological transparency window.42 Therefore, 800 nm emission from UCNPs loaded into biological tissue can be imaged by exciting with 980 nm light.
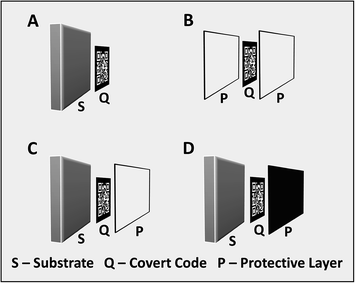
We propose four scenarios for the use of NIR-to-NIR upconversion inks for security printing, as illustrated in Fig. 1: (A) covert codes printed on a substrate; (B) covert codes embedded within a polymer; (C) covert codes printed on a hard substrate and subsequently coated with a transparent polymer; and (D) covert codes printed on a hard substrate and subsequently coated with an polymer that is opaque to visible light.
Protected codes or symbols encoding critical product information are ideally suited for safeguarding integrated circuits (ICs) and electronic circuit boards from counterfeiting threats. Tampering with markings integrated within the IC packaging without damaging the component would be a difficult task. Integrated circuits are often enclosed in an epoxy case (or mold compound) and electronic circuit boards are frequently coated with polymer films. We choose an epoxy polymer as the protecting layer in our experiments. Epoxy is chemical resistant and insoluble in common solvents, making it difficult to remove in order to reach the embedded code. Moreover, it is highly transmissive in the NIR region. We use electronic circuit boards as an example substrate to demonstrate the potential application of NIR-to-NIR upconversion in security printing and anti-counterfeiting.
Experimental section
Materials
Yttrium oxide (99.99%), ytterbium oxide (99.9%), thulium oxide (99.9%) and oleic acid (90%) were obtained from Alfa Aesar; 1-octadecene (90%) from Aldrich; methyl benzoate (99%), sodium acetate trihydrate (99%) from Acro Organics; sodium fluoride (99%) from Fisher Scientific; acetic acid (glacial, 99.9%) from Pharm Co.; poly(methyl methacrylate) beads (MW 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) from Scientific Polymer Products. Black thermo-bubble-jet ink was purchased from Hobbicolors (DJC-45060-0-A). Ready-to-mix epoxy resins were purchased from commercial retailers.
000) from Scientific Polymer Products. Black thermo-bubble-jet ink was purchased from Hobbicolors (DJC-45060-0-A). Ready-to-mix epoxy resins were purchased from commercial retailers.
Synthesis of UCNPs
The UCNPs synthesis procedure is similar to the procedure reported by Lin et al. with a few modifications.44 Yttrium oleate (Y(OA)3), ytterbium oleate (Yb(OA)3) and thulium oleate (Tm(OA)3) precursor solutions, 25 mL each, were prepared as described by Lin et al.44 The resulting Y(OA)3, Yb(OA)3, and Tm(OA)3 solutions have concentrations of 0.2 mmol mL−1 of Y3+, 0.1 mmol mL−1 of Yb3+, and 0.01 mmol mL−1 of Tm3+, respectively.To prepare UCNPs, CH3COONa·3H2O (2 mmol), NaF (4 mmol), 1-ODE (16 mL) and appropriate amounts of Y(OA)3, Yb(OA)3, and Tm(OA)3 precursor solutions were combined in a 50 mL flask. The composition of the precursor solution determines the doping concentration of Yb3+/Tm3+. For 25% Yb3+/0.3% Tm3+: 3.74 mL Y(OA)3, 2.5 mL Yb(OA)3 and 0.3 mL Tm(OA)3; for 25% Yb3+/2% Tm3+: 3.65 mL Y(OA)3, 2.5 mL Yb(OA)3 and 2 mL Tm(OA)3; for 48% Yb3+/2% Tm3+: 2.5 mL Y(OA)3, 4.8 mL Yb(OA)3 and 2 mL Tm(OA)3; and for 46% Yb3+/4% Tm3+: 2.5 mL Y(OA)3, 4.6 mL Yb(OA)3 and 4 mL Tm(OA)3 were added. Additional oleic acid was added to bring the total volume of the reaction mixture to 32 mL. The reaction mixture was heated under vacuum at 100 °C for 1 hour to remove volatile solvents and then the vacuum was replaced with a gentle stream of Ar gas. The temperature was increased to 320 °C and reaction was continued for another 2.5 hours. The UCNP product was washed three times by precipitation with acetone, re-dispersing in toluene and precipitating with acetone. The UCNPs were dried under vacuum at 65 °C for 24 hours. The size and morphology of the UCNPs were characterized by TEM (FEI Tecnai, 120 kV) (see ESI, Fig. S1†). The UCNP exhibit a hexagonal morphology with an average size of 112 ± 12 nm. The product UCNPs are phase pure β-NaYF4 nanocrystals (see ESI, Fig. S2†) as determined by PXRD.
Spectroscopy
Steady-state spectroscopy was performed by exciting 1 wt% dispersions of UCNPs in toluene with a 980 nm CW laser (MDL-N-980, Opto Engine LLC) with power densities between 5–35 W cm−2. Emission spectra were recorded with a miniature spectrophotometer (BLUE-Wave, StellarNet). An 850 nm short-pass filter (4 OD, Edmund, #64-334), was used to block the excitation light from the spectrophotometer. Emission spectra were corrected for the instrument response to give relative photon flux per wavelength interval.Epoxy film preparation
Epoxy films of desired thickness were prepared from ready-to-mix epoxy resins. A commercially available epoxy (Loctite Quick Set Epoxy) was used to prepare epoxy films. The epoxy comes in a two-syringe dispenser with epoxy resin in one syringe and hardener in other syringe. A thin coat of epoxy was prepared by mixing the hardener and the epoxy resin and coating the epoxy mixture on a substrate using a glass slide as an applicator. The black epoxy was prepared by mixing 0.06 mL of black inkjet ink (Hobbicolors) to approximately 2 mL of epoxy mixture. Epoxy thin film was prepared by applying a smear of epoxy mixture on a polypropylene sheet and the film was carefully peeled-off after drying.Preparation of inks and printing
The upconversion inks were prepared as described by Blumenthal et al.22 In brief, 5 wt% of UCNPs were dispersed in a solution of 1 wt% of PMMA in a mixture of toluene/methyl benzoate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The inks were printed on plain paper, an electronic circuit board, and an epoxy film using an Optomec aerosol-jet printer.
1). The inks were printed on plain paper, an electronic circuit board, and an epoxy film using an Optomec aerosol-jet printer.
NIR imaging
NIR imaging was performed with a point-and-shoot camera modified to detect NIR signal. The NIR filter in front of the CCD chip was carefully removed from a Canon A470 point-and-shoot camera to capture NIR images. Unless specified, all images were captured using a 1 second exposure, ISO 400, f/5.8 exciting with a 980 nm CW laser (1.5 W cm−2) in ambient lighting from a low-wattage lamp. An 850 nm short-pass filter was used to block excitation light from entering into the camera.Results and discussion
Optimization of doping composition for NIR-to-NIR upconversion
Fig. 2 shows the energy level diagram and relevant optical processes for the Yb3+/Tm3+ doped upconversion system. The Yb3+ ion acts as a sensitizer and the Tm3+ ion acts as an activator, producing visible and NIR emission. The Yb3+ sensitizers absorb 980 nm excitation light and transfer energy to adjacent Tm3+ activator ions. Multiple sequential Yb3+ → Tm3+ energy transfer events promote the Tm3+ ions to successively higher energy levels. There is some doubt as to how the 1D2 state of Tm3+ is populated, so that this excitation pathway is not labelled in Fig. 2 (it has been suggested that the final step to populate 1D2 requires Tm3+ → Tm3+ energy transfer).45,46 Blue emission is observed from the 1D2 and 1G4 excited states of Tm3+ ion, corresponding to the 1D2 → 3F4 (450 nm) and 1G4 → 3H6 (475 nm) transitions. NIR emission is observed from the 3H4 and 1G4 emitting levels, corresponding to the 3H4 → 3H6 (800 nm) and 1G4 → 3H5 (780 nm) transition, the latter of which is not labelled in Fig. 2. Weak red emission is also observed, corresponding to the 1G4 → 3F4 (650 nm) transition.Yb3+/Tm3+-doped β-NaYF4 nanoparticles have been studied primarily relative to their NIR-to-blue upconversion properties, with the doping levels chosen to optimize the blue upconversion emission. This blue emission is excited via three- and four-photon processes, whereas the NIR-to-NIR 3H4 → 3H6 emission at 800 nm is excited via a two-photon process. As a result, the 800 nm NIR-to-NIR emission is much more intense than the NIR-to-blue emission, particularly at modest excitation power densities, Pexc. For example, even for β-NaYF4: 25% Yb3+/0.3% Tm3+, which is optimized for blue emission, NIR emission is 400× more intense than blue emission at Pexc = 6.2 W cm−2 and 100× more intense at Pexc = 35 W cm−2 (see Fig. 3).
 | ||
| Fig. 3 (A) Comparison of upconversion emission, Ic, spectra of β-NaYF4 nanoparticles with various Yb3+/Tm3+ doping levels dispersed in toluene. Excitation was at 980 nm (35 W cm−2). The spectra represent a valid comparison of relative brightness of samples (in terms of number photons emitted per constant wavelength interval) corrected to equal number densities of nanoparticles (see eqn (1)). The inset shows a 100× magnification of the visible region of the spectra. (B) log–log plot of the dependence of the 800 nm NIR emission intensity on excitation power density, Pexc. (C) NIR-to-blue emission intensity ratio, INIR/IBlue for UCNPs (48-2) as a function of excitation power density, Pexc. | ||
For the initial phase of this study, we determine the optimum doping levels for maximizing the absolute brightness of 800 nm emission, with the secondary purpose of minimizing the blue emission. The absolute brightness of the 800 nm emission determines the minimum Pexc required to view the NIR-to-NIR upconversion images shown later in this article. For print applications for which no opaque coatings are applied, minimizing the blue emission is necessary in order to maintain the covert nature of the images. When using opaque coatings, only the absolute brightness of 800 nm emission is of interest.
We synthesized UCNPs with four doping combinations of Yb3+ and Tm3+: 25% Yb3+/0.3% Tm3+ (UCNPs (25-0.3)), 25% Yb3+/2% Tm3+ (UCNPs (25-2)), 48% Yb3+/2% Tm3+ (UCNPs (48-2)), and 46% Yb/4% Tm3+ (UCNPs (46-4)). The emission spectra were recorded by exciting the UCNPs with a 980 nm CW laser using a range of excitation power densities, Pexc = 5–35 W cm−2. Fig. 3A compares the UC emission spectra of the four compounds dispersed in toluene using Pexc = 35 W cm−2. The inset shows the NIR-to-visible portion of the spectrum magnified by 100×. Clearly, even at the highest Pexc values used, the spectra are completely dominated by the 800 nm emission.
The peak assignments in Fig. 3A are largely consistent with those reported in the literature.4,46 The NIR emission is dominated by the 3H4 → 3H6 transition at 800 nm, which is excited via a two-photon process. At higher excitation power densities (not used here), a significant NIR contribution at 780 nm from the 1G4 → 3H5 transition, excited via a three-photon process, becomes evident, usually appearing as a strong shoulder to the 800 nm peak.47 The blue peaks at 450 nm and 475 nm correspond to the 1D2 → 3F4 and 1G4 → 3H6 transitions, excited via four- and three-photon processes, respectively.
There has been some confusion regarding the origin of the red emission at 650 nm, which has been variously assigned in the literature as arising from 3F3,2 or from 1G4. Yan et al. report that the 475 nm and 650 nm emission intensity exhibit similar dependence on excitation power, consistent with both transitions arising from 1G4.48 Moreover, we observe that the relative intensities of 475 nm and 650 nm emission remain constant at all doping concentrations, indicating that these transitions experience the same quenching effects, and, therefore, originate from the same emitting state. Finally, we also observe that the time profiles of 475 nm and 650 nm emission following pulsed 980 nm excitation are nearly identical, strongly supporting the assignment of the 650 nm emission arising from the 1G4 state. Therefore, the 650 nm emission is assigned as being predominately due to the 1G4 → 3F4 transition.
The emission at 700 nm is tentatively assigned to 3F3,2 → 3H6 based on the fact that this is the expected wavelength for this emission, and on the fact that this peak shows a power dependence similar to that of the 800 nm emission, 3H4 → 3H6 (see ESI†). Referring to Fig. 2, emission from 3F3,2 and 3H4 are expected to have similar power dependence, because 3H4 is fed through the 3F3,2 states. We note, however, that the 700 nm peak is observed in some spectra reported in the literature, but not in others. The reason for this is unclear to us. We note, however, that the relative intensity of the 700 nm transition will be very sensitive to multi-phonon quenching because the 3F3,2–3H4 energy gap is so small. This emission may then vary somewhat from sample to sample due to otherwise minor differences in sample properties.
The spectra in Fig. 3A represent the relative brightness of the different sample dispersions corrected to equal number densities of nanoparticles. Corrected spectra, IC, were generated from the measured spectra, IM, using the following formula:
| IC = IM × %Yb/A (980 nm) | (1) |
| %Yb | %Tm | Ic (NIR) | Ic (blue) × 102 | Ic NIR/Ic blue |
|---|---|---|---|---|
| 25 | 0.3 | 1.0 | 0.97 | 103 |
| 25 | 2 | 1.3 | 0.03 | 4366 |
| 48 | 2 | 5.8 | 0.09 | 6207 |
| 46 | 4 | 0.4 | 0.01 | 3146 |
Referring to Fig. 3A, several important trends are evident. First, blue emission is increasingly quenched with increasing Tm concentration, whereas the NIR (800 nm) emission initially increases marginally, and then drops precipitously. These observations are consistent with a previous study by Quintanilla et al.46 that reported strong Tm concentration quenching of both 1D2 and 1G4 with a more modest effect on emission from 3F2,3 and 3H4. Similarly, Zhao et al. also reported a decrease in 800 nm emission with increasing Tm concentration in 20% Yb3+/0.2–4% Tm3+ doped β-NaYF4 crystals at 10 W cm−2 excitation power density.29 Thus, the optimal Tm concentration for our purposes, quenching blue emission while having little or no detrimental effect on 800 nm emission, can be identified as being quite close to the 2% level. With regard to %Yb concentration, over the range of excitation power densities used here, the corrected intensity, IC, of 800 nm emission continues to increase as Yb concentration is raised beyond 25%. However, we limit the Yb doping to 48% because it becomes increasingly difficult to control the size and phase stability of the nanocrystals at higher doping levels, as discussed by Damasco, et al., and references therein.49
Fig. 3C shows the ratio of 800 nm-to-blue emission for UCNPs (48-2) as a function of Pexc over the range of excitation power densities used in this study. Even at the highest Pexc values, 800 nm emission is >6000× more intense than the blue emission. As noted below, 800 nm luminescent images can be read easily with a modified point-and-shoot camera using laser power densities at which little or no visible emission is detectable by eye.
NIR-to-NIR imaging of printed patterns
The NIR-optimized UCNPs (48-2) were formulated into inks and used to print 1 cm × 1 cm QR codes on a variety of substrates. Fig. 4 shows photographs of these QR codes, under ambient lighting and 980 nm excitation, printed on plain paper (Fig. 4A and B), laminated between transparent epoxy films (Fig. 4C and D), and printed on an electronic circuit board (Fig. 4E and F).The locations of the printed areas in the ambient-lighting photographs are indicated by a red dotted box. In all cases, the printed QR codes are invisible to the naked eye under ambient light. The images of the QR codes under ambient light were captured with smart-phone camera (Fig. 4A, C and E), whereas the 800 nm luminescent images generated by 980 nm excitation (Fig. 4B, D and F) were captured with a point-and-shoot camera, modified as described above. At the excitation power densities used to acquire the luminescent images shown in Fig. 4 (Pexc = 1.5 W cm−2), blue emission is either undetectable or very faint.
The QR code printed on a plain paper (Fig. 4A) generates a very bright, sharp 800 nm luminescent image (Fig. 4B) even under relatively low excitation flux (Pexc = 1.5 W cm−2).
Fig. 4C shows an ambient-light photograph of a sample prepared by printing a QR code on a 0.2 mm thick transparent epoxy layer followed by coating with another 0.2 mm thick transparent epoxy layer. The laminated sample was placed on a ruler to assist the reader in judging its size and optical quality. The NIR luminescent image of the QR code was captured with the NIR sensitive camera using 980 nm excitation Pexc = 1.5 W cm−2 (Fig. 4D). The image is not as bright or sharp as that of the QR code on paper due to the imperfect optical quality of the epoxy matrix, caused in part by air bubbles entrapped in the epoxy film while mixing the hardener and resin, and by lensing effects arising from the topography of the epoxy surface.
Fig. 4E is a photograph of the upconversion ink printed on an electronic circuit board and then coated with a protective thin film (0.4 mm) of transparent epoxy. The QR code retained its integrity after coating with the epoxy. A distinct NIR luminescent image of QR code was captured by exciting the printed region with 980 nm light, Pexc = 1.5 W cm−2 (Fig. 4F). The epoxy film has high degree of transparency and does not significantly attenuate the transmission of excitation (980 nm) or emission (800 nm) light. The experiment demonstrates the ability to protect the security feature with durable coatings without adversely affecting functionality.
Imaging printed features through opaque coatings
Because the imaging proposed herein utilizes emission and excitation in the NIR, it is possible to coat and protect these images with materials that are visibly opaque. This strategy strengthens the covert nature of the print/read system and offers some additional advantages over bare prints or transparent-layer protective coatings. First, even if otherwise viewable intensities of visible emission are generated by the NIR excitation, the opaque coating will block these wavelengths. Second, the invisibility of the latent print image on the uncoated substrate is no longer a requirement. This permits the use of higher particle loadings or thicker print coatings, which, in turn, lower the threshold of Pexc required to view and capture the image.We use two methods to apply opaque coatings to the UC printed features. Both methods use opaque coatings based on commercially available, water-based inkjet-printer ink from Hobbicolor (black). The transmission spectrum of the ink (20 μL of ink in 5 mL of water) measured across 1 cm path length has <1% transmission in the visible region and >95% transmission in the NIR region (see Fig. 5). Also, for this set of experiments, we use inks activated with the UCNPs (25-0.3) taggants, optimized for blue emission, in order to judge the ability of the opaque coatings to block visible emission from the printed images.
In the first method, an inkjet printer loaded with the Hobbicolor black cartridge is used to print over UC print features that were previously applied to the paper. In the second method, a UC print feature is coated with a layer of black epoxy (dyed with 0.03 mL of Hobbicolor ink per 1 mL epoxy). The transmission spectra of a 0.4 mm thick epoxy layer before and after dying with black ink are shown in Fig. 5. The undyed epoxy film has a high degree of transmittance in the visible as well as NIR region. The black epoxy film has <1% transmittance in the visible region, but retains >80% transmittance in the relevant NIR region.
Fig. 6A and B are images of the UCNPs (25-0.3) printed on a plain paper, acquired with a NIR-sensitive camera under ambient lighting. The black arrows indicate the positions of the printed features. The lower-left printed image in Fig. 6A is being illuminated with 980 nm laser excitation, and the UC luminescent image of the QR code is clearly visible. Similarly, the UC luminescent image of University of South Dakota logo was captured in Fig. 6B. For Fig. 6A and B, it is possible to detect very faint blue emission with the naked eye (blue emission becomes visible when excitation power densities in the range of Pexc = 4.0–5.5 W cm−2 are used).20,21 This blue emission is not evident in the camera images of Fig. 6A and B, because it is overwhelmed by the intensity of the 800 nm emission. Fig. 6C and D show images corresponding to Fig. 6A and B after the same sheet of paper was passed through an inkjet printer and coated with black ink. The NIR images can be easily captured through the printed coatings with no significant attenuation relative to those obtained prior to coating. The faint blue emission is now, however, completely blocked and no trace of the images are evident to the naked eye, even upon increasing excitation power densities to levels normally used to view blue upconversion (Pexc = 5 W cm−2). Fig. 6E and F are magnified images (5×) of the luminescent images shown in the Fig. 6C and D, respectively. The images retain good detail even after coating with black inkjet ink.
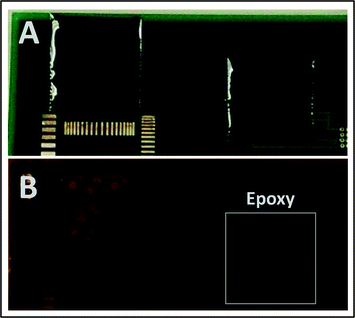
In the second method, we use a dyed epoxy resin as the opaque protective coating. A 1 cm × 1 cm QR code was printed with blue-optimized upconversion ink on an electronic circuit board. This print was then coated with a 0.4 mm layer of black-ink-doped epoxy overcoat. The transmittance spectrum of this layer is shown in Fig. 5. Fig. 7A shows an ambient-lighting photograph of the epoxy-coated electronic circuit taken with a smartphone. The UCNPs printed region was excited with a 980 nm laser (Pexc = 1.5 W cm−2) through the black epoxy layer. Although the printed features are not visible to the naked eye, they are easily captured using a NIR sensitive camera (Fig. 7B). The blue emission, however, was completely blocked by the black epoxy coating, even at Pexc = 5 W cm−2. The highlighted box in Fig. 7B shows another QR code coated with the black epoxy, which was not excited with 980 nm light, and, therefore, shows no NIR emission.
We note that the luminescent images shown in Fig. 6 and 7 are not as bright as those shown in Fig. 4, due to the fact that blue-optimized nanoparticles (UCNPs (25-0.3)) were used to formulate the inks for these samples in order to test the efficacy of the opaque coatings in blocking any visible blue emission.
Fig. 8 shows examples of covert images printed using NIR-optimized inks (UCNPs (48-2)) on a circuit board. The images on the left are coated with black epoxy, whereas the images on the right are coated with transparent epoxy. Bright, distinct, NIR luminescent images from both print areas, produced with modest 980 nm excitation density (Pexc = 1.5 W cm−2), are easily captured with our modified point-and-shoot camera. The epoxy coatings do not adversely affect the integrity of the printed images. Moreover, the image acquired through the black epoxy coating is nearly as bright as that acquired through the transparent epoxy.
Conclusion
The print-and-read security printing system described herein, based on NIR-to-NIR inks, offers a high level of covertness and can be made tamper-evident when combined with hard, opaque coatings. The inks are activated with Yb3+/Tm3+ doped β-NaYF4 UCNPs, and it is determined that the optimal doping levels for maximizing the brightness of 800 nm upconversion and minimizing the undesired blue upconversion is 48% Yb3+/2% Tm3+. For the NIR-optimized UCNPs, the NIR-to-NIR intensity is greater than 6000× that of the NIR-to-blue emission, even at relatively high excitation power densities (35 W cm−2). The upconversion images, generated with 980 nm excitation, cannot be seen with the naked eye, but are easily captured with an inexpensive, modified point-and-shoot digital camera. The 800 nm upconversion emission is near perfect for detection with a digital camera, since this wavelength is at or near the peak sensitivity of the CCD chip. All images tested are easily captured with a modified point-and-shoot camera using modest excitation power densities (1.5 W cm−2).Electronic circuit boards, plain paper, and epoxy-resin films are shown to be suitable substrates to demonstrate the application. Inkjet printing or epoxy coatings applied over the upconversion prints do not erode the print integrity. It is demonstrated that the NIR-to-NIR images can be read through opaque coatings of inkjet printing or black epoxy resin, with no significant loss of brightness. The use of durable opaque coatings offers a significant advantage to the covert print system for three reasons: (1) it deters tampering; (2) it increases the covert nature of the marking, both in terms of the latent image, and in terms of the luminescent image (by blocking visible emission); and (3) it relaxes the requirement that the bare printed image be completely covert.
The ability to read the covert code or symbol across an opaque protective layer makes the current system attractive for numerous security and anti-counterfeiting applications, including electronic circuit boards and high-value integrated circuits.
Abbreviations
| UCNPs | Upconversion nanoparticles |
| UCNPs (25-0.3) | 25% Yb3+/0.3% Tm3+ doped β-NaYF4 UCNPs |
| UCNPs (25-2) | 25% Yb3+/2% Tm3+ doped β-NaYF4 UCNPs |
| UCNPs (48-2) | 48% Yb3+/2% Tm3+ doped β-NaYF4 UCNPs |
| UCNPs (46-4) | 46% Yb3+/4% Tm3+ doped β-NaYF4 UCNPs |
Acknowledgements
The authors acknowledge generous support from NSF (EPS-0903804, DGE-0903685, CHE-0840507, IIP 1414211). C. Douma and D. Langerman were supported by an NSF:REU award to Crawford and May (EEC 1263393). P. S. M. acknowledges support from NASA (Cooperative Agreement Number: NNX10AN34A). Additional support was provided through a Collaboration grant to J. Kellar from the State of South Dakota, Governor's Office of Economic Development.References
- E. M. Chan, D. J. Gargas, P. J. Schuck and D. J. Milliron, J. Phys. Chem. B, 2012, 116, 10561–10570 CrossRef CAS PubMed
.
- Q. Meng, R. J. Witte, Y. Gong, E. L. Day, J. Chen, P. S. May and M. T. Berry, Chem. Mater., 2010, 22, 6056–6064 CrossRef CAS
.
- F. Wang, D. Banerjee, Y. Liu, X. Chen and X. Liu, Analyst, 2010, 135, 1839–1854 RSC
.
- S. Heer, K. Kömpe, H. U. Güdel and M. Haase, Adv. Mater., 2004, 16, 2102–2105 CrossRef CAS
.
- F. Vetrone and J. A. Capobianco, Int. J. Nanotechnol., 2008, 5, 1306–1339 CrossRef CAS
.
- M. Haase and H. Schäfer, Angew. Chem., Int. Ed., 2011, 50, 5808–5829 CrossRef CAS PubMed
.
- F. Wang and X. Liu, Chem. Soc. Rev., 2009, 38, 976–989 RSC
.
- C. Hazra, V. N. K. B. Adusumalli and V. Mahalingam, ACS Appl. Mater. Interfaces, 2014, 6, 7833–7839 CAS
.
- L. Zhao, J. Peng, M. Chen, Y. Liu, L. Yao, W. Feng and F. Li, ACS Appl. Mater. Interfaces, 2014, 6, 11190–11197 CAS
.
- G. Chen, H. Qiu, P. N. Prasad and X. Chen, Chem. Rev., 2014, 114, 5161–5214 CrossRef CAS PubMed
.
- Y. Ma, S. Huang, M. Deng and L. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 7790–7796 CAS
.
- S. Liu, L. Zhang, T. Yang, H. Yang, K. Y. Zhang, X. Zhao, W. Lv, Q. Yu, X. Zhang, Q. Zhao, X. Liu and W. Huang, ACS Appl. Mater. Interfaces, 2014, 6, 11013–11017 CAS
.
- Q. Liu, J. Peng, L. Sun and F. Li, ACS Nano, 2011, 5, 8040–8048 CrossRef CAS PubMed
.
- L. Zhou, B. He, J. Huang, Z. Cheng, X. Xu and C. Wei, ACS Appl. Mater. Interfaces, 2014, 6, 7719–7727 CAS
.
- Q. Zhan, J. Qian, H. Liang, G. Somesfalean, D. Wang, S. He, Z. Zhang and S. Andersson-Engels, ACS Nano, 2011, 5, 3744–3757 CrossRef CAS PubMed
.
- Y.-F. Wang, G.-Y. Liu, L.-D. Sun, J.-W. Xiao, J.-C. Zhou and C.-H. Yan, ACS Nano, 2013, 7, 7200–7206 CrossRef CAS PubMed
.
- G. Chen, J. Shen, T. Y. Ohulchanskyy, N. J. Patel, A. Kutikov, Z. Li, J. Song, R. K. Pandey, H. Ågren, P. N. Prasad and G. Han, ACS Nano, 2012, 6, 8280–8287 CrossRef CAS PubMed
.
- Z. Hou, X. Li, C. Li, Y. Dai, P. a. Ma, X. Zhang, X. Kang, Z. Cheng and J. Lin, Langmuir, 2013, 29, 9473–9482 CrossRef CAS PubMed
.
- L. He, L. Feng, L. Cheng, Y. Liu, Z. Li, R. Peng, Y. Li, L. Guo and Z. Liu, ACS Appl. Mater. Interfaces, 2013, 5, 10381–10388 CAS
.
- J. M. Meruga, A. Baride, W. Cross, J. J. Kellar and P. S. May, J. Mater. Chem. C, 2014, 2, 2221–2227 RSC
.
- J. M. Meruga, W. M. Cross, P. S. May, Q. Luu, G. A. Crawford and J. J. Kellar, Nanotechnology, 2012, 23, 395201 CrossRef PubMed
.
- T. Blumenthal, J. Meruga, P. S. May, J. Kellar, W. Cross, K. Ankireddy, S. Vunnam and Q. N. Luu, Nanotechnology, 2012, 23, 185305 CrossRef PubMed
.
- N. M. Sangeetha, P. Moutet, D. Lagarde, G. Sallen, B. Urbaszek, X. Marie, G. Viau and L. Ressier, Nanoscale, 2013, 5, 9587–9592 RSC
.
- M.-K. Tsang, G. Bai and J. Hao, Chem. Soc. Rev., 2015, 44, 1585 RSC
.
- J. Lee, P. W. Bisso, R. L. Srinivas, J. J. Kim, A. J. Swiston and P. S. Doyle, Nat. Mater., 2014, 13, 524–529 CrossRef CAS PubMed
.
- Y. Zhang, L. Zhang, R. Deng, J. Tian, Y. Zong, D. Jin and X. Liu, J. Am. Chem. Soc., 2014, 136, 4893–4896 CrossRef CAS PubMed
.
- B. Yoon, J. Lee, I. S. Park, S. Jeon, J. Lee and J.-M. Kim, J. Mater. Chem. C, 2013, 1, 2388–2403 RSC
.
- W. J. Kim, M. Nyk and P. N. Prasad, Nanotechnology, 2009, 20, 185301 CrossRef PubMed
.
- J. Zhao, D. Jin, E. P. Schartner, Y. Lu, Y. Liu, A. V. Zvyagin, L. Zhang, J. M. Dawes, P. Xi, J. A. Piper, E. M. Goldys and T. M. Monro, Nat. Nanotechnol., 2013, 8, 729–734 CrossRef CAS PubMed
.
- J.-C. Boyer, F. Vetrone, L. A. Cuccia and J. A. Capobianco, J. Am. Chem. Soc., 2006, 128, 7444–7445 CrossRef CAS PubMed
.
- J.-C. Boyer, L. A. Cuccia and J. A. Capobianco, Nano Lett., 2007, 7, 847–852 CrossRef CAS PubMed
.
- F. Auzel and D. Pecile, J. Lumin., 1976, 11, 321–330 CrossRef CAS
.
- S. Gai, C. Li, P. Yang and J. Lin, Chem. Rev., 2014, 114, 2343–2389 CrossRef CAS PubMed
.
- R. Lv, G. Yang, F. He, Y. Dai, S. Gai and P. Yang, Nanoscale, 2014, 6, 14799–14809 RSC
.
- R. Lv, C. Zhong, R. Li, P. Yang, F. He, S. Gai, Z. Hou, G. Yang and J. Lin, Chem. Mater., 2015, 27, 1751–1763 CrossRef CAS
.
- R. H. Page, K. I. Schaffers, P. A. Waide, J. B. Tassano, S. A. Payne, W. F. Krupke and W. K. Bischel, J. Opt. Soc. Am. B, 1998, 15, 996–1008 CrossRef CAS
.
- L. Zhengquan and Z. Yong, Nanotechnology, 2008, 19, 345606 CrossRef PubMed
.
- J. Zhang, H. Zhao, X. Zhang, X. Wang, H. Gao, Z. Zhang and W. Cao, J. Phys. Chem. C, 2014, 118, 2820–2825 CAS
.
- F. Wang and X. Liu, J. Am. Chem. Soc., 2008, 130, 5642–5643 CrossRef CAS PubMed
.
- Q. Liu, Y. Sun, T. Yang, W. Feng, C. Li and F. Li, J. Am. Chem. Soc., 2011, 133, 17122–17125 CrossRef CAS PubMed
.
- J.-C. Boyer, M.-P. Manseau, J. I. Murray and F. C. J. M. van Veggel, Langmuir, 2009, 26, 1157–1164 CrossRef PubMed
.
- N.-N. Dong, M. Pedroni, F. Piccinelli, G. Conti, A. Sbarbati, J. E. Ramírez-Hernández, L. M. Maestro, M. C. Iglesias-de la Cruz, F. Sanz-Rodriguez, A. Juarranz, F. Chen, F. Vetrone, J. A. Capobianco, J. G. Solé, M. Bettinelli, D. Jaque and A. Speghini, ACS Nano, 2011, 5, 8665–8671 CrossRef CAS PubMed
.
- M. Nyk, R. Kumar, T. Y. Ohulchanskyy, E. J. Bergey and P. N. Prasad, Nano Lett., 2008, 8, 3834–3838 CrossRef CAS PubMed
.
- C. Lin, M. T. Berry, R. Anderson, S. Smith and P. S. May, Chem. Mater., 2009, 21, 3406–3413 CrossRef CAS
.
- T. Riedener, H. U. Güdel, G. C. Valley and R. A. McFarlane, J. Lumin., 1995, 63, 327–337 CrossRef CAS
.
- M. Quintanilla, N. O. Nunez, E. Cantelar, M. Ocana and F. Cusso, Nanoscale, 2011, 3, 1046–1052 RSC
.
- Q. Luu, A. Hor, J. Fisher, R. B. Anderson, S. Liu, T.-S. Luk, H. P. Paudel, M. Farrokh Baroughi, P. S. May and S. Smith, J. Phys. Chem. C, 2014, 118, 3251–3257 CAS
.
- A. Yin, Y. Zhang, L. Sun and C. Yan, Nanoscale, 2010, 2, 953–959 RSC
.
- J. A. Damasco, G. Chen, W. Shao, H. Ågren, H. Huang, W. Song, J. F. Lovell and P. N. Prasad, ACS Appl. Mater. Interfaces, 2014, 6, 13884–13893 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra20785a |
| This journal is © The Royal Society of Chemistry 2015 |