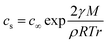Aceclofenac nanocrystals for improved dissolution: influence of polymeric stabilizers
Satyanarayan Pattnaik*a,
Kalpana Swaina,
Jupally Venkateswar Raoa,
Talla Varuna,
K. Baikuntha Prustya and
Sanjeev Kumar Subudhib
aFormulation Development and Drug Delivery Systems, Department of Pharmaceutics, Talla Padmavathi College of Pharmacy, Kareemabad, Warangal, India. E-mail: saty3000@yahoo.com; Tel: +91-7386752616
bTalla Padmavathi Pharmacy College, Orus, Warangal, India
First published on 23rd October 2015
Abstract
With the increasingly lipophilic nature of candidate drugs, solubility and dissolution rates have become the limiting factors that affect bioavailability of oral and parenteral formulations. The purpose of the study is to improve the dissolution rate of aceclofenac through a nanonization technique. In the present work, particle engineering was carried out to obtain pure drug nanocrystals of aceclofenac using a standard, simple and scalable bottom up technique to overcome its poor dissolution behavior employing different classes of polymeric stabilizers. The physicochemical properties were evaluated including particle size distribution, powder X-ray diffractometry, scanning electron microscopy and in vitro dissolution studies. Intestinal absorption studies were also carried out to assess the effectiveness of the fabricated nanocrystals. The concentration and type of polymer influenced the particle size and dissolution velocity of aceclofenac. Among the stabilizers studied, methyl cellulose (0.3% w/v) was found most efficient which lead to highest dissolution (88.27 ± 2.053%) with significant reduction in particle size. The intestinal permeation studies indicated significantly (p < 0.05) higher permeation of drug from the nanocrystals. Semi synthetic non ionic polymers were found very effective in reducing the particle size (preventing growth of crystals), improving the dissolution and intestinal permeation of aceclofenac from the engineered nanocrystals.
1. Introduction
Effective delivery of most of the new or already available pharmaceuticals to the human body has always been a challenge to the formulation scientist. Over the last decade, there has been brain storming on the various physicochemical properties required for successful delivery of drug candidates.1–3 Unlike phenotypic approach, often the physicochemical and biological issues are ignored during drug discovery through target based approach.4 One such challenging issue related to the physicochemical property of drugs is the aqueous solubility. With the increasingly hydrophobic nature of the candidate drugs, solubility and dissolution rates have become the limiting factors that affect bioavailability of oral and parenteral formulations.5 Particle size reduction is a very promising strategy to improve the dissolution rate and hence oral bioavailability of poorly soluble drugs. According to the Noyes–Whitney equation, when particle size is reduced to the nanometer size range, the dissolution rate (kinetic improvement) is increased due to increased surface area.5,6 Besides that, an enhancement of saturation solubility (thermodynamic improvement) is observed in accordance to the Ostwald–Freundlich equation, which is normally not observed with micronized particles.6 The particle size can also affect the interaction between the particles and the cells, e.g. by enabling an improved payer's patch uptake.7Four different synthesis techniques for drug nanoparticles can be distinguished namely, chemical reactions, bottom-up or precipitation techniques, top-down or size reduction techniques and combinative approaches.8–11 The latter ones combine bottom-up with top-down steps for an enhanced particle size reduction effectiveness.12 However, stabilization of the small nanosized drug crystals is more crucial than creating them during bottom-up synthesis. Molecular interactions between the nascent particles and the surface stabilizer molecules play a crucial role in the process of stabilization. Presence of stabilizers decreases the surface tension at the solid–liquid interface and thereby increases the nucleation rate.13 Recently, precipitation in the presence of special polymers as stabilizers to prevent crystal growth was successfully applied for some APIs, such as ibuprofen, itraconazole and ketoconazole.14,15
Aceclofenac is an orally effective non-steroidal anti-inflammatory drug (NSAID) of the phenyl acetic acid group, which possesses remarkable anti-inflammatory, analgesic and antipyretic properties.16,17 Among the NSAIDs, aceclofenac is considered to be well tolerated with a lower incidence of gastrointestinal adverse effects.
Owing to poor aqueous solubility, aceclofenac exhibits low bioavailability after oral administration.18,19 Therefore, improvement of dissolution from its oral solid dosage forms is an important issue for enhancing bioavailability and therapeutic efficacy of aceclofenac. Co-crystals20 and surface solid dispersions21 has been attempted to improve the dissolution rate of aceclofenac. However, a few attempts has been made to prepare pure drug nanocrystals of aceclofenac for possible improvement of dissolution velocity and hence bioavailability.
In the present work, particle engineering was carried out to obtain pure drug nanocrystals of aceclofenac using a standard, simple and scalable bottom up technique to overcome its water insolubility and poor dissolution behavior, and the optimum particle was obtained through the screening of different polymeric stabilizers. The stabilizers used in this study were from diverse classes like semisynthetic non-ionic polymers [methylcellulose (MC) and hydroxypropyl methylcellulose (HPMC)], semisynthetic ionic polymer [sodium carboxy methyl cellulose (NaCMC)] and synthetic linear polymers [polyvinyl pyrrolidone (PVP) and polyvinyl alcohol (PVA)]. The physicochemical properties were evaluated including particle size distribution, powder X-ray diffractometry (PXRD), scanning electron microscopy (SEM) and in vitro dissolution studies. Intestinal permeation studies in isolated goat intestine were also carried out.
2. Materials and methods
2.1. Materials
Aceclofenac was obtained as a gift sample from Cipla Ltd (Mumbai, India). HPMC (Hydroxypropyl Methyl Cellulose, methoxyl content: 28–30%, hydroxypropyl content: 7–12%, nominal viscosity of a 2% (w/v) aqueous solution of 5 mPa s) was obtained as gift sample from Colorcon Asia Pvt. Limited (Goa, India). NaCMC (sodium content: 6.5–9.5%, degree of substitution type: 0.9, viscosity of 2% w/v solution: 25–36 mPa s) and methylcellulose (methoxyl substitution: 27.5–31.5%, viscosity of a 2% aqueous solution: 12–18 mPa s) were obtained from The Dow Chemical Company, Michigan, USA. Polyvinylalcohol (PVA, MW 125![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was obtained from SD Fine-Chem. Ltd., Boisar, India. Polyvinylpyrrolidone (PVP; K value: 26–35) was purchased from HiMedia Laboratories Pvt. Ltd., Mumbai, India.
000) was obtained from SD Fine-Chem. Ltd., Boisar, India. Polyvinylpyrrolidone (PVP; K value: 26–35) was purchased from HiMedia Laboratories Pvt. Ltd., Mumbai, India.
2.2. Preparation of aceclofenac nanocrystals
Nanocrystals were produced by adding aqueous solution (0.1%, 0.3% or 0.5% w/v) of polymers such as HPMC, MC, NaCMC, PVA and PVP in acetone solution of aceclofenac (10% w/v solution) under magnetic stirring. Simultaneous cooling was also applied. Rate of antisolvent addition, temperature and mixing rate were kept constant. Centrifugation was carried out to separate the drug particles followed by vacuum drying.2.3. Particle size analysis
The size of drug nanoparticles was measured by dynamic laser light scattering (Nanoparticle size analyzer, Microtrac flex). Before analysis, the drug suspension was diluted by purified water to 0.2 mg ml−1. Poly dispersion index (PDI), graphic mean size (Mz) & calculated surface area (Cs) were used to interpret the results of particle size analysis.2.4. In vitro dissolution studies
The in vitro dissolution studies were carried out using USP Type I (basket type) dissolution apparatus. The dissolution media used was 900 ml of 1% (w/v) sodium lauryl sulfate (SLS) solution in distilled water. The dissolution studies were carried out for 60 minutes. The dissolution medium was kept in thermostatically controlled water bath, maintained at 37 ± 0.05 °C. Basket rotation was adjusted to 100 rpm. At definite intervals, 5 ml samples were withdrawn and analyzed spectrophotometrically at 274 nm for the drug release. At each time of withdrawal, 5 ml of fresh corresponding medium was replaced into the dissolution flask to maintain the sink condition.2.5. Scanning electron microscopy (SEM)
Scanning electron microscopy was used to characterize the particle morphology of the unprocessed drug as well as the fabricated drug nanoparticles. A small fraction of each drug powder sample was fixed on a double-sided conductive carbon tape and sputter-coated with 5 nm of a Pt–Pd alloy. Micrographs were obtained on a Jeol Scanning Electron Microscope (Model: JSM 5200, Japan).2.6. Powder X-ray diffraction (PXRD)
Samples of unprocessed drug and the fabricated nanocrystals were assessed for crystallinity using X-ray diffractometer (Model: SEIFERT, C-3000, Germany) using Nickel-filtered CuKα radiation (λ = 1.54 Å). The voltage and current were 30 kV and 15 mA, respectively. Measurements were carried out in the angular scan range from 5° to 40° (2θ) at a scan speed of 1° min−1.2.7. Ex vivo intestinal permeation studies
The method adopted earlier by our research group was followed to estimate the intestinal permeation of the samples under investigation.22 In brief; small intestine of goat was collected from a local slaughter house for the study, kept in buffer fluid (Krebs–Ringer solution) and used immediately without storing for a prolonged period. The tissue sample was cleaned properly to separate the mesentery, rinsed with the buffer and then cut in different sections. Each section was everted on a Teflon rod, and fixed on its location by means of a thread. The experimental set up followed as mentioned by Meriani et al.23 with slight modification. Intestinal holder was cylindrical glass vessel connected to a “U” glass tube whose one portion was represented by the intestine. Intestine holders (four in one set) filled with buffer fluid represented the receiver environment (4 × 12 ml) and the holder placed in the donor environment. Both receiver and donor phases were continuously aerated to keep the intestine cells alive during experimentation. At regular interval of time, after beginning of the permeation test, 4 ml of the receiver phase were sampled from each intestine holder and replaced with pure buffer, for a time duration of 120 min. Ibuprofen concentration in each of four liquid phases sampled was estimated by UV spectrophotometer (UV-160, Shimadzu, Japan).2.8. Statistical data analysis
The data was subjected to one way analysis of variance followed by all pair-wise multiple comparison procedures (Holm-Sidak method) at overall significance level of 0.05 using Sigma Stat software (Sigma Stat 3.5, SPSS Inc, Chicago, IL).3. Results and discussion
3.1. Particle size analysis
The particle size analysis for the raw aceclofenac, recrystallized drug (ReD) and fabricated nanoparticles using various stabilizers revealed that presence of stabilizer influenced the particle size. Poly dispersion index (PDI), graphic mean (Mz) & calculated surface area (Cs) were used to interpret the results of particle size analysis (Table 1). Graphic mean provides a less coarse-particle weighted mean particle size than mean diameter of the volume distribution. While it includes the median value, it can provide a different and possibly better control value since both small particles and large particles are included in the calculation. Smaller graphic mean (Mz) values indicating smaller particles were found when MC was used as stabilizer (Table 1). The Mz value for the raw aceclofenac was found maximum (213.8 nm) indicating bigger particles. The concentration of polymeric stabilizer found to influence the particle size. Increasing the concentration of most of the studied polymers from 0.1 to 0.3% decreased the particle size except for Na CMC. Polymeric stabilizers help in preventing growth and stabilize the particles essentially by the same way as of surfactants, i.e. adsorption at the solid–liquid interface and reduction of the interfacial tension leading to an increased rate of nucleation. Moreover, polymers also get accumulated in the hydrodynamic layer between the particles and hinder their collision and subsequent growth.24,25 During the crystal formation stage, adsorption of polymers on the particles further helps in stabilization by providing steric hindrance. In the present investigation, the particle size of all the fabricated nanocrystals, except those with Na CMC, were found significantly less compared to raw drug and recrystallized drug indicating excellent stabilization of nanoparticles. Sodium carboxy methylcellulose is an ionic polymer and ionic polymers generally have a higher solubility in water compared to non-ionic counterparts like HPMC or MC. Hence, according to Lundlius' rule, the quantity of polymer on the adsorption surface is decreased owing to the enhancement of the polymer solubility.26 This enhances the particle agglomeration and increases the final particle size. Moreover, because of the higher solubility of these polymers, they increase the drug solubility, reduce the degree of supersaturation and increase the particle size as a result of a reduced nucleation rate. This could be the probable reason for formation of comparatively larger particles with Na CMC. This is in good agreement with the findings of Dalvi and Dave, where polymer JR 400 (more soluble) increased the particle size compared to HPMC, a less soluble polymer.27| Product code | Stabilizer (conc. in %) | Cumulative % drug dissolved at 1 h | Particle size analysis | ||
|---|---|---|---|---|---|
| Polydispersion index (PDI) | Graphic mean size (Mz, nm) | Calculated surface area (Cs, m2 cm−3) | |||
| Raw drug | — | 42.63 ± 2.131 | 1.3811 | 213.8 | 57.82 |
| Recrystallized drug (ReD) | — | 59.27 ± 1.572 | 1.4271 | 207.4 | 57.22 |
| MC1 | MC (0.1) | 86.14 ± 1.226 | 0.2517 | 79.2 | 68.23 |
| MC3 | MC(0.3) | 88.27 ± 2.053 | 0.2581 | 78.5 | 70.51 |
| MC5 | MC(0.5) | 85.33 ± 1.831 | 0.2734 | 79.6 | 65.07 |
| Na CMC1 | CMC(0.1) | 49.01 ± 1.811 | 1.8346 | 208.7 | 58.24 |
| Na CMC3 | CMC(0.3) | 47.2 ± 1.349 | 1.9934 | 211.5 | 58.11 |
| Na CMC5 | CMC(0.5) | 46.8 ± 1.621 | 1.8461 | 212.3 | 57.88 |
| HPMC1 | HPMC(0.1) | 69.1 ± 2.331 | 0.2955 | 137.6 | 59.46 |
| HPMC3 | HPMC(0.3) | 76.8 ± 2.126 | 0.2836 | 110.2 | 60.57 |
| HPMC5 | HPMC(0.5) | 81.64 ± 1.973 | 0.2243 | 92.3 | 62.43 |
| PVP1 | PVP(0.1) | 64.60 ± 1.592 | 0.3616 | 142.6 | 58.38 |
| PVP3 | PVP(0.3) | 68.63 ± 2.668 | 0.2761 | 139.5 | 58.46 |
| PVP5 | PVP(0.5) | 59.16 ± 1.271 | 0.5018 | 146.2 | 57.77 |
| PVA1 | PVA(0.1) | 67.91 ± 2.062 | 0.3462 | 143.8 | 58.61 |
| PVA3 | PVA(0.3) | 69.37 ± 1.642 | 0.2916 | 133.5 | 58.73 |
| PVA5 | PVA(0.5) | 41.30 ± 1.553 | 0.4624 | 279.3 | 56.15 |
PVA and PVP failed to retard the growth of nanocrystals and the particles were found to be bigger. These synthetic linear polymers used in this study were less viscous than HPMC or MC. The viscosity of the polymers also plays a vital role in controlling the particle size. High viscous polymers reduce the mobility of nuclei and thereby reduce the collision frequency and hence reduce the final particle size.
Poly dispersion index (PDI) indicates the width of particle size distribution curve. Smaller PDI indicate narrow particle size distribution which is quite often desired for synthesis of drug nanoparticles. Use of stabilizers like MC, HPMC, PVP and PVA resulted in a narrow particle size distribution with PDI values less than 0.5 (Table 1). Raw drug, recrystallized drug without stabilizer and fabricated particles in presence of Na CMC as stabilizer resulted in broader particle size distribution (PDI values greater than 1).
Calculated surface area (Cs) is an indication of specific surface area. The Cs values were found to increase when stabilizers are used. The value was found maximum with MC stabilizer indicating smaller particles (Table 1).
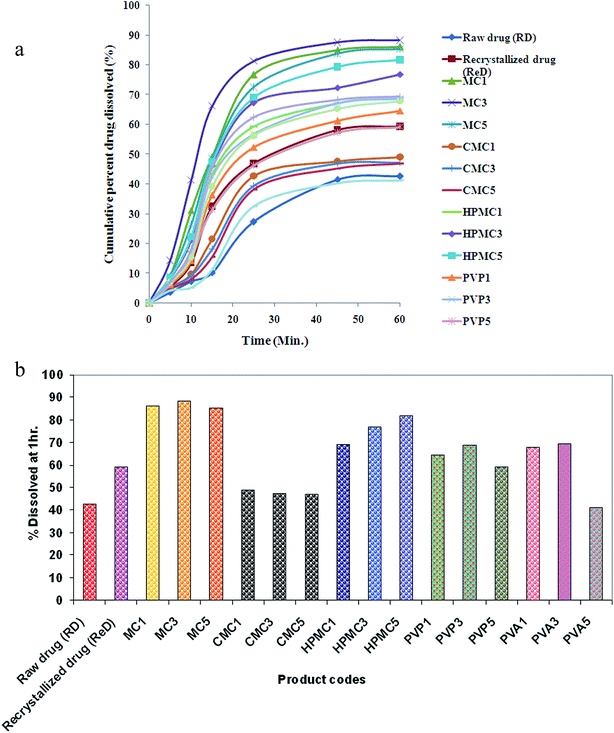
3.2. In vitro dissolution studies
In vitro dissolution studies of raw aceclofenac, recrystallized aceclofenac and the synthesized aceclofenac particles were carried out in distilled water containing 1% sodium lauryl sulfate (SLS). The surface tension is lowered by addition of SLS in an attempt to mimic in vivo conditions.28,29The studies revealed improved dissolution of aceclofenac through the process of nanonization carried out in presence of various stabilizers (Fig. 1a and b). Among the stabilizers studied in this investigation, MC was found most efficient which lead to highest dissolution (MC3; 88.27 ± 2.053%). The performance of stabilizers with respect to dissolution of aceclofenac nanocrystals is as follows; MC > HPMC > PVA > PVP > NaCMC (Table 1). The particle size of the engineered nanocrystals also followed the same order. Hence, the extent of improvement in dissolution is directly related to the extent of reduction in particle size. Reduction in particle size results in greater effective surface area available for dissolution of drug. In a similar study, such reduction in particle size of clarithromycin to as small as about 400 nm using precipitation–lyophilization–homogenization method, improved the in vitro dissolution up to 80%.30 Moreover, for smaller particles, the thermodynamic solubility (cs) of the particle is enhanced due to the high curvature at the particle interface according to the Kelvin equation31
Hence, the increased surface area and solubility enhanced dissolution velocity of drug from the fabricated nanoparticles.
3.3. Scanning electron microscopy (SEM)
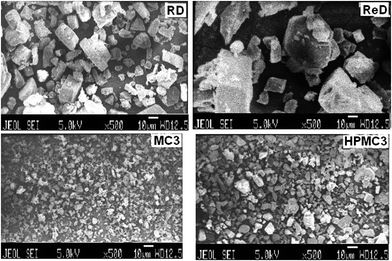
The assessment of morphology of the particles using SEM indicated slab like crystals in raw aceclofenac and recrystallized drug (Fig. 2). It is evident from the SEM study that the crystal engineering process to obtain nanocrystals significantly altered the shape and size of aceclofenac particles. Though spherical shaped particles are often desired, irregular shaped particles were found in our study.3.4. Powder X-ray diffraction (PXRD)
The PXRD indicated that all the samples had the same polymorph with similar peak positions (Fig. 3). The samples exhibited the characteristic diffraction peaks at 2θ values of 18.48°, 19.4°, 22.28°, 24.56°, 23.96°, 24.36°, 25.92°, 32.16°, 38.00°, etc., indicating the crystalline nature of aceclofenac. However, there were some differences among the samples in the relative intensities of each peak. These differences can be explained by preferred orientation, in which the distribution of crystal orientation is nonrandom and crystals may tend to grow greater or lesser degree to specific orientation.32 This result might be due to a difference, not of polymorph, but of crystal habit. The difference could be because the abundance of the planes exposed to the X-ray source would have been changed, thus producing the change in the relative intensities of the peak.333.5. Ex vivo intestinal permeation
Intestinal permeation studies using isolated goat intestine is a standard method to assess the rate of permeation of drugs upon oral administration.23,34 The study conducted for two hours revealed a significant (p < 0.05) improvement of rate and extent of permeation of aceclofenac from MC3 nanocrystals when compared to raw aceclofenac or recrystallized aceclofenac samples.Raw aceclofenac (RD) and recrystallized aceclofenac sample (ReD) samples showed lower percent of permeation (21.12 ± 2.75% and 23.49 ± 3.24% respectively). However, the polymer stabilized nanocrystals (MC3) exhibited significantly (p > 0.05) higher intestinal permeation (62.10 ± 3.72%). The differences in percent permeation was found statistically significant at a level of significance of 0.05 when the observed data were subjected to one way ANOVA followed by post hoc Tukey test.
Drug dissolution in gastrointestinal fluid is a prerequisite step in oral absorption of drugs. The oral absorption of ibuprofen is dissolution rate limited and hence approaches to improve dissolution of such dissolution rate limited drugs will improve oral bioavailability.5 The polymeric stabilizers studied in this research demonstrated significant improvement in dissolution of aceclofenac which has resulted in improved intestinal permeation.
4. Conclusion
In this study, aceclofenac nanocrystals were prepared using a very simple bottom up approach and various polymeric stabilizers like MC, HPMC, Na CMC, PVP and PVA were used to inhibit crystal growth and aggregation. Both the concentration and type of polymer influenced the particle size and dissolution velocity of aceclofenac. Semi-synthetic non ionic polymers like MC and HPMC were found very effective in reducing the particle size (preventing growth of crystals) and improving the dissolution of aceclofenac from the engineered nanocrystals. Intestinal permeation studies revealed statistically significant improvement of efficacy of fabricated nanocrystals (MC3 and HPMC3) in terms of percentage permeation of aceclofenac indicating enhanced bioavailability through improved dissolution of aceclofenac nanocrystals.Conflicts of interest
The authors disclose no conflicts of interest.References
- G. Vistoli, A. Pedretti and B. Testaet, Drug Discovery Today, 2008, 13, 285–294 CrossRef CAS PubMed.
- M. Q. Zhang and B. Wilkinson, Curr. Opin. Biotechnol., 2007, 18, 478–488 CrossRef CAS PubMed.
- S. Mallick, S. Pattnaik, K. Swain, P. K. de, A. Saha, G. Ghoshal and A. Mondal, Eur. J. Pharm. Biopharm., 2008, 68, 346–351 CrossRef CAS PubMed.
- K. Kawakami, Adv. Drug Delivery Rev., 2012, 64, 480–495 CrossRef CAS PubMed.
- S. Mallick, S. Pattnaik, K. Swain and P. K. de, Drug Dev. Ind. Pharm., 2007, 33, 865–873 CrossRef CAS PubMed.
- F. Kesisoglou, et al., Adv. Drug Delivery Rev., 2007, 59, 631–644 CrossRef CAS PubMed.
- M. P. Desai, V. Labhasetwar, G. L. Amidon and R. J. Levy, Pharm. Res., 1996, 13, 1838–1845 CrossRef CAS.
- M. S. Kim, S. J. Jin, J. S. Kim, H. J. Park, H. S. Song, R. H. H. Neubert and S. J. Hwang, Eur. J. Pharm. Biopharm., 2008, 69, 454–465 CrossRef CAS PubMed.
- M. Kondo, T. Niwa, H. Okamoto and K. Banjo, Chem. Pharm. Bull., 2009, 57, 657–662 CrossRef CAS.
- X. Chen, T. J. Young, M. Sarkari, R. O. Williams III and K. P. Johnston, Int. J. Pharm., 2002, 242, 3–14 CrossRef CAS.
- N. Rasenack and B. W. Muller, Pharm. Dev. Technol., 2004, 9, 1–13 CrossRef CAS PubMed.
- R. Shegokar and R. H. Muller, Int. J. Pharm., 2010, 399, 129–139 CrossRef CAS PubMed.
- S. Dalvi and R. Dave, Ind. Eng. Chem. Res., 2009, 48, 7581–7593 CrossRef CAS.
- N. Rasenack and B. W. Müller, Int. J. Pharm., 2002, 245, 9–24 CrossRef CAS.
- N. Rasenack and B. W. Müller, Pharm. Res., 2002, 19, 1894–1900 CrossRef CAS.
- British Pharmacopoeia, The Stationary Office, MHRA, British Pharmacopoeial Commission Office, 2005, vol. 1, London.
- K. Parfitt, Analgesics anti-inflammatory and antipyretics, in Martindale: The Complete Drug Reference, ed. J. E. F. Reynolds, Massachusetts, 32nd edn, 1999, pp. 2–12 Search PubMed.
- B. Lee and H. Jung, AAPS Annual Meeting, New Orleans, LA, USA, Pharm. Sci., 1999, supplement 1(4): S-614.
- T. Kim, J. Shin and B. Lee, in AAPS, Annual Meeting, Denver, Colorado, USA, 2001 Search PubMed.
- S. Mutalik, P. Anju, K. Manoj and A. N. Usha, Int. J. Pharm., 2008, 350, 279–290 CrossRef CAS PubMed.
- F. A. Maulvi, S. J. Dalwadi, V. T. Thakkar, T. G. Soni, M. C. Gohel and T. R. Gandhi, Powder Technol., 2011, 207, 47–54 CrossRef CAS PubMed.
- S. Pattnaik, K. Swain, J. V. Rao, V. Talla, K. B. Prusty and S. K. Subudhi, RSC Adv., 2015, 5, 74720–74725 RSC.
- F. Meriani, N. Coceani, C. Sirotti, D. Voinovich and M. Grassi, J. Pharm. Sci., 2004, 93, 540–552 CrossRef CAS PubMed.
- S. L. Raghavan, A. Trividic, A. F. Davis and J. Hadgraft, Int. J. Pharm., 2001, 212, 213–221 CrossRef CAS.
- S. L. Raghavan, K. Schuessel, A. Davis and J. Hadgraft, Int. J. Pharm., 2003, 261, 153–158 CrossRef CAS.
- R. Duro, C. Souto, J. L. Gómez-Amoza, R. Martínez-Pacheco and A. Concheiro, Drug Dev. Ind. Pharm., 1999, 25, 817–829 CrossRef CAS PubMed.
- S. Dalvi and R. Dave, Ind. Eng. Chem. Res., 2009, 48, 7581–7593 CrossRef CAS.
- B. L. Pedersen, A. Mu llertz, H. Brondsted and H. G. Kristensen, Pharm. Res., 2000, 17, 891–894 CrossRef CAS.
- S. Mallick, S. Pattnaik, K. Swain, P. K. de, A. Saha, P. Mazumdar and G. Ghoshal, Drug Dev. Ind. Pharm., 2008, 34, 726–734 CrossRef CAS PubMed.
- B. Morakul, J. Suksiriworapong, M. T. Chomnawang, P. Langguth and V. B. Junyaprasert, Eur. J. Pharm. Biopharm., 2014, 88, 886–896 CrossRef CAS PubMed.
- L. M. Skinner and J. R. Sambles, Aerosol Sci., 1972, 3, 199–210 CrossRef CAS.
- E. Cho, W. Cho, K. H. Cha, J. Park, M. S. Kim, J. S. Kim, H. J. Park and S. J. Hwang, Int. J. Pharm., 2010, 396, 91–98 CrossRef CAS PubMed.
- A. Nokhodchi, N. Bolourtchian and R. Dinarvand, Int. J. Pharm., 2003, 250, 85–97 CrossRef CAS.
- S. Mallick, S. Pattnaik, K. Swain and P. K. de, Drug Dev. Ind. Pharm., 2007, 33, 535–541 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2015 |