A multifunctional multi-walled carbon nanotubes/ceramic membrane composite filter for air purification†
Abstract
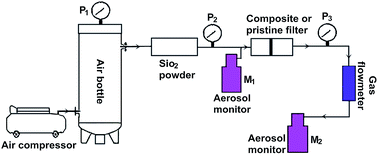
Carbon nanotubes (CNTs) are very small diameter fibers that have the potential to be integrated into filters to further increase particle capture efficiency. In this work, we used a chemical vapor deposition (CVD) method to create the carbon nanotubes/ceramic composite filter by growing multi-walled carbon nanotubes (MWCNTs) on a porous alumina ceramic membrane. Compared with the pristine alumina ceramic membrane, although the mean pore size and porosity of composite filter decreased 9.2% and 11.0% respectively, the resulting composite filter showed significant improvements in air filtration performance, owing to the dramatical increase of specific area by two order of magnitude and enhancement of wall slip flow effect over CNTs. The pressure drop across the composite filters decreased about 62.9% with respect to that of the pristine filters, while the filtration efficiency of the composite filters at the most penetrating particle size (MPPS) has been increased to 99.9999% (reached the standard of ULPA filters), leading to an obvious higher quality factor (Qf). The presence of CNTs strongly inhibits the propagation of bacteria on the filters with an antibacterial rate of 97.86% and show high water repellency (water contact angle of 148.2°). These results make the composite filter very promising for multifunctional air filtration applications.

 Please wait while we load your content...
Please wait while we load your content...