Adjusting phase transition of titania-based nanotubes via hydrothermal and post treatment†
Abstract
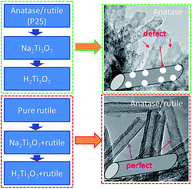
Titania-based nanotubes are prepared via hydrothermal and post treatment of titania with different anatase/rutile ratios. Commerical TiO2 P25 with an anatase/rutile ratio of 80/20 changes to sodium titanate, hydrogen titanate and anatase after hydrothermal treatment at 130 °C over 2 h, ion exchange by washing with water and calcination at 400 °C for 5 h. The final tubular structure has a lot of defects owing to a great quantity of dehydration. Pure rutile is more stable than P25. Lamellars exfoliate from (110) facets of rutile at 130 °C hydrothermal treatment to roll up into nanotubes. Only a small amount of rutile transfers to amorphous sodium titanate within 24 h, and it recrystallizes on the surface of nanotubes and results in a perfect tubular structure with a small amount of dehydration. The dissolution–recrystallization mechanism plays an important role in the composition and crystal structure of titania-based nanotubes.

 Please wait while we load your content...
Please wait while we load your content...