Gold nanoparticle-based fluorescent “turn-on” sensing system for the selective detection of mercury ions in aqueous solution†
Sohee Choi and
Youngmi Kim*
Department of Chemistry, Institute of Nanosensor and Biotechnology, Dankook University, 152 Jukjeon-ro, Suji-gu, Yongin-si, Gyeonggi-do 448-701, Korea. E-mail: youngmi@dankook.ac.kr; Fax: +82-31-8021-7199; Tel: +82-31-8005-3156
First published on 29th October 2015
Abstract
In this study, a simple and straightforward fluorometric assay using dye-adsorbed gold nanoparticles (AuNPs) was used in the highly selective and sensitive detection of mercury ions in aqueous buffer solution. Through the strong affinity of Hg atoms for Au, Hg atoms in situ generated by the reduction of Hg(II) ions in the presence of citrate ions on the surface of AuNPs displaced cationic BODIPY dye (1-PPh3+) from the fluorescence-quenched AuNP/1-PPh3+ adsorbate, leading to a significant increase in fluorescence intensity. The AuNP/1-PPh3+ adsorbate-based sensing system provided a rapid response upon the addition of Hg(II) ions, with highly fluorescent turn-on signals and excellent selectivity.
Monitoring the levels of toxic metal ions, especially Hg(II) ions, is important because contamination by these ions can cause detrimental effects on the environment and on human health, even at very low concentrations.1 As a result, efficient methods for determining Hg(II) ions in environmental, toxicological, and biological samples are highly desirable. Conventional methods for monitoring Hg(II) ions include atomic absorption/emission spectroscopy,2 cold vapor atomic fluorescence spectrometry,3 and inductively coupled plasma mass spectrometry (ICP-MS).4 Although these methods allow sensitive and quantitative determination of Hg(II) ion levels, they require expensive and sophisticated instrumentation, along with tedious sample preparation procedures. Due to the need for simple, rapid, and inexpensive on-site tracking, a number of fluorometric and/or colorimetric approaches using organic chromophores,5 conjugated polymers,6 quantum dots,7 carbon nanodots,8 and metal nanoparticles9 have been developed to detect Hg(II) ions. However, some of these systems have several important drawbacks: they exhibit poor selectivity toward Hg(II) over other metal ions,5i,5j,9f,9h require the use of media that contain a high proportion of organic solvents,5h,5i or necessitate extended reaction times at elevated temperatures.5b,5j Therefore, the development of new Hg(II)-sensing methods that overcome these limitations still remains a challenge.
Gold nanoparticles (AuNPs) are attractive materials for designing sensory schemes, due to their unique optical properties, such as surface plasmon resonance (SPR) absorption sensitive to size, shape, and inter-particle distance of the AuNPs.10 In addition, advantageous optical characteristics such as extremely high molar extinction coefficients (up to 109 M−1 cm−1) and a broad energy bandwidths allow for AuNPs to act as superquenchers through efficient energy- and/or electron-transfer processes between fluorescent molecules and AuNPs.11 Chromophore-functionalized AuNPs have been used in fluorescence “turn on” sensing schemes for detecting various analytes such as metal ions and biomolecules based on modulation of the efficiency of energy transfer between fluorescent dyes and AuNPs.12 Recently, our group used this superquenching property of AuNPs to conduct fluorometric assays of polyamines13 and biological thiols.14 As we continue exploring new sensing systems, we present here a simple and rapid fluorometric assay for the selective detection of Hg(II) ion in aqueous buffer solution, using fluorophore-adsorbed AuNPs as probes. Many AuNP-based sensing systems for the detection of Hg(II) ions utilize the specific interactions between Hg(II) ions and chelating ligands bound to the AuNP surface, resulting in Hg(II)-induced aggregation of functionalized AuNPs.9 Unlike this strategy, our sensing protocol takes advantage of well-known strong Hg–Au metallophilic interactions.15 We envisioned that coupling the Hg–Au metallophilic interaction with the previously reported observation that citrate ions on the surface of AuNPs can reduce Hg(II) ions to Hg(0) on the surface of AuNPs, forming Au–Hg alloys16,17a would serve as a Hg(II) ion-sensor in aqueous media.
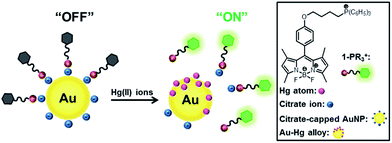
As proposed in Scheme 1, the fluorescence of a cationic organic dye, which adsorbs onto anionic citrate-capped AuNPs through electrostatic interactions, is initially efficiently quenched by energy and/or electron transfer from the fluorophore to AuNPs. Then, Hg atoms, which are generated in situ by the reduction of Hg(II) ions with citrate ions on the AuNP surfaces, deposit onto the surface of the AuNPs, leading to the release of the adsorbed dye from the AuNPs. As a result, the liberated dye recovers its original fluorescence signal rapidly, thereby enabling efficient detection of Hg(II) ions. Similar approaches for determining Hg(II) based on the high affinity of Hg atoms for Au have been reported by several groups.17 However, their selectivity toward Hg(II) over other metal ions is not excellent. As a result, it became necessary to modify the AuNP surfaces with thiol ligands such as mercaptopropionic acid or thioglycolic acid and to perform the assays in the presence of a chelating ligand such as 2,6-pyridinedicarboxylic acid or ethylenediamine tetraacetic acid in order to achieve good selectivity.18 By contrast, our sensing system exhibits excellent selectivity toward Hg(II) against other metal ions, as well as a high degree of simplicity, using as-prepared citrate-capped AuNPs without any additional modification to the AuNP surface and without the introduction of a chelating ligand.
 | ||
| Scheme 1 Proposed sensing approach for Hg(II) ions using fluorescence-quenched AuNP/1-PPh3+ adsorbate; chemical structure of cationic BODIPY derivative 1-PPh3+. | ||
As a choice of fluorophore, we selected a cationic boron dipyrromethane (BODIPY) dye, 1-PPh3+, which in our earlier work demonstrated an excellent ability to form tight electrostatic adsorbate with citrate-capped AuNPs.19 The absorption and emission spectra of 1-PPh3+ show absorption maximum at 496 nm and emission maximum at 511 nm, which is superimposed on the surface plasmon resonance (SPR) band of AuNPs at 520 nm (Fig. S1†). The AuNP/1-PPh3+ adsorbate was prepared by mixing 13 nm citrate-capped AuNPs (3 nM) and 1-PPh3+ (1 μM) for two hours in HEPES buffer (10 mM, pH 7.4, 5% EtOH) at 25 °C and centrifuging. The fluorescence of 1-PPh3+ adsorbed onto the AuNPs was effectively quenched (>98%) relative to that of the unbound pure 1-PPh3+, due to an efficient energy transfer process (Fig. S2†). The AuNP/1-PPh3+ adsorbate has a fluorescence quantum yield of 0.001.
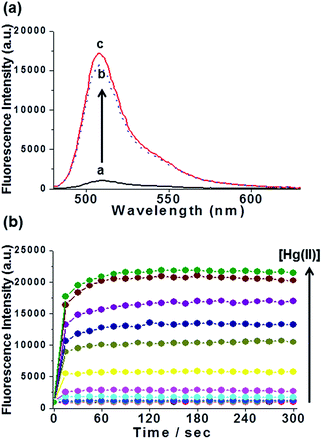
The sensing behavior of the AuNP/1-PPh3+ adsorbate was investigated by adding HgCl2 to a solution of AuNP/1-PPh3+ adsorbate in phosphate buffer (50 mM, pH 8.0) at 25 °C.20 Upon the addition of HgCl2, the increase in intensity of the emission band at λmax,em = 510 nm, which is characteristic of cationic BODIPY 1-PPh3+, occurred in less than 30 s, and it reached saturation within 2 min (Fig. 1). As much as a 22-fold enhancement in the fluorescence intensity at 510 nm was observed upon the addition of 50 μM of HgCl2 to a solution of AuNP/1-PPh3+ adsorbate. After centrifugation of the assay mixture solution, the fluorescence spectrum of the supernatant was almost identical to that of an assay mixture of suspended AuNP/1-PPh3+ adsorbate and HgCl2 (Fig. 1a, lines b and c), suggesting that the 1-PPh3+ liberated from the AuNP/1-PPh3+ adsorbate in the presence of Hg(II) ions is highly fluorescent.
Control experiments showed that the sole addition of 50 μM of Hg(II) ions to pure 1-PPh3+ in phosphate buffer (50 mM, pH 8.0, 5% EtOH, 25 °C) does not directly cause fluorescence quenching or fluorescence enhancement (Fig. S5†). These results indicate that the enhanced fluorescence of the assay solution was mainly caused by the displacement of 1-PPh3+ from the AuNP/1-PPh3+ adsorbate in the presence of Hg(II) ions. Importantly, in the absence of Hg(II) ions, AuNP/1-PPh3+ adsorbate in aqueous phosphate buffer (50 mM, pH 8.0, 25 °C) is stable for more than 1 week without any indication of displacement of 1-PPh3+ from the adsorbate (Fig. S6†).
In addition to the fluorescence change, a noticeably red-shifted SPR band in the UV-vis absorption spectrum of AuNP/1-PPh3+ in the presence of Hg(II) ions (Fig. S7†) demonstrated that adding Hg(II) ions can induce further aggregation of AuNPs through the reduction to Hg atoms, and subsequently, the deposition of Hg atoms onto the AuNP surfaces. This observation is consistent with our working model (Scheme 1), which implies that the surface charge of the AuNPs becomes less negatively charged, losing its affinity for the cationic 1-PPh3+ and becoming less water-soluble during the sensing event.
Fig. 2 shows the fluorescence turn-on response of the AuNP/1-PPh3+ adsorbate to concentrations of HgCl2 ranging from 0 to 50 × 10−6 mol L−1 upon incubation for 2 min. As expected, the fluorescence intensities at 510 nm increased as the HgCl2 concentrations increased. Subsequent data analysis revealed a linear relationship (R2 = 0.992) between the normalized fluorescence intensities at 510 nm and HgCl2 concentrations in the 0.01 × 10−6 mol L−1 to 10 × 10−6 mol L−1 range (Fig. 2, inset). The limit of detection of the AuNP/1-PPh3+ adsorbate was about 5.7 × 10−8 mol L−1 (11 ppb Hg content) for Hg(II),21 on a 3σ/slope basis (Fig. S8†), which was comparable to other previously reported values.
We obtained similar results with other mercury salts, such as Hg(NO3)2 and Hg(ClO4)2, under the same assay conditions (50 mM phosphate buffer, pH 8.0, 25 °C), suggesting that the counter anions exerted a negligible effect on our sensing system (Fig. S9†). Therefore, the AuNP/1-PPh3+ adsorbate is a simple means of determining toxic Hg(II) levels in aqueous solution.
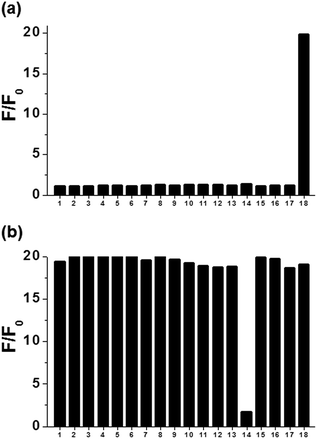
To test the selectivity of our sensing system, the fluorescence response of the AuNP/1-PPh3+ adsorbate toward other metal ions were investigated by monitoring the fluorescence spectra of the AuNP/1-PPh3+ adsorbate upon addition of each metal ion in phosphate buffer (50 mM, pH 8.0) at 25 °C. Fluorescence intensity at 510 nm, measured 2 min after addition of 50 μM of each of the metal ions, showed that a significant fluorescence turn-on response occurred only in the presence of Hg(II) ions. Meanwhile, none of the other metal species, including Ca(II), Cd(II), Cu(II), Co(II), Cr(II), Fe(II), Fe(III), Pb(II), Mg(II), Mn(II), Ni(II), Pd(II), K(I), Ag(I), Na(I), Zn(II), and Al(III), caused positive fluorescence signal changes (Fig. 3a). These results clearly demonstrate that the AuNP/1-PPh3+ adsorbate-based fluorescence sensor is highly selective toward Hg(II) over other metal ions. The excellent selectivity displayed by the AuNP/1-PPh3+ adsorbate is attributed to the highly specific interaction between Hg and Au atoms, which displaced 1-PPh3+ from the AuNP/1-PPh3+ adsorbate, and its aptitude to be reduced to Hg(0) by the citrate-capped AuNPs. However, the fluorescence enhancement induced by Hg(II) ions was interfered by coexisting Ag(I) ions (Fig. 3b). For example, the addition of Hg(II) to a solution of AuNP/1-PPh3+ adsorbate containing Ag(I) led to negligible increases in fluorescence intensity at 510 nm. This phenomenon might be a consequence of the ability of these metal ions to promote the oxidization of Hg(0) to form Hg(II), thus preventing specific interaction between Hg(0) and AuNPs. However, other interfering interactions of silver with AuNP or with the deposition of mercury on their surface cannot currently be entirely ruled out.
To demonstrate potential for the analysis of Hg(II) ions in real samples, we conducted a fluorometric assay of Han river water samples taken in the city of Seoul to which were added different concentrations of Hg(II) ions. The increase in fluorescence intensity of AuNP/1-PR3+ was linearly related with the increasing concentration of Hg(II) ions (0 to 5 μM) in synthetic Han river water samples, offering similar responses to those observed in aqueous phosphate buffer (Fig. S10†).
Conclusions
In summary, we have demonstrated a fluorometric method for detecting Hg(II) ions with high sensitivity and selectivity using an AuNP/1-PPh3+ electrostatic adsorbate in aqueous solution. Based on the high affinity between Hg and Au, the reduced Hg(0) atoms interacted with the AuNPs and efficiently displaced the cationic BODIPY dye 1-PPh3+ from the AuNP/1-PPh3+ adsorbate, resulting in highly fluorescent turn-on signals. This sensing platform exhibits several important features. First, the assay presented herein is simple in design and offers a convenient “mix-and-detect” protocol for rapid detection (within 2 min) of Hg(II) ions. In addition, no further chemical modification of the AuNPs or additional chelating reagent is required, thereby offering a simple and convenient approach as a fluorescence “turn-on” probe for the detection of Hg(II) ions in aqueous media.Acknowledgements
This research was supported by the research fund of Dankook University in 2013.Notes and references
- (a) I. Hoyle and R. D. Handy, Aquat. Toxicol., 2005, 72, 147 CrossRef CAS PubMed; (b) D. P. Wojcik, M. E. Godfrey, D. Christie and B. E. Haley, Neuroendocrinol. Lett., 2006, 27, 415 CAS; (c) S. Koenig, M. Solé, C. Fernández-Gómez and S. Diez, Sci. Total Environ., 2013, 442, 329 CrossRef CAS PubMed.
- (a) Y.-L. Yu, Z. Du and J.-H. Wang, J. Anal. At. Spectrom., 2007, 22, 650 RSC; (b) J. L. Gómez-Ariza, F. Lorenzo and T. García-Barrera, Anal. Bioanal. Chem., 2005, 382, 485 CrossRef PubMed.
- W. Geng, T. Nakajima, H. Takanashi and A. Ohki, J. Hazard. Mater., 2008, 154, 325 CrossRef CAS PubMed.
- (a) Y. Li, C. Chen, B. Li, J. Sun, J. Wang, Y. Gao, Y. Zhao and Z. Chai, J. Anal. At. Spectrom., 2006, 21, 94 RSC; (b) B. M. W. Fong, T. S. Siu, J. S. K. Lee and S. Tam, J. Anal. Toxicol., 2007, 31, 281 CrossRef CAS PubMed.
- (a) E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS PubMed; (b) F. Song, S. Watanabe, P. E. Floreancig and K. Koide, J. Am. Chem. Soc., 2008, 130, 16460 CrossRef CAS PubMed; (c) S. Ando and K. Koide, J. Am. Chem. Soc., 2011, 133, 2556 CrossRef CAS PubMed; (d) C. Chen, R. Wang, L. Guo, N. Fu, H. Dong and Y. Yuan, Org. Lett., 2011, 13, 1162 CrossRef CAS PubMed; (e) K. Tsukamoto, Y. Shinohara, S. Iwasaki and H. Maeda, Chem. Commun., 2011, 47, 5073 RSC; (f) H. Dai, Y. Yan, Y. Guo, L. Fan, Z. Che and H. Xu, Chem.–Eur. J., 2012, 18, 11188 CrossRef CAS PubMed; (g) W. Xuan, C. Chen, Y. Cao, W. He, W. Jiang, K. Liu and W. Wang, Chem. Commun., 2012, 48, 7292 RSC; (h) Y.-K. Yang, K.-J. Yook and J. Tae, J. Am. Chem. Soc., 2005, 127, 16760 CrossRef CAS PubMed; (i) X. Zhang, Y. Xiao and X. Qian, Angew. Chem., Int. Ed., 2008, 47, 8025 CrossRef CAS PubMed; (j) Y.-S. Cho and K. H. Ahn, Tetrahedron Lett., 2010, 51, 3852 CrossRef CAS.
- (a) N. Y. Kwon, D. Kim, J. H. Son, G. S. Jang, J. H. Lee and T. S. Lee, Macromol. Rapid Commun., 2011, 32, 1061 CrossRef CAS PubMed; (b) X. Liu, Y. Tang, L. Wang, J. Zhang, S. Song, C. Fan and S. Wang, Adv. Mater., 2007, 19, 1471 CrossRef CAS; (c) W. Huang and W. Wu, J. Appl. Polym. Sci., 2012, 124, 2055 CrossRef CAS.
- (a) M. Li, Q. Wang, X. Shi, L. A. Hornak and N. Wu, Anal. Chem., 2011, 83, 7061 CrossRef CAS PubMed; (b) L. E. Page, X. Zhang, A. M. Jawaid and P. T. Snee, Chem. Commun., 2011, 47, 7773 RSC.
- L. Zhou, Y. Lin, Z. Huang, J. Ren and X. Qu, Chem. Commun., 2012, 48, 1147 RSC.
- (a) Y.-W. Lin, C.-C. Huang and H.-T. Chang, Analyst, 2011, 136, 863 RSC; (b) C.-W. Liu, C.-C. Huang and H.-T. Chang, Langmuir, 2008, 24, 8346 CrossRef CAS PubMed; (c) H. Wang, Y. Wang, J. Jin and R. Yang, Anal. Chem., 2008, 80, 9021 CrossRef CAS PubMed; (d) C.-C. Huang, Z. Yang, K.-H. Lee and H.-T. Chang, Angew. Chem., Int. Ed., 2007, 46, 6824 CrossRef CAS PubMed; (e) W. Y. Xie, W. T. Huang, J. R. Zhang, H. Q. Luo and N. B. Li, J. Mater. Chem., 2012, 22, 11479 RSC; (f) Y. Kim, R. C. Johnson and J. T. Hupp, Nano Lett., 2001, 1, 165 CrossRef CAS; (g) Y.-R. Kim, R. K. Mahajan, J. S. Kim and H. Kim, ACS Appl. Mater. Interfaces, 2010, 2, 292 CrossRef CAS PubMed; (h) C.-J. Yu and W.-L. Tseng, Langmuir, 2008, 24, 12717 CrossRef CAS PubMed; (i) Y. Xu, L. Deng, H. Wang, X. Ouyang, J. Zheng, J. Li and R. Yang, Chem. Commun., 2011, 47, 6039 RSC; (j) G. K. Darbha, A. K. Singh, U. S. Rai, E. Yu, H. Yu and P. C. Ray, J. Am. Chem. Soc., 2008, 130, 8038 CrossRef CAS PubMed; (k) C. C. Huang and H. T. Chang, Chem. Commun., 2007, 1215 RSC.
- (a) M. Hu, J. Chen, Z.-Y. Li, L. Au, G. V. Hartland, X. Li, M. Marqueze and Y. Xia, Chem. Soc. Rev., 2006, 35, 1084 RSC; (b) M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293 CrossRef CAS PubMed.
- C. H. Fan, S. Wang, J. W. Hong, G. C. Bazan, K. W. Plaxco and A. J. Heeger, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 6297 CrossRef CAS PubMed.
- (a) K. Saha, S. S. Agasti, C. Kim, X. Li and V. M. Rotello, Chem. Rev., 2012, 112, 2739 CrossRef CAS PubMed; (b) K. G. Thomas and P. V. Kamat, Acc. Chem. Res., 2003, 36, 888 CrossRef CAS PubMed; (c) B. I. Ipe, K. Yoosaf and K. G. Thomas, J. Am. Chem. Soc., 2006, 128, 1907 CrossRef CAS PubMed; (d) X. R. He, H. B. Liu, Y. L. Li, S. Wang, Y. J. Li, N. Wang, J. C. Xiao, X. H. Xu and D. B. Zhu, Adv. Mater., 2005, 17, 2811 CrossRef CAS; (e) N. Zhang, Y. Y. Liu, L. L. Tong, K. H. Xu, L. H. Zhuo and B. Tang, Analyst, 2008, 133, 1176 RSC; (f) C. C. Huang, C. T. Chen, Y. C. Shiang, Z. H. Lin and H. T. Chang, Anal. Chem., 2009, 81, 875 CrossRef CAS PubMed.
- T.-I. Kim, J. Park and Y. Kim, Chem.–Eur. J., 2011, 17, 11978 CrossRef CAS PubMed.
- S. Choi, H. Kim, Y. Choi and Y. Kim, Sens. Actuators, B, 2013, 177, 467 CrossRef CAS.
- (a) E. E. Huber, Appl. Phys. Lett., 1966, 8, 169 CrossRef CAS; (b) A. W. Castleman Jr and J. J. Conti, Phys. Rev. A, 1970, 2, 1975 CrossRef; (c) A. Henglein and M. Giersig, J. Phys. Chem. B, 2000, 104, 5056 CrossRef CAS; (d) T. Morris, H. Copeland, E. McLinden, S. Wilson and G. Szulczewski, Langmuir, 2002, 18, 7261 CrossRef CAS.
- (a) Y. J. Long, Y. F. Li, Y. Liu, J. J. Zheng, J. Tanga and C. Z. Huang, Chem. Commun., 2011, 47, 11939 RSC; (b) C.-I. Wang, C.-C. Huang, Y.-W. Lin, W.-T. Chen and H.-T. Chang, Anal. Chim. Acta, 2012, 745, 124 CrossRef CAS PubMed; (c) L. Yan, Z. Chen, Z. Zhang, C. Qu, L. Chen and D. Shen, Analyst, 2013, 138, 4280 RSC.
- (a) C.-Y. Lin, C.-J. Yu, Y.-H. Lin and W.-L. Tseng, Anal. Chem., 2010, 82, 6830 CrossRef CAS PubMed; (b) M. Rex, F. E. Hernandez and A. D. Campiglia, Anal. Chem., 2006, 78, 445 CrossRef CAS PubMed.
- (a) C.-C. Huang and H.-T. Chang, Anal. Chem., 2006, 78, 8332 CrossRef CAS PubMed; (b) G. K. Darbha, A. Ray and P. C. Ray, ACS Nano, 2007, 1, 208 CrossRef CAS PubMed; (c) J. Chen, A. Zheng, A. Chen, Y. Gao, C. He, X. Kai, G. Wu and Y. Chen, Anal. Chim. Acta, 2007, 599, 134 CrossRef CAS PubMed; (d) A. Zheng, J. Chen, G. Wu, H. Wei, C. He, X. Kai, G. Wu and Y. Chen, Microchim. Acta, 2009, 164, 17 CrossRef CAS; (e) H.-Y. Chang, T.-M. Hsiung, Y.-F. Huang and C.-C. Huang, Environ. Sci. Technol., 2011, 45, 1534 CrossRef CAS PubMed.
- At equilibrium, under these initial conditions, the proportion of free 1-PPh3+ to 1-PPh3+ bound to AuNPs is less than 1 in 150. See Fig. S11† and also ref. 13 and 14.
- The fluorometric assay conditions were optimized by monitoring the fluorescence enhancement of the AuNP/1-PPh3+ adsorbate upon addition of Hg(II) ions in assay solutions made of various buffers and at varying pHs as well as concentration of 1-PPh3+ (Fig. S3 and S4†).
- Mercury Update: Impact on Fish Advisories: EPA Fact Sheet EPA-823-F-01–011, EPA, Office of Water, Washington, DC, 2001, the maximum allowable level of mercury in drinking water is 2 ppb.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and characterization and additional absorption and emission spectra. See DOI: 10.1039/c5ra20152g |
| This journal is © The Royal Society of Chemistry 2015 |