Aluminum borohydride–ethylenediamine as a hydrogen storage candidate†
Xiongfei Zheng‡
a,
Xuefeng Huang‡b,
Yuanzhou Songa,
Xiaohua Maac and
Yanhui Guo*a
aDepartment of Materials Science, Fudan University, Shanghai 200433, China. E-mail: gyh@fudan.edu.cn
bSchool of Science, Hangzhou Dianzi University, Hangzhou 310018, China
cCenter of Special Materials and Technology, Fudan University, Shanghai 200433, China
First published on 27th November 2015
Abstract
A series of novel hydrogen storage compounds (Al(BH4)3·nC2H8N2, n = 4, 3, 2, 1) were synthesized. This system turned out to be a reliable hydrogen storage candidate and a maximum of 10.4 wt% pure hydrogen was achieved below 350 °C. Further investigation revealed that the coordination number has a significant impact on their dehydrogenation properties, enabling tunable dehydrogenation of the system.
Solid-state hydrogen storage systems have shown a great prospect for the solution of hydrogen storage problems. One of the most popular systems comprises the BNH compounds, which can release hydrogen via the reaction of the N–Hδ+⋯Hδ−–B dihydrogen bond and have achieved the most impressive dehydrogenation properties for hydrogen storage capacity, dehydrogenation temperature, kinetics and purity.1–12 For the BNH compounds, the N–H mainly comes from ammonia,2,11 amine,4–6 and hydrazine,7 and the B–H mainly comes from borane (BH3), borohydrides (BH4−)8,9 or polyboranes (B3H7, B4H10). Up to now, BNH compounds comprising various N–H and B–H sources have been explored and diverse candidates such as ammonia–borane (AB), M(NH2BH3)m (M = Li, Na, Ca, Mg), ethane-1,2-di-amineborane,10 N2H4BH3, metal borohydride ammoniates (MBAs),13 C(NH2)3BH4, LiBH4·nN2H4, NH3B3H7, etc.7,14–19 have been developed.
According to previous studies on N–H sources, ammines are indicated to be promising for the design of BNH compounds. There are various ammines available, favoring the design of BNH compounds with extensively tunable and customized dehydrogenation. Ammines are found to be efficient in promoting hydrogen emission, e.g. 1,2-di-amineborane releases pure hydrogen without foaming which is superior to ammonia borane (AB).5,10 More importantly, reversibility has been achieved with CBN heterocycles containing the ammine group.1,20 In terms of the B–H sources, borohydrides are used and a series of distinctive MBAs have been synthesized and unique strategies for metal ion replacement,18 and coordination number adjustment2 have been developed to tailor their hydrogen storage properties. Although ammine and borohydride have been justified as reliable N–H and B–H sources, investigation of the combination of ammine with borohydride to produce BNH hydrogen storage compounds has been reported recently.12 The pioneering investigations on Mg(BH4)2·nC2H8N2, n = 4, 3, 2 suggested that amine metal borohydride may serve as a hydrogen storage material.12 However, the onset decomposition temperature of 170 °C for Mg(BH4)2·nC2H8N2 is high and the purity of hydrogen evolution is not satisfactory. Since central metal cations have been demonstrated to play a crucial role in developing BNH hydrogen storage compounds such as metal borohydride ammoniates and metal borohydride amides,21 a similar strategy may be utilized to explore ammine metal borohydrides with a high hydrogen capacity and excellent dehydrogenation properties.
Herein we report the synthesis and detailed hydrogen storage properties of a series of novel ethylene diamine (EDA) aluminum borohydrides – Al(BH4)3·nC2H8N2, n = 4, 3, 2, 1, denoted as EAB. Our results indicate that encouraging dehydrogenation including pure hydrogen evolution without foaming and a low dehydrogenation temperature can be achieved with these compounds. Meanwhile, the thermodynamics and kinetics of the hydrogen emission are adjustable through varying the coordination number.
Synthesis of Al(BH4)3·nC2H8N2, n < 6, was simply realized by reaction of an Al(BH4)3 stream and EDA stream via the following three reactions.
| AlCl3(s) + 3Li(Na)BH4(s) → 3Li(Na)Cl(s) + Al(BH4)3(g) | (1) |
| Al(BH4)3(g) + nC2H8N2(g) → Al(BH4)3·nC2H8N2(s), n < 6 | (2) |
| Al(BH4)3(g) + Al(BH4)3·nC2H8N2 → Al(BH4)3·mC2H8N2(s), m < n | (3) |
Al(BH4)3 is obtained by the reaction of either sodium borohydride or lithium borohydride by reaction (1) (ref. 22) and is carried on a stream of purified N2. Firstly, excessive EDA is used and EAB with a high coordination number is synthesized by reaction (2) within simple apparatus as shown in Fig. 1. To determine the coordination numbers of the product, the Al and active hydrogen content in the products was firstly determined by the methods of Al3+ titration and active hydrogen analysis.23,24 Then, the Al(BH4)3 and EDA content in the as-prepared Al(BH4)3·nC2H8N2 was calculated to determine the coordination number. By further reaction of the above product with Al(BH4)3 via reaction (3), a series of Al(BH4)3·nC2H8N2 compounds with n = 5, 4, 3, 2, 1 were produced. As much as 5 g of Al(BH4)3·nC2H8N2 can be produced at one time in our laboratory.
 | ||
| Fig. 1 Preparation of Al(BH4)3·nC2H8N2. Reactor I and reactor II correspond to the reactions of eqn (1) and (2), respectively. | ||
These white products were firstly characterized by XRD measurements and Fourier transform infrared spectroscopy. It was observed that the XRD patterns of Al(BH4)3·5C2H8N2 and Al(BH4)3·4C2H8N2 were almost the same, while the XRD patterns for the Al(BH4)3·nC2H8N2 compounds (n = 4, 3, 2, 1) varied from each other. These results may suggest that Al(BH4)3 could only combine with 4 equivalent EDA molecules. The extra EDA in Al(BH4)3·5C2H8N2 may be only physically absorbed by Al(BH4)3·4C2H8N2 which may be supported by its independent endothermic desorption at low temperature according to the DSC results (Fig. S3†).
The infrared spectra of Al(BH4)3·nC2H8N2 (n = 4, 3, 2, 1) were recorded in the frequency range 3500–400 cm−1 as shown in Fig. S4.† By comparing with the infrared absorption bands of EDA and [BH4]−, the characteristic bands of EAB were determined. The large and strong bands in the range of 2250–2240 cm−1 correspond to the B–H stretching modes. The bands observed in the range of 1125–1262 cm−l were in the region characteristic of B–H bending frequencies. The fine bands centred around 3200 cm−l are the characteristic bands of the NH stretching modes. In the 1570–1620 cm−1 region the large bands are assigned to the bending vibrations of NH2 groups. Two little weak bands at 2992–2946 cm−1 and 2908–2892 cm−1 are assigned to the C–H antisymmetric stretching mode and symmetric stretching mode respectively. A strong absorption band which appears in the range of 1014–1001 cm−1 belongs to the C–N stretching mode. The infrared spectra deliver some evidence for the interaction of EDA with Al(BH4)3. Compared with pristine EDA, an apparent red shift of the N–H deformation modes in Al(BH4)3·nC2H8N2 (n = 4, 3, 2, 1) was observed (Table S1†), while the vibration modes assigned to CH2 bending, C–C, and C–N stretching did not vary much, indicating the coordination of ammine groups with Al cations.12 Moreover, the characteristic bands for Al(BH4)3·nC2H8N2 with different coordination numbers were quite similar to each other, suggesting a minor impact of coordination number on the infrared absorption of these compounds. Meanwhile, since no evidence for B–H and N–H evolution was present, these compounds are probably the coordination compounds that consist of EDA ligand attached to Al(BH4)3.
To study the dehydrogenation performance of these compounds, comprehensive investigations were conducted using TG/MS (Fig. 2a, S5†), TPD (Fig. 2b) and DSC (Fig. S3†) measurements. The TG analysis of Al(BH4)3·nC2H8N2 (n = 4, 3, 2, 1) shows stepwise dehydrogenation with 18.3%, 10.2%, 9.3% and 10.8% weight loss reached at 350 °C (Tables 1 and S5†), respectively. The MS results indicate that H2 is the predominant product for these complexes. The two-step dehydrogenations are quite evident in that the first step always releases half the amount of the total H2 according to the TPD results, and the gas evolution amount of Al(BH4)3·nC2H8N2 (n = 4, 3, 2, 1) increases with the increase of coordination number (Fig. 2b). Interestingly, the gas released in the first step is always half the amount of the total H2 capacity. For example, Al(BH4)3·2C2H8N2 and Al(BH4)3·3C2H8N2 gave about 4.8 wt% and 4.3 wt% H2 in the first step compared to their H2 capacity of 9.4 and 8.7 wt%, respectively.
| Samplesa | 1st peak T (°C) | 2nd peak T (°C) | 1st step H2 evolutionb (wt%) | H2 capacityb (wt%) | H2 purityb (%) |
|---|---|---|---|---|---|
| a Non-isothermal heat treatment of the samples corresponding to Fig. 1 and S3 (see ESI).b The capacity and purity of H2 emission gas were determined using gravimetric and volumetric results, with the assumption that the impurity was only EDA to facilitate calculation. The wt% is on a material basis. | |||||
| Al(BH4)3·4C2H8N2 | 149 | 198 | 4.8 | 8.4 | 96.1 |
| Al(BH4)3·3C2H8N2 | 145 | 197 | 4.3 | 8.7 | 99.5 |
| Al(BH4)3·2C2H8N2 | 135 | 197 | 4.8 | 9.4 | 99.6 |
| Al(BH4)3·C2H8N2 | 125 | 197 | 5.0 | 10.4 | 99.9 |
Moreover, with the decrease of the coordination number, the first dehydrogenation peak moves to lower temperatures of only 135 °C, and 125 °C for Al(BH4)3·2C2H8N2 and Al(BH4)3·C2H8N2 (Fig. 3). The purities of the gaseous products were estimated using a combination of TG and TPD analysis.9 It is found that the hydrogen purity is also tunable. For Al(BH4)3·4C2H8N2, a trace amount of EDA impurity was detected in the temperature range from 60 °C to 150 °C along with H2 evolution, while on decreasing the coordination numbers to 2 and 1, pure H2 evolution without detectable impurity was observed in the MS results and confirmed by the good agreement between weight and the volumetric results. The above results suggest that the coordination number has significant impacts on the dehydrogenation properties of hydrogen capacity, dehydrogenation temperature and purity, offering an adjustable strategy to control the dehydrogenation of this system. Meanwhile, it’s noteworthy that no foaming was observed for all the series of samples during the decomposition. Compared to Mg(BH4)2·nC2H8N2 (n = 4, 3, 2),12 Al(BH4)3·nC2H8N2 (n = 4, 3, 2, 1) exhibits more outstanding dehydrogenation properties in terms of hydrogen capacity, hydrogenation temperature and H2 purity. The distinction of these two systems in terms of dehydrogenation may be attributed to the diverse cation electronegativity in which the ability of the Al3+ cation to attract N is stronger than that of Mg2+, providing a more convenient pathway for the N–Hδ+⋯Hδ−B combination.18 However, DSC analysis (Fig. S3†) shows that the two dehydrogenation steps for EAB were weakly exothermic, suggesting that dehydrogenation is thermodynamically infeasible to achieve a direct rehydrogenation.
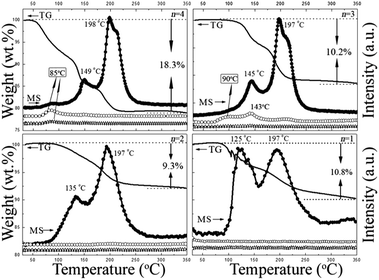 | ||
| Fig. 3 TG (solid line) and MS (symbols) profiles of Al(BH4)3·nC2H8N2 (n = 4, 3, 2, 1), with a heating rate of 5 °C min−1 in argon. ● H2, m/z = 2, ○ EDA, m/z = 30, ☆ B2H6, m/z = 26. | ||
To gain a better understanding of the dehydrogenation process of the products, Al(BH4)3·2C2H8N2 which has the best desorption properties, was investigated using XRD and in situ FTIR spectra. The XRD peaks for Al(BH4)3·2C2H8N2 shift a little to higher degrees at 135 °C, and then turn out to be amorphous after heating at 350 °C (Fig. S5, ESI†). This indicates that a minor change on its framework may occur after the first step, and then the crystal structure was totally destroyed after the second step. In situ FTIR spectra of the Al(BH4)3·2C2H8N2 composite enable an insight into the evolution of the chemical bonds, as shown in Fig. 4. During the first step below 135 °C, a gradual decrease in the intensity of all the bands for both [BH4]− (peaks at 1098, 1168, 2352 and 2249 cm−1)25 and NH2 (peaks at 1318, 1341, 1555 and 1578 cm−1)5 was observed, suggesting that both [BH4]− and NH2 groups contribute to the dehydrogenation. However, the characteristic bands for the NH2 groups and [BH4]− groups still remain at 135 °C (Fig. 4), suggesting the partial consumption of the NH2 and [BH4]− in the first step. Moreover, since the H2 released in the first steps is half the amount of the total H2 capacity, it is supposed that only one of the two terminal NH2 groups of EDA participates in the first dehydrogenation step. In the following step above 135 °C, the intensities of all the bands for [BH4]− (peaks at 1098, 1168, 2352 and 2249 cm−1)25 continue to decrease, but still exist at 350 °C. Meanwhile, the intensity of the residual NH2 group peaks at 1578 and 1555 cm−1 reaches a minimum at 350 °C (Fig. S6†). The decrease in intensity of the peaks corresponding to both the B–H and residual N–H bonds indicates that dehydrogenation through the reaction of N–H bonds with B–H bonds may continue during the second stage. Moreover, combining the evolution of the B–H and N–H bonds with the dehydrogenation results, it is supposed that the residue NH2 group is completely consumed in the second step. Meanwhile, a broad band ranging from 1130 to 1600 cm−1, which can be assigned to the vibration of sp2-kind [–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B
B![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–] species, was intensified after the dehydrogenation of Al(BH4)3·2C2H8N2 at 135 °C.26 Regarding the C–H bonds, their asymmetrical absorption peak at 2992–2946 cm−1 and symmetrical absorption peak at 2908–2892 cm−1 still remain during the heating process as shown in Fig. S6 and S7,† which indicates that the C–H bond was not involved in the dehydrogenation. The above results may suggest that a stepwise combination of the NH2 and [BH4]− groups occurs during the dehydrogenation of Al(BH4)3·2C2H8N2. During the first step below 135 °C, it is supposed that one of the two NH2 terminal groups combines with some of the [BH4]− groups in the compound, through the recombination of N–Hδ+⋯Hδ−–B dihydrogen bonds, then another NH2 group further reacts with [BH4]− at elevated temperature. The recombination of the N–Hδ+⋯Hδ−–B dihydrogen bonds is found to be different to that of the metal borohydride ammoniates (MBAs) in which only two N–H bonds of the NH3 groups combine with B–H bonds at low temperature and the residual N–H bonds react further with excess B–H bonds at elevated temperature.9 This difference strongly suggests that the ligand, depending on the species, has a varied influence on the dehydrogenation of BNH compounds.
N–] species, was intensified after the dehydrogenation of Al(BH4)3·2C2H8N2 at 135 °C.26 Regarding the C–H bonds, their asymmetrical absorption peak at 2992–2946 cm−1 and symmetrical absorption peak at 2908–2892 cm−1 still remain during the heating process as shown in Fig. S6 and S7,† which indicates that the C–H bond was not involved in the dehydrogenation. The above results may suggest that a stepwise combination of the NH2 and [BH4]− groups occurs during the dehydrogenation of Al(BH4)3·2C2H8N2. During the first step below 135 °C, it is supposed that one of the two NH2 terminal groups combines with some of the [BH4]− groups in the compound, through the recombination of N–Hδ+⋯Hδ−–B dihydrogen bonds, then another NH2 group further reacts with [BH4]− at elevated temperature. The recombination of the N–Hδ+⋯Hδ−–B dihydrogen bonds is found to be different to that of the metal borohydride ammoniates (MBAs) in which only two N–H bonds of the NH3 groups combine with B–H bonds at low temperature and the residual N–H bonds react further with excess B–H bonds at elevated temperature.9 This difference strongly suggests that the ligand, depending on the species, has a varied influence on the dehydrogenation of BNH compounds.
In summary, a series of Al(BH4)3·nC2H8N2 compounds, n = 4, 3, 2, 1 were successfully synthesized by facile processes. The dehydrogenation peaks of these compounds were found to decrease gradually with decreasing coordination number, and all the impurities were suppressed at the coordination numbers of 2 and 1. For Al(BH4)3·C2H8N2 as much as 10.4 wt% pure H2 can be achieved at 350 °C, suggesting amine metal borohydride can also be an effective hydrogen storage candidate. Our findings may provide a new strategy for designing future B–N–H systems with more extensively tunable and possibly customized dehydrogenation.
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (51201035, 51471052, U1201241 and 51276053), the doctoral program of higher education of China (20110071120008).Notes and references
- Z. G. Huang and T. Autrey, Energy Environ. Sci., 2012, 5, 9257–9268 CAS.
- Y. H. Guo, Y. X. Jiang, G. L. Xia and X. B. Yu, Chem. Commun., 2012, 48, 4408–4410 RSC.
- Y. Yang, Y. Liu, H. Wu, W. Zhou, M. Gao and H. Pan, Phys. Chem. Chem. Phys., 2014, 16, 135–143 RSC.
- F. Leardini, D. M. Gattia, A. Montone, F. Cuevas, E. Perez-Mayoral, M. J. Valero-Pedraza, M. A. Banares and R. Cantelli, Int. J. Hydrogen Energy, 2015, 40, 2763–2767 CrossRef CAS.
- F. Leardini, M. J. Valero-Pedraza, E. Perez-Mayoral, R. Cantelli and M. A. Banares, J. Phys. Chem. C, 2014, 118, 17221–17230 Search PubMed.
- Z. X. Yang, F. Y. Cheng, Z. L. Tao, J. Liang and J. Chen, Int. J. Hydrogen Energy, 2012, 37, 7638–7644 CrossRef CAS.
- T. Hugle, M. F. Kuhnel and D. Lentz, J. Am. Chem. Soc., 2009, 131, 7444–7446 CrossRef PubMed.
- I. Dovgaliuk, C. S. Le Duff, K. Robeyns, M. Devillers and Y. Filinchuk, Chem. Mater., 2015, 27, 768–777 CrossRef CAS.
- Y. Z. Song, F. L. Wu, X. F. Zheng, X. H. Ma, F. Fang and Y. H. Guo, Chem. Commun., 2015, 51, 1104–1107 RSC.
- D. Neiner, A. Karkamkar, M. Bowden, Y. J. Choi, A. Luedtke, J. Holladay, A. Fisher, N. Szymczak and T. Autrey, Energy Environ. Sci., 2011, 4, 4187–4193 CAS.
- X. Chen, X. Bao, J.-C. Zhao and S. G. Shore, J. Am. Chem. Soc., 2011, 133, 14172–14175 CrossRef CAS PubMed.
- J. Chen, Y. S. Chua, H. Wu, Z. Xiong, T. He, W. Zhou, X. Ju, M. Yang, G. Wu and P. Chen, Int. J. Hydrogen Energy, 2015, 40, 412–419 CrossRef CAS.
- L. H. Jepsen, M. B. Ley, Y. S. Lee, Y. W. Cho, M. Dornheim, J. O. Jensen, Y. Filinchuk, J. E. Jorgensen, F. Besenbacher and T. R. Jensen, Mater. Today, 2014, 17, 129–135 CrossRef CAS.
- Z. T. Xiong, C. K. Yong, G. T. Wu, P. Chen, W. Shaw, A. Karkamkar, T. Autrey, M. O. Jones, S. R. Johnson, P. P. Edwards and W. I. F. David, Nat. Mater., 2008, 7, 138–141 CrossRef CAS.
- T. J. Groshens and R. A. Hollins, Chem. Commun., 2009, 3089–3091, 10.1039/b900376b.
- T. He, H. Wu, G. T. Wu, J. H. Wang, W. Zhou, Z. T. Xiong, J. E. Chen, T. Zhang and P. Chen, Energy Environ. Sci., 2012, 5, 5686–5689 CAS.
- G. Soloveichik, J. H. Her, P. W. Stephens, Y. Gao, J. Rijssenbeek, M. Andrus and J. C. Zhao, Inorg. Chem., 2008, 47, 4290–4298 CrossRef CAS PubMed.
- Y. H. Guo, H. Wu, W. Zhou and X. B. Yu, J. Am. Chem. Soc., 2011, 133, 4690–4693 CrossRef CAS PubMed.
- Y. J. Yang, Y. F. Liu, Y. Li, M. X. Gao and H. G. Pan, Chemistry - An Asian Journal, 2013, 8, 476–481 CrossRef CAS PubMed.
- W. Luo, L. N. Zakharov and S.-Y. Liu, J. Am. Chem. Soc., 2011, 133, 13006–13009 CrossRef CAS PubMed.
- L. H. Jepsen, M. B. Ley, Y.-S. Lee, Y. W. Cho, M. Dornheim, J. O. Jensen, Y. Filinchuk, J. E. Jørgensen, F. Besenbacher and T. R. Jensen, Mater. Today, 2014, 17, 129–135 CrossRef CAS.
- H. I. Schlesinger, H. C. Brown and E. K. Hyde, J. Am. Chem. Soc., 1953, 75, 209–213 CrossRef CAS.
- P. H. Bird and M. G. Wallbrid, J. Chem. Soc., 1965, 3923–3928, 10.1039/jr9650003923.
- J. K. Ruff and M. F. Hawthorne, J. Am. Chem. Soc., 1961, 83, 535–538 CrossRef CAS.
- F. Fang, Y. T. Li, Y. Song, D. L. Sun, Q. G. Zhang, L. Z. Ouyang and M. Zhu, J. Phys. Chem. C, 2011, 115, 13528–13533 CAS.
- C. Gervais, J. Maquet, F. Babonneau, C. Duriez, E. Framery, M. Vaultier, P. Florian and D. Massiot, Chem. Mater., 2001, 13, 1700–1707 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra20005a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2015 |


