Vanadium(v) tetra-phenolate complexes: synthesis, structural studies and ethylene homo-(co-)polymerization capability†
Abstract
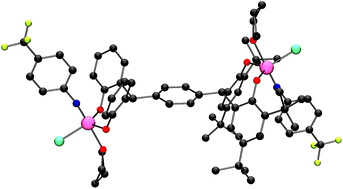
Reaction of α,α,α′,α′-tetrakis(3,5-di-tert-butyl-2-hydroxyphenyl)-p-xylene (p-L1H4) with two equivalents of [VO(OR)3] (R = nPr, tBu) in refluxing toluene afforded, after work-up, the complexes {[VO(OnPr)(THF)]2(μ-p-L1)}·2(THF) (1·2(THF)) or {[VO(OtBu)]2(μ-p-L1)}·2MeCN (2·2MeCN), respectively in moderate to good yield. A similar reaction using the meta pro-ligand, namely α,α,α′,α′-tetrakis(3,5-di-tert-butyl-2-hydroxyphenyl)-m-xylene (m-L2H4) afforded the complex {[VO(OnPr)(THF)]2(μ-p-L2)} (3). Use of [V(Np-R1C6H4)(tBuO)3] (R1 = Me, CF3) with p-L1H4 led to the isolation of the oxo–imido complexes {[VO(tBuO)][V(Np-R1C6H4) (tBuO)](μ-p-L1)} (R1 = Me, 4·CH2Cl2; CF3, 5·CH2Cl2), whereas use of [V(Np-R1C6H4)Cl3] (R1 = Me, CF3) in combination with Et3N/p-L1H4 or p-L1Na4 afforded the diimido complexes {[V(Np-MeC6H4)(THF)Cl]2(μ-p-L1)}·4toluene (6·4toluene) or {[V(Np-CF3C6H4)(THF)Cl]2(μ-p-L1)} (7). For comparative studies, the complex [(VO)(μ-OnPr)L3]2 (8) has also been prepared via the interaction of [VO(nPrO)3] and 2-(α-(2-hydroxy-3,5-di-tert-butylphenyl)benzyl)-4,6-di-tert-butylphenol (L3H2). The crystal structures of 1·2THF, 2·2MeCN, 3, 4·CH2Cl2, 5·CH2Cl2, 6·4toluene·THF, 7 and 8 have been determined. Complexes 1–3 and 5–8 have been screened as pre-catalysts for the polymerization of ethylene in the presence of a variety of co-catalysts (with and without a re-activator), including DMAC (dimethylaluminium chloride), DEAC (diethylaluminium chloride), EADC (ethylaluminium dichloride) and EASC (ethylaluminium sesquichloride) at various temperatures and for the co-polymerization of ethylene with propylene; results are compared versus the benchmark catalyst [VO(OEt)Cl2]. In some cases, activities as high as 243 400 g mmol−1 V−1 h−1 (30.43 kgPE mmol V−1 h−1 bar−1) were achievable, whilst it also proved possible to obtain higher molecular weight polymers (in comparable yields to the use of [VO(OEt)Cl2]). In all cases with dimethylaluminium chloride (DMAC)/ethyltrichloroacetate (ETA) activation, the activities achieved surpassed those of the benchmark catalyst. In the case of the co-polymerization of ethylene with propylene, complexes 1–3 and 5–8 showed comparable or higher molecular weight than [VO(OEt)Cl2] with comparable catalytic activities or higher in the case of the imido complexes 6 and 7.

 Please wait while we load your content...
Please wait while we load your content...